Abstract
Background
Cardiac resynchronization therapy is an important therapeutic modality in drug refractory symptomatic patients of heart failure with wide QRS (≥120 ms) on electrocardiogram. However, wide QRS (considered as a marker of electrical dyssynchrony) occurs in only 30% of heart failure patients, making majority of drug refractory heart failure patients ineligible for resynchronization therapy. Significant numbers of patients with narrow QRS have echocardiographic evidence of left ventricular dyssynchrony. However, there is sparse data about additional features on the surface ECG which can predict intraventricular dyssynchrony. This study was undertaken to assess the utility of fragmented narrow QRS complex to predict significant intraventricular dyssynchrony in symptomatic patients of non-ischemic dilated cardiomyopathy.
Method
100 symptomatic patients of non-ischemic dilated cardiomyopathy with narrow QRS complexes (50 each with fragmented and normal QRS) were recruited. Tissue Doppler imaging was used to assess intraventricular dyssynchrony as per ‘Yu index’.
Results
78% patients (n = 39) in fQRS complex group and 14% (n = 7) in normal QRS complex group had significant intraventricular dyssynchrony (χ2 = 20.61; p < 0.000005). fQRS complexes had 84.78% sensitivity, 79.62% specificity, a positive predictive value of 78% and negative predictive value of 86% to detect intraventricular dyssynchrony. fQRS also had sensitivity and specificity of 93% and 90% respectively to localize the dyssynchronous segment.
Conclusion
fQRS is a marker of electrical dyssynchrony, which results in significant intraventricular dyssynchrony in patients of non-ischemic dilated cardiomyopathy and a narrow QRS interval. fQRS localizes the dyssynchronous segment and might be useful in identifying patients who can benefit from cardiac resynchronization therapy.
Keywords: Cardiomyopathy, Intraventricular dyssynchrony, Fragmented QRS complex, Tissue Doppler imaging
1. Introduction
Cardiac resynchronization therapy (CRT) is a useful therapeutic strategy in drug refractory symptomatic patients of heart failure (HF) with wide QRS (≥120 ms) on electrocardiogram (ECG).1 Though QRS duration ≥120 ms has been adopted as a marker of electrical dyssynchrony responsible for presence of left ventricular (LV) dyssynchrony and subsequent eligibility for CRT, studies suggest that QRS widening ≥120 ms occurs in only 30% of HF patients.2,3 In patients of HF with normal QRS duration, LV dyssynchrony has been reported in 20–50% patients.4–7 However, there are not enough studies available analyzing additional features on the surface ECG that can be taken as marker of electrical dyssynchrony resulting in significant intraventricular dyssynchrony (IVD) in patients of HF with narrow QRS. Till date only two studies have reported that fQRS on the surface ECG is a predictor of significant IVD in patients of non-ischemic DCM.8,9 We undertook this study to assess the sensitivity, specificity and positive predictive value (PPV) of fQRS complex on the surface ECG to detect significant IVD in symptomatic patients of non-ischemic DCM.
2. Methods
2.1. Patients selection
This study was conducted in GB Pant Hospital, New Delhi, a tertiary care centre, located in the Northern part of India. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
A total of 100 patients of chronic HF due to non-ischemic dilated cardiomyopathy (DCM), with LV ejection fraction of less than 35% and sinus rhythm having narrow QRS complexes (<120 ms), were prospectively recruited. These included 50 patients with fQRS complexes and another 50 without fragmentation of QRS complexes. All patients included in the study had LV dysfunction of at least 2 years and were symptomatic (New York Functional Class II or above) in spite of optimal medical therapy for at least 6 months (Table 1). The patients were evaluated carefully for their functional capacities and 12 lead ECGs were obtained. Coronary angiogram was done in all patients to rule out the presence of significant (≥50% stenosis in any epicardial coronary artery) coronary artery disease (CAD). Patients with organic valvular heart disease, history suggestive of CAD, atrial fibrillation, kidney failure, liver failure and on permanent pacemakers were excluded from the study. The study protocol was approved by the institutional review board. Written informed consent was obtained from all participants.
