Abstract
Introduction
Immediate and acute stent recoil has been observed following balloon deflation in normal and diseased coronary arteries, and the degree varies by stent design.
Methods
A total of 19 patients, who underwent elective stent implantation for single de novo native coronary artery lesions, were enrolled: all patients treated with the biodegradable polymer-coated sirolimus-eluting cobalt–chromium coronary stent system (Supralimus-Core®). The immediate, acute and cumulative stent recoil was assessed by quantitative coronary angiography. The cumulative stent recoil was measured at 24 h of stent implantation.
Results
The absolute late loss due to recoil was found 0.08 ± 0.19 mm for Immediate Stent Recoil (ISR), 0.05 ± 0.21 mm for Acute Stent Recoil (ASR) and 0.11 ± 0.25 mm for Cumulative Stent Recoil (CSR) respectively.
Conclusions
In vivo acute stent recoil of the Supralimus-Core® has higher radial strength compared to other available standard drug-eluting stents.
Keywords: Biodegradable, Coronary artery disease, Recoil, Sirolimus-eluting stent, Cobalt–chromium
1. Introduction
In Percutaneous Coronary Intervention (PCI), coronary stent has been used as standard for treatment of Coronary Artery Disease (CAD). They provide vessel wall scaffolding and prevent early elastic recoil and restenosis as compared to balloon angioplasty.1 The Geometric arterial remolding may be a contributing factor to restenosis in human coronary artery.2–4 Acute stent recoil reflects apparent inadequate stent expansion and decreased final stent area which is a significant forecaster of subsequent restenosis and probably clinical events.5 Drug-coated stents which use bioabsorbable polymer may have more elastic recoil because polymers are more elastic than metal.6 Acute recoil reduces the mean lumen diameter immediately and it may be a contributor for late loss of Minimal Luminal Diameter (MLD) and restenosis.7 It has also been demonstrated that thin-strut Cobalt–Chromium (Co–Cr) stents may lead to lower restenosis than thick strut stainless steel stent. Minimum recoil should be an essential property of an ideal stent. Low recoil (the ability of a stent to maintain its initial expansion diameter) is clinically desirable to reduce the risk of subsequent malapposition or restenosis. In the present study, we determined the acute recoil of the vessel wall immediately and early after Supralimus-Core® Sirolimus-eluting stent system (Sahajanand Medical Technologies Pvt. Ltd., Surat, India) implantation.
2. Materials and methods
This was a prospective, single-centre and non-randomized study. A total 19 patients were enrolled and treated with 19 Supralimus-Core® Sirolimus-eluting coronary stent systems for de novo native coronary artery lesion. Patients with de novo native coronary artery lesions located in epicardial vessel who qualify for percutaneous coronary intervention were included according to the pre-specified inclusion and exclusion criteria for this study as mentioned below. Primary endpoint was immediate and acute recoil following Supralimus-Core® Sirolimus-eluting coronary stent system implantation for de novo native coronary artery lesions. This study was approved by the local ethics committee and all patients gave written informed consent.
During percutaneous coronary intervention procedure, heparin boluses were intravenously administered and were additionally given if needed. All study procedure for target lesion were performed electively and treated using standard interventional techniques, with mandatory pre-dilatation with balloon size not less than 80% of reference vessel diameter. The stent diameter was chosen nearly equal to reference vessel diameter and not exceeding more than 15% of reference vessel diameter. Stents were deployed at 15 atmospheres. Post-dilatation if required was performed with balloon length less than stent length with pressure not exceeding 16 atmospheres. Any patients requiring post-dilatation using higher pressure or higher size balloon were designed to be excluded.
2.1. Inclusion criteria
Patients included in the study were more than 18 years of age with symptomatic ischemic heart disease with de novo stenotic coronary lesion with Reference Vessel Diameter (RVD) of ≥2.5 and ≤4.0 mm. Patient or patient's legal representative was informed of the nature of the study and agreed to its provisions and had provided written informed consent as notified/approved by the Institutional Review Board/Ethics Committee of the clinical site.
This way all included patients had standard deployment pressure of 15 atmospheres. All lesions were pre-dilated with a balloon of same diameter. All stents were deployed using a pressure of 15 atmospheres.