Table 1.
Demographic, clinical, electrocardiographic and basic echocardiographic parameters.
| Fragmented QRS (n = 50) | Normal QRS (n = 50) | Chi square value | p value | |
|---|---|---|---|---|
| Age (in years) | 45.88 ± 13.70 | 47.84 ± 14.07 | 0.48 | |
| Sex (M:F) | 32:28 | 28:22 | 0.33 | 0.56 |
| NYHA CLASS (II:III-IV) | 27:23 | 36:14 | 4.45 | 0.22 |
| Beta blocker use n (%) | 45 (90%) | 42 (84%) | 0.40 | 0.53 |
| ACE-inhibitor use n (%) | 43 (86%) | 45 (90%) | 0.19 | 0.66 |
| Digoxin n (%) | 45 (90%) | 43 (86%) | 0.19 | 0.66 |
| Heart rate (BPM) | 93.08 ± 17.21 | 92.32 ± 21.28 | 0.84 | |
| PR interval (ms) | 150.18 ± 29.78 | 143.90 ± 29.88 | 0.30 | |
| QRS duration (ms) | 99.42 ± 13.05 | 90.10 ± 13.75 | <0.001* | |
| LVEDD (mm) | 62.42 ± 8.10 | 60.68 ± 8.01 | 0.28 | |
| LVESD (mm) | 54.1 ± 7.85 | 51.80 ± 7.32 | 0.13 | |
| LVEF % (Simpson's) | 22.24 ± 5.08 | 24.24 ± 5.92 | 0.07 | |
| EPSS (mm) | 20.66 ± 3.14 | 20.09 ± 3.02 | 0.36 | |
| MR regurgitant volume (ml) | 31.39 ± 15.72 | 31.95 ± 15.11 | 0.86 | |
| TR jet gradient (mm Hg) | 28.04 ± 9.05 | 29.09 ± 10.13 | 0.61 | |
| Mitral E/A | 2.45 ± 1.47 | 2.13 ± 1.24 | 0.23 | |
| IVRT (ms) | 97.92 ± 23.04 | 79.98 ± 18.91 | <0.05* | |
| Septal s (cm/sec) | 2.88 ± 0.77 | 2.71 ± 0.71 | 0.25 | |
| Septal e (cm/sec) | 2.98 ± 1.09 | 3.13 ± 1.06 | 0.51 | |
| Septal a (cm/sec) | 3.81 ± 1.01 | 3.94 ± 0.97 | 0.50 | |
| Lateral s (cm/sec) | 2.85 ± 0.76 | 2.78 ± 0.75 | 0.62 | |
| Lateral e (cm/sec) | 4.96 ± 1.72 | 4.68 ± 1.79 | 0.42 | |
| Lateral a (cm/sec) | 3.12 ± 1.00 | 3.14 ± 1.01 | 0.92 | |
| E/e septal | 33.69 ± 17.69 | 30.13 ± 15.81 | 0.29 | |
| E/e lateral | 21.70 ± 15.24 | 21.72 ± 14.05 | 0.99 | |
| SPWD (ms) | 87.94 ± 64.22 | 100.58 ± 67.33 | 0.34 | |
| RVOT PEP (ms) | 96.76 ± 19.65 | 98.60 ± 18.03 | 0.63 | |
| Aortic PEP (ms) | 111.96 ± 21.32 | 113.32 ± 19.54 | 0.74 | |
| Aortic – RVOT PEP (ms) | 15.2 ± 14.46 | 14.72 ± 11.25 | 0.85 |
Data are as mean ± SD for continuous variable and absolute no. (%) for categorical variable; A-Late diastolic velocity; a-Tissue Doppler imaging late diastolic velocity; ACE-angiotensin converting enzyme; E-Early diastolic velocity; EPSS-E point septal separation; e-Tissue Doppler imaging early diastolic velocity; IVRT-Iso-volumetric relaxation time; LVEDD-Left ventricular end-diastolic diameter; LVEF-Left ventricular ejection fraction; LVESD-Left ventricular end-systolic diameter; MR-Mitral regurgitation; NYHA-New York Heart Association functional classification; PEP-Pre ejection period; RVOT-Right ventricular outflow tract; s-Tissue Doppler imaging systolic velocity; SPWD-Septal to posterior wall delay; TR-Tricuspid regurgitation. *Statistically significant difference in values between two groups.
2.2. ECG
The resting 12-lead ECG (filter range, 0.05–100 Hz; A.C. filter, 60 Hz, 25 mm/s, 10 mm/mV) was analyzed by two independent clinicians who were blinded to echocardiographic data. The fQRS included various RSR' patterns and was defined by the presence of an additional R-wave (R′ prime), notching in nadir of the S-wave, notching of R-wave, or the presence of more than one R prime (fragmentation) in two contiguous leads corresponding to a major myocardial segment as previously described by Das et al (Fig. 1).10 The presence of fQRS in ≥2 contiguous anterior leads (V1–V5) were assigned to anterior myocardial segments, in ≥2 contiguous lateral leads (I, aVL, and V5, V6) to the lateral myocardial segments, in ≥2 contiguous inferior leads (II, III, and aVF) to the inferior myocardial segments and in both V1 and V2 only to the posterior myocardial segments.
Fig. 1.
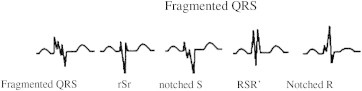
Various morphologies of QRS on 12-lead ECG defined as fQRS in present study originally proposed by Das et al.10
2.3. Echocardiography
Standard echocardiography with Doppler studies was performed by using a commercially available system (Philips iE33 system, Andover, Massachusetts, USA). LV dimensions were measured by two-dimensional guided M-mode echocardiography according to the guidelines of the American Society of Echocardiography.11 LV volumes were determined by the use of biplane Simpson's method of disks, and the ejection fraction was calculated with a formula calling for the subtraction of the end-systolic volume from the end-diastolic volume and the difference divided by the end-diastolic volume. The effective orifice area and the regurgitant volume of the functional mitral regurgitation jet (MR volume) were calculated by ‘proximal iso-velocity surface area’ method.12
Tissue Doppler imaging (TDI) was performed in the apical views (four-chamber, two-chamber and long-axis) for the long axis motion of the left ventricle. Two-dimensional echocardiography with tissue Doppler color imaging was performed with a 2.5 MHz phase array transducer. Myocardial regional velocity curves were constructed from the digitized images as previously described13 (Fig. 2B). In this way septal, anteroseptal, anterior, lateral, inferior and posterior segments were interrogated at both basal and middle levels. Aortic opening and closure were determined by sampling the flow through the LV outflow tract with the use of pulsed Doppler echocardiography. For the purpose of measurement, the beginning of the QRS complex was used as the reference point, from where the time to peak myocardial sustained systolic (Ts) and early diastolic (Te) velocities were calculated. The higher peak in velocity was selected when double peaks were encountered within the aortic ejection period.
Fig. 2.
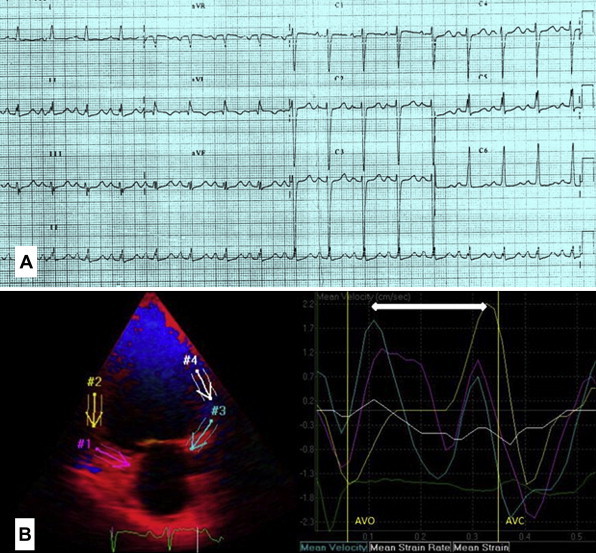
(A): 12-lead ECG showing fragmented narrow QRS complexes in inferior leads (B): Colour coded TDI (post processing image) of the same patient showing systolic dyssynchronous inferior wall segments.