2.2. Exclusion criteria
Patients were excluded if any of the following conditions were present: General exclusion criteria: 1) Women of child bearing potential; 2) Acute or chronic renal dysfunction (creatinine >2.0 mg/dl or >150 μmol/L); 3) Any patient who has a platelet count <100,000 cells/mm3 or > 700,000 cells/mm3, a WBC of <3000 cells/mm3; 4) Evidence of an acute Q-wave or non-Q-wave myocardial infarction within 72 h preceding the index procedure; 5) Restenosis or significant lesion in SVG. 6) Patient with a life expectancy less than 12 months; 7) Known allergies to aspirin, clopidogrel bisulphate (Plavix®), ticlopidine (Ticlid®), heparin or cobalt–chromium; 8) Any significant medical condition which in the investigator's opinion may interfere with the patient's optimal participation in the study; 9) Currently participating in an investigational drug or another device study, or subject to inclusion in another investigational drug or another device study during follow-up; 10) Previous bare metal stenting (less than 1 year) anywhere within the target vessel or previous drug-eluting stenting anywhere within any epicardial vessel.
Angiographic Exclusion criteria: 1) Unprotected left main coronary artery disease with ≥50% stenosis; 2) Angiographic evidence of thrombus 3) Ejection fraction ≤25%; 4) Heavily calcified lesion and/or calcified lesion which cannot be successfully pre-dilated.
2.3. Description of the study stent
Supralimus-Core® (Sahajanand Medical Technologies Pvt. Ltd., Surat, India) has L605 Co–Cr alloy as its stent platform having strut thickness of 60 μm with biodegradable polymers and drug load of 1.4 μg/mm2. About 70% of drug is released within 7 days and remaining drug is released over a period of 48 days. The coating layer comprises of drug Sirolimus blended together with biodegradable polymeric matrix. This matrix includes different biodegradable polymers – poly l-lactide, 50/50 poly dl-lactide-co-glycolide and polyvinylpyrrolidone to control the drug elution from stent coating. After releasing the drug within 48 days, these polymers eventually degrade naturally and are excreted from the body in the form of their metabolites. The average coating thickness of Supralimus-Core® stent is between 5 and 6 μm.
2.4. Interventional procedure and adjunctive medications
All patients were on aspirin in a dose of 75–150 mg at least 24 h prior to the procedure. A loading dose of 300 mg of clopidogrel was given 24 h prior to procedure or 600 mg on the day of the procedure was given to patients before procedure. During the procedure initial dose of 100 IU/kg bolus of heparin was given to the patient. Additional heparin was used if necessary during procedure to achieve activated coagulation time >250 s. Administration of GP IIb/IIIa inhibitor was left to the investigator's discretion.
Pre-procedural pictures were recorded into two orthogonal views. After pre-dilating lesion stent was deployed at 15 atmospheres and a picture of inflated balloon was recorded in two angiographic views. Immediately after stent deployment and after last balloon dilatation angiographic pictures were recorded in two orthogonal views similar to pre-dilatation angiograms. Angiography was performed at 24 h after the stent procedure and pictures were recorded in similar orthogonal views.
2.5. Quantitative coronary angiography analysis
QCA was performed using CAAS V analysis system (Pie Medical BV, Maastricht, the Netherlands). The following QCA parameters were computed: Minimal Luminal Diameter (MLD), Reference Vessel Diameter (RVD), and percent diameter stenosis for pre, immediate post-procedure and 24 h angiogram. Balloon diameter was measured on the inflated balloon picture. Immediate, acute and cumulative stent recoil was calculated by following formula (Fig. 1):
-
1)
Immediate Stent Recoil (ISR): immediate stent recoil was defined as expanded stent mean lumen diameter during balloon inflation (A) minus stent mean lumen diameter at immediate angiography after balloon deflation (B). Percent immediate stent recoil was defined as (A − B)/A × 100
-
2)
Acute Stent Recoil (ASR): acute stent recoil was defined as stent mean lumen diameter at immediate angiography after balloon deflation (B) minus stent lumen diameter at 24hr after angiography (C). Percent acute stent recoil was defined as (B − C)/B × 100
-
3)
Cumulative Stent Recoil (CSR): cumulative stent recoil was defined as expanded stent lumen diameter during balloon inflation (A) minus stent lumen diameter at 24 h angiography (C). Percent cumulative stent recoil was defined as (A − C)/A × 100.