To assess global LV function, the myocardial sustained systolic (s), early diastolic (e) and late diastolic (a) velocities from the basal septal and basal lateral segments were calculated. For the assessment of synchronicity, the standard deviations of Ts (Ts-SD) of all 12 LV segments were calculated. Significant systolic IVD was defined as Ts-SD >32.6 ms proposed by Yu et al and popularly known as ‘Yu index’.14 Fig. 2B depicts color coded TDI (post processing image) of a patient with the ECG shown in Fig. 2A, showing systolic dyssynchronous inferior wall segments.
We also looked for the association of fQRS and the most delayed contracting LV segment on echocardiography. ‘Dyssynchronic segments’ were defined as those LV segments that contracted later than 100 ms following the earliest contracting LV segment (which had the earliest Ts) and ‘most delayed segment’ was the last contracting myocardial segment (which had the latest Ts).
2.4. Statistical analysis
Analysis was performed using a statistical software program (SPSS for windows, version 17.0, SPSS Inc. Chicago, IL, USA). Data has been presented as mean ± SD and compared using the paired student's t test. Mann–Whitney U test has been used when the distribution was not normal. Categorical data between two or more groups were compared by Pearson χ2 test. P value of <0.05 was considered to be significant. Sensitivity was defined by the number of true positives for the presence of fQRS complexes and IVD, both. Specificity was defined by the number of true negatives with no fQRS complexes and the absence of dyssynchrony. Intra-observer (the mean difference between two independent measurements) and inter-observer (the mean difference between 2 independent observers) variabilities were analyzed in 10 randomly selected patients and expressed as the mean percent error (difference divided by number of observations).
3. Results
The overall study group comprised of 40 women (40%) and 60 men (60%), with mean (±SD) age of 46.86 ± 13.85 years. The estimated inter-and intra-observer variabilities for fragmentation in ECG were 2.5% and 1.8% and for TDI measurements on echocardiography were 4.8% and 3.1%, respectively. The demographic, clinical, electrocardiographic and echocardiographic features of patients with fragmented or normal QRS complexes have been shown in Tables 1 and 2. The two groups were well matched with respect to age, sex, New York Heart Association (NYHA) functional class and use of drugs like beta blockers, ACE-inhibitor, diuretics and digoxin. There was no significant difference in severity of LV dilatation, LV ejection fraction and mitral regurgitation between the two groups.
Table 2.
Comparison of time to peak myocardial sustained systolic velocity (Ts) (in ms) between patients with fQRS and without fQRS.
| Segment | Fragmented QRS (n = 50) | Normal QRS (n = 50) | p value |
|---|---|---|---|
| Basal septal | 158.52 ± 37.86 | 160.26 ± 33.09 | 0.80 |
| Mid septal | 156.24 ± 37.56 | 160.80 ± 33.75 | 0.52 |
| Basal lateral | 177.28 ± 49.88 | 164.88 ± 34.25 | 0.15 |
| Mid lateral | 182.18 ± 49.23 | 164.16 ± 32.79 | 0.03* |
| Basal inferior | 187.48 ± 48.44 | 169.64 ± 32.64 | 0.03* |
| Mid inferior | 188.18 ± 47.05 | 169.08 ± 29.24 | 0.01* |
| Basal anterior | 177 ± 47.21 | 161.36 ± 28.38 | 0.048* |
| Mid anterior | 183.24 ± 50.14 | 162.18 ± 29.55 | 0.01* |
| Basal posterior | 172.16 ± 40.25 | 167.08 ± 29.08 | 0.47 |
| Mid posterior | 173.76 ± 45.23 | 169.84 ± 31.05 | 0.61 |
| Basal anteroseptal | 170.04 ± 37.11 | 162.94 ± 26.94 | 0.27 |
| Mid anteroseptal | 169.7 ± 38.52 | 169.90 ± 34.23 | 0.98 |
| Ts-SD (Yu index) | 35.64 ± 12.79 | 20.45 ± 11.17 | <0.001* |
| Yu index positive patient n (%) | 39 (78%) | 7 (14%) | 0.000005* |
Data are as mean ± SD; Ts Max- Time to peak systolic velocity for the most delayed segment; Ts-SD- Standard deviation of all 12 Ts values. *Statistically significant difference in values between two groups.