Fig. 1.
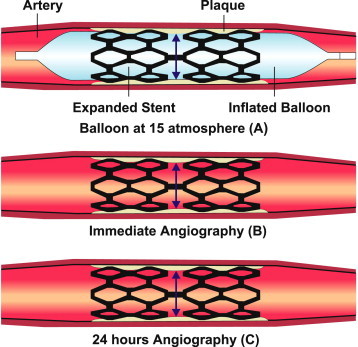
Immediate, acute and cumulative stent recoil.
2.6. Statistical analysis
Discrete variables are expressed as counts and percentages and were compared in terms of relative risks with 95% confidence intervals. Count variables were summarized by the count and the percentage. Continuous variables are expressed as means ± SD. The normality of the distributions of mean acute recoil after deflation of balloon was tested with Shapiro–Wilk test.
3. Results
3.1. Baseline patient characteristics and follow-up
The baseline demographics, procedural and lesion characteristics of the Supralimus-Core® recoil study population have been summarized (Tables 1 and 2).
Table 1.
Baseline demographics data (n = 19).
| Patient characteristics | |
|---|---|
| Age (mean ± SD, years) | 57.3 ± 9.9 |
| Male, n (%) | 15 (78.9%) |
| Diabetes mellitus, n (%) | 11 (57.9%) |
| Hypertension, n (%) | 12 (63.2%) |
| Dyslipidemia, n (%) | 16 (84.2%) |
| Smoking, n (%) | 1 (5.3%) |
| Prior myocardial infarction n (%) | 1 (5.3%) |
| Unstable angina, n (%) | 19 (100%) |
Table 2.
Lesion and procedural characteristics (n = 19).
| Lesion location | |
| Right coronary artery, n (%) | 10 (52.6%) |
| Left circumflex, n (%) | 1 (5.3%) |
| Left anterior descending, n (%) | 8 (42.1%) |
| Lesion type (ACC/AHA) | |
| A, n (%) | 1 (5.3%) |
| B1, n (%) | 4 (21.1%) |
| B2, n (%) | 10 (52.6%) |
| C, n (%) | 4 (21.1%) |
| Stent to artery ratio | 0.92 ± 0.12 |
| Average lesion length, mm | 10.68 ± 3.16 |
| Clinical success | 19 (100%) |
ACC = American College of Cardiology; AHA = American Heart Association.
3.2. Angiographic and clinical outcomes
Total 19 patients (19 lesions) were implanted with 19 stents with average lesion length of 10.68 ± 3.16 mm. All angiograms were evaluated by readers blinded to the study stent, by an independent core laboratory (Zeus Data Power LLP). Using the formula as stated above the ISR, ASR and CSR were found to be 2.42 ± 7.48%, 1.12 ± 7.24% and 3.24 ± 8.27% respectively. Twenty four hours angiographic follow-up was performed in all 19 patients (Table 3). As shown in Tables 4 and 5, acute and cumulative stent recoil was calculated during mean diameter of balloon at the highest pressure, mean diameter of stent immediately after balloon deflation and mean diameter of stent at 24 h follow-up (Fig. 2).
Table 3.
Quantitative coronary angiographic analysis (n = 19).
| QCA parameters | Mean ± SD |
|---|---|
| Pre-PCI | n = 19 |
| Lesion length, mm | 10.68 ± 3.16 |
| Reference vessel diameter, mm | 2.61 ± 0.44 |
| Minimum lumen diameter, mm | 1.11 ± 0.32 |
| Diameter stenosis, % | 55.84 ± 15.87 |
| Post-PCI | n = 19 |
| Minimum lumen diameter, mm | 2.37 ± 0.36 |
| Diameter stenosis, % | 13.32 ± 4.47 |
| Acute gain, mm | 1.26 ± 0.54 |
| Follow-up PCI (24 h) | n = 19 |
| Minimum lumen diameter, mm | 2.30 ± 0.34 |
| Diameter stenosis, % | 16.13 ± 5.85 |
| Late loss, mm | 0.04 ± 0.10 |
Table 4.
Angiographic parameters related with acute stent recoil assessments (n = 19).
| Angiographic parameters | Mean ± SD (mm) |
|---|---|
| Mean diameter of balloon/stent at the highest pressure (mm) | 2.81 ± 0.41 |
| Mean diameter of stent immediately after balloon deflation (mm) | 2.73 ± 0.37 |
| Immediate stent recoil (mm) | 0.08 ± 0.19 |
| Immediate percent recoil (%) | 2.42 ± 7.48 |
Table 5.
Stent recoil (n = 19).
| Mean lumen diameter | Mean ± SD (mm) | % |
|---|---|---|
| Mean stent expansion minus mean post-stent expansion, (mm) (immediate stent recoil) | 0.08 ± 0.19 | 2.42 ± 7.48 |
| Mean post-stent expansion minus mean follow-up stent expansion, (mm) (acute stent recoil) | 0.05 ± 0.21 | 1.12 ± 7.24 |
| Mean stent expansion minus mean follow-up stent expansion, (mm) (cumulative stent recoil) | 0.11 ± 0.25 | 3.24 ± 8.27 |
Fig. 2.
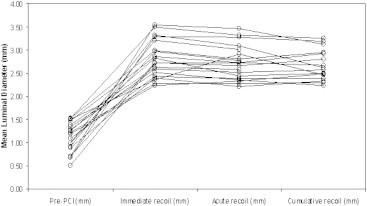
Sequential measurements of minimal luminal diameter of pre-PCI, stent expansion, post-stent expansion and follow-up (each line represents the data from a single patient).
The absolute late loss due to recoil was found 0.08 ± 0.19 mm for ISR, 0.05 ± 0.21 mm for ASR and 0.11 ± 0.25 mm for CSR respectively.
The normality of the distributions of mean acute recoil after deflation of balloon was tested with Shapiro–Wilk test (p = 0.001) and left skewness (−2.083) (Fig. 3).
Fig. 3.
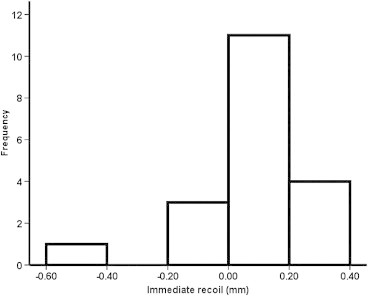
Normality distribution of mean acute recoil.
We tried to establish the correlation of certain variables with the stent recoil. We studied the relationship of reference vessel diameter and lesion length with the immediate recoil using Pearson correlation coefficient. The relationship of reference vessel diameter and lesion length is shown in Figs. 4 and 5. The figures show that there is no strong positive correlation between reference vessel diameter with immediate recoil and very weak positive correlation between lesion lengths with immediate recoil.
Fig. 4.
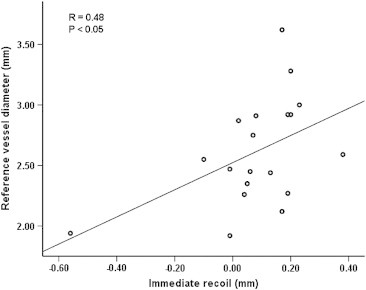
Lesion length vs. immediate recoil.
Fig. 5.
Reference vessel diameter vs. immediate recoil.
We also tried to study the possible effect of diabetes mellitus on recoil as diabetes could affect the elastic property of vessel wall. In our study there were 11 (57.9%) diabetics. The comparison of diabetics and non-diabetics was done using Mann–Whitney U test which is depicted graphically in Fig. 6.
Fig. 6.
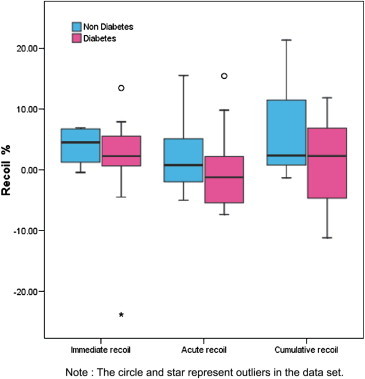
Recoil box plot in diabetic and non-diabetic patients.