However, there was significant difference in the QRS duration in patients with fQRS complexes as compared to those with normal QRS, though QRS duration in both the groups was <120 ms (99.42 ± 13.05 ms vs. 90.10 ± 13.75 ms; p < 0.001).
In the 50 patients with fQRS complex, the distribution of fragmentation was as follows; inferior segment in 18 (36%), anterior in 12 (24%) and lateral in 10 (20%). The fQRS was seen in more than one myocardial segment in 10 (20%) patients and the distribution of fragmentation was as follows: inferior and lateral segments in 5 (10%), anterior and inferior in 4 (8%), and anterior and lateral in one patient (2%).
Table 2 shows the regional Ts in individual LV segments and it was significantly delayed in some LV segments in fQRS group compared to normal QRS group. Significant systolic IVD (‘Yu index’ Ts-SD > 32.6 ms) was seen in 39 patients (78%) in fQRS complex group and 7 patients (14%) in normal QRS complex group (Pearson's χ2 = 20.61; p < 0.000005) [Table 2].
The presence of fQRS complexes in the basal ECG was found to detect systolic IVD as defined by the ‘Yu index’ (Ts-SD > 32.6 ms) with sensitivity of 84.78%, specificity of 79.62%, positive predictive value (PPV) of 78% and a negative predictive value (NPV) of 86% (Table 3).
Table 3.
Relationship between fragmented QRS and intraventricular systolic dyssynchrony.
| Group | Significant systolic dyssynchrony present (n) | No significant systolic dyssynchrony (n) | Total (n) |
|---|---|---|---|
| Fragmented QRS | 39 (true positives) | 11 (false positives) | 50 |
| Normal QRS | 7 (false negatives) | 43 (true negatives) | 50 |
| Total | 46 | 54 | 100 |
The 50 patients with fQRS complexes were sub-divided into two groups with either significant systolic dyssynchrony (group 1, n = 39) or without dyssynchrony (group 2, n = 11) on the basis of Ts-SD greater than or lesser than 32.6 ms. Table 4 shows the clinical, demographic and echocardiographic characteristics of patients in group 1 and 2. The demographic and clinical parameters between the two groups were not statistically different. The patients in group 1 had significantly longer isovolumetric relaxation time (IVRT) in comparison to patients in group 2 (105.23 ± 20.36 vs. 72.00 ± 8.90 ms; p < 0.001). There was no significant difference in any other echocardiographic parameters between the two groups. Among 39 patients of group 1 with significant systolic dyssynchrony, 30 (76.92%) patients had ECG fragmentation in the maximal dyssynchronic segment and six (15.39%) patients had ECG fragmentation in one of the dyssynchronic segments. In three (7.69%) patients, fragmented segments and dyssynchronic segments did not correlate. Among the dyssynchronic patients, the sensitivity and specificity of a fragmented segment on ECG to detect the most dyssynchronic segment or one of the dyssynchronic segments were 93% and 88%, respectively.
Table 4.
Characteristics of patients with fQRS with (group 1) and without (group 2) significant systolic dyssynchrony.