There are no in-hospital events and stent thrombosis events reported. No clinical events reported at 6 months.
4. Discussion
The main function of metallic stents is to scaffold the vessel wall and prevent early elastic recoil and acute vessel closure.8 Minimum stent recoil is a desirable property of a stent since stent recoil can contribute to reduced acute gain of MLD and possible contribute to higher late loss of lumen.
Despite advances in polymer and drug technology, the underlying stent platform remains a key determinant of clinical outcome. Reduction in stent strut thickness has been associated with improved stent deliverability, improved procedural outcome, and lower rates of subsequent restenosis. Newer-generation 316L-SS stent designs have enabled reduced strut thickness while retaining radial strength and minimizing recoil. Cobalt–chromium alloys have enabled a reduction in stent strut thickness to around 60–90 mm while retaining modest radiopacity, but due to higher elastic properties, have been possibly associated with greater stent recoil.9
In previous human clinical trials, acute stent recoil varied between 3% and 15% following Wiktor or Palmaz–Schatz stent implantation.10–13 Stent recoil was usually defined as the difference between the minimum balloon diameter and the MLD post-stent implantation. However, usage of minimum variables, proposed by previous investigators, has the potential for assessing only a part of the stented segment, because the balloon does not expand uniformly, causing asymmetric stent expansion. To reflect the behavior of the vessel wall of the entire stented segment, we used mean variables and defined acute stent recoil as the difference between the mean diameter of the last inflated balloon and the mean luminal diameter immediately after the last balloon deflation. Our study demonstrated that the immediate stent recoil of the Supralimus-Core® was 0.08 ± 0.19 mm (2.42%), which is lower than previously reported in vivo conventional metallic stent recoil.
The CSR for Supralimus-Core® stent was 3.24 ± 8.27%. This is lower compared to most available cobalt–chromium stents. This is likely to contribute to better long-term results using Supralimus-Core® stents. However this needs to be established by studying actual clinical and angiographic outcomes.
The comparative stent recoil of the different stents is shown in Fig. 7. Unlike previous studies we studied the immediate, acute and cumulative stent recoil separately. The results bring out very interesting observation that the acute stent recoil (additional recoil at 24 h after immediate recoil) contributed minimally to the cumulative stent recoil and immediate stent recoil reflected most of the total recoil. This has a very important bearing for the future studies of this nature as it obviates the need for 24 h angiogram.
Fig. 7.
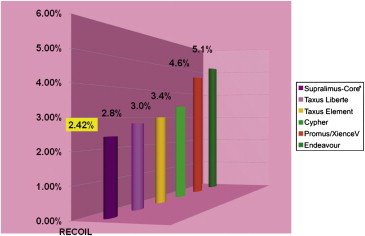
Recoil comparisons across current stent platforms.
We studied the effects of reference vessel diameter and lesion length and found that they have no bearing on the recoil. This suggests that the recoil is probably not affected whether the vessel is large or small in diameter (Figs. 4 and 5). The figures show that there is no strong positive correlation between reference vessel diameter with immediate recoil and very weak positive correlation between lesion lengths with immediate recoil. This however needs to be established in larger numbers as it may have a bearing on future stent designing.
The comparison between diabetics and non-diabetics revealed that presence of diabetes did not impact the recoil. However the small size of this study limits drawing hard conclusions in this regard but opens up interesting probabilities of exploring these aspects further.
The ISR, ASR and CSR of Supralimus-Core® were 2.42 ± 7.48%, 1.12 ± 7.24% and 3.24 ± 8.27% respectively, which is comparable to or lower than recoil of most Co–Cr DES. Stent recoil was not affected by vessel diameter or lesion length.
5. Conclusions
In vivo acute stent recoil of the Supralimus-Core® has higher radial strength compared to other available standard drug-eluting stent.
Funding
This study was supported by Sahajanand Medical Technologies Pvt. Ltd., Surat, India.
Conflicts of interest
Ashok Thakkar is an employee of Sahajanand Medical Technologies Private Limited. Dr. Atul Abhyankar has received consulting fees from Sahajanand Medical Technologies Private Limited.
Footnotes
The institution at which the work was performed: Shree B.D.M. Mahavir Heart Institute, Athwagate, Ring Road, Surat 395001, Gujarat, India.Tel.: +91 9824145738; fax: +91 261 2254669.
References
- 1.Tanimoto S., Serruys P.W., Thuesen L. Comparison of in vivo acute stent recoil between the bioabsorbable everolimus-eluting coronary stent and the everolimus-eluting cobalt chromium coronary stent: insights from the ABSORB and SPIRIT trials. Catheter Cardiovasc Interv. 2007;70(4):515–523. doi: 10.1002/ccd.21136. [DOI] [PubMed] [Google Scholar]
- 2.Painter J.A., Mintz G.S., Wong S.C. Serial intravascular ultrasound studies fail to show evidence of chronic Palmaz–Schatz stent recoil. Am J Cardiol. 1995;75(5):398–400. doi: 10.1016/s0002-9149(99)80564-5. [DOI] [PubMed] [Google Scholar]
- 3.Post M.J., Borst C., Kuntz R.E. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. A study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation. 1994;89(6):2816–2821. doi: 10.1161/01.cir.89.6.2816. [DOI] [PubMed] [Google Scholar]
- 4.Isner J.M. Vascular remodeling. Honey, I think I shrunk the artery. Circulation. 1994;89(6):2937–2941. doi: 10.1161/01.cir.89.6.2937. [DOI] [PubMed] [Google Scholar]
- 5.Regar E., Schaar J., Serruys P.W. Images in cardiology. Acute recoil in sirolimus eluting stent: real time, in vivo assessment with optical coherence tomography. Heart. 2006;92(1):123. doi: 10.1136/hrt.2005.065151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanimoto S., Bruining N., van Domburg R.T. Late stent recoil of the bioabsorbable everolimus-eluting coronary stent and its relationship with plaque morphology. J Am Coll Cardiol. 2008;52(20):1616–1620. doi: 10.1016/j.jacc.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez A., Santaera O., Larribeau M., Sosa M.I., Palacios I.F. Early decrease in minimal luminal diameter after successful percutaneous transluminal coronary angioplasty predicts late restenosis. Am J Cardiol. 1993;71(16):1391–1395. doi: 10.1016/0002-9149(93)90598-7. [DOI] [PubMed] [Google Scholar]
- 8.Farb A., Weber D.K., Kolodgie F.D., Burke A.P., Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002;105(25):2974–2980. doi: 10.1161/01.cir.0000019071.72887.bd. [DOI] [PubMed] [Google Scholar]
- 9.Menown I.B., Noad R., Garcia E.J., Meredith I. The platinum chromium element stent platform: from alloy, to design, to clinical practice. Adv Ther. 2010;27(3):129–141. doi: 10.1007/s12325-010-0022-9. [DOI] [PubMed] [Google Scholar]
- 10.Serruys P., De Jaegere P., Bertrand M. Morphologic change in coronary artery stenosis with the Medtronic Wiktor stent: initial results from the core laboratory for quantitative angiography. Cathet Cardiovasc Diagn. 1991;24(4):237–245. doi: 10.1002/ccd.1810240403. [DOI] [PubMed] [Google Scholar]
- 11.de Jaegere P., Serruys P.W., van Es G.A. Recoil following Wiktor stent implantation for restenotic lesions of coronary arteries. Cathet Cardiovasc Diagn. 1994;32(2):147–156. doi: 10.1002/ccd.1810320210. [DOI] [PubMed] [Google Scholar]
- 12.Haude M., Erbel R., Issa H., Meyer J. Quantitative analysis of elastic recoil after balloon angioplasty and after intracoronary implantation of balloon-expandable Palmaz–Schatz stents. J Am Coll Cardiol. 1993;21(1):26–34. doi: 10.1016/0735-1097(93)90713-b. [DOI] [PubMed] [Google Scholar]
- 13.Rechavia E., Litvack F., Macko G., Eigler N.L. Influence of expanded balloon diameter on Palmaz–Schatz stent recoil. Cathet Cardiovasc Diagn. 1995;36(1):11–16. doi: 10.1002/ccd.1810360105. [DOI] [PubMed] [Google Scholar]