| Characteristic | Group 1 (with systolic dyssynchrony) (n = 39) | Group 2 (no systolic dyssynchrony) (n = 11) | Chi square value | p value |
|---|---|---|---|---|
| Age (in years) | 46.13 ± 14.24 | 45.00 ± 12.20 | 0.80 | |
| Sex (M:F) | 23:16 | 9:2 | 0.43 | 0.51 |
| NYHA class n (%) | 2.74 | 0.09 | ||
| II | 23 (59%) | 4 (57%) | ||
| III-IV | 16 (41%) | 7 (43%) | ||
| Heart rate (BPM) | 91.67 ± 17.74 | 98.09 ± 14.91 | 0.24 | |
| PR interval (ms) | 148.59 ± 29.52 | 155.82 ± 31.49 | 0.51 | |
| QRS duration (ms) | 99.08 ± 13.32 | 100.64 ± 12.62 | 0.72 | |
| LVEF % (Simpson's) | 22.31 ± 5.30 | 22.00 ± 4.43 | 0.85 | |
| EPSS (mm) | 20.47 ± 3.44 | 21.33 ± 1.70 | 0.26 | |
| MR regurgitant volume (ml) | 31.61 ± 13.45 | 30.66 ± 22.41 | 0.90 | |
| TR jet gradient (mm Hg) | 27.09 ± 9.40 | 31.10 ± 7.46 | 0.18 | |
| Mitral E/A | 2.29 ± 1.39 | 3.03 ± 1.70 | 0.21 | |
| IVRT (ms) | 105.23 ± 20.36 | 72.00 ± 8.90 | <0.001* | |
| Septal s (cm/sec) | 2.87 ± 0.81 | 2.93 ± 0.65 | 0.81 | |
| Septal e (cm/sec) | 2.99 ± 1.12 | 2.97 ± 1.04 | 0.97 | |
| Septal a (cm/sec) | 3.84 ± 0.96 | 3.69 ± 1.21 | 0.71 | |
| Lateral s (cm/sec) | 2.94 ± 0.81 | 2.55 ± 0.48 | 0.06 | |
| Lateral e (cm/sec) | 5.01 ± 1.59 | 4.71 ± 2.05 | 0.66 | |
| Lateral a (cm/sec) | 3.21 ± 0.98 | 2.81 ± 1.05 | 0.27 | |
| E/e septal | 32.46 ± 16.97 | 38.08 ± 20.32 | 0.42 | |
| E/e lateral | 20.28 ± 14.43 | 27.05 ± 17.68 | 0.26 | |
| Ts-SD (ms) | 41.67 ± 5.96 | 14.25 ± 4.63 | <0.001* |
Data are as mean ± SD for continuous variable and absolute no. (%) for categorical variable; A-Late diastolic velocity; a-Tissue Doppler imaging late diastolic velocity; E-Early diastolic velocity; EPSS-E point septal separation; e-Tissue Doppler imaging early diastolic velocity; IVRT-Isovolumetric relaxation time; LVEF-Left ventricular ejection fraction; MR-Mitral regurgitation; NYHA-New York Heart Association functional classification; s-Tissue Doppler imaging systolic velocity; TR-Tricuspid regurgitation; Ts-SD-Standard deviation of time to peak systolic velocity of 12 LV segments. *Statistically significant difference in values between two groups.
4. Discussion
CRT is an established treatment option for patients with drug refractory heart failure. The predominant mechanism of benefit from CRT appears to be related to the presence of IVD due to electrical conduction delay and subsequent resynchronization after CRT.15–19 The presence of significant IVD at baseline secondary to electrical dyssynchrony is therefore, mandatory for the response to CRT. QRS duration of ≥120 ms has been adopted as a cut-off point for electrical dyssynchrony resulting in LV systolic dyssynchrony in CRT trials20,21 and subsequently by treatment guidelines.1 However, studies suggest that QRS widening of ≥120 ms occurs in only 30% of HF patients making majority of HF patients ineligible for CRT. On the other hand, significant IVD by TDI has been reported in 36–51%14,22 of patients with HF and QRS <120 ms, and these patients also showed benefit from CRT.23,24 Similar prevalence of IVD has also emerged from strain imaging25 and radionuclide phase analysis.26 The aim of our study was to see whether fQRS on the surface ECG could be used as a marker of electrical dyssynchrony to predict the presence of significant IVD and refer the patients accordingly for subsequent echocardiographic assessment.
Das et al10 first reported the significance of fQRS in patients of CAD where he showed it to be a better predictor of myocardial scar than Q waves on ECG, in patients with narrow QRS complexes. fQRS in CAD patients occurs due to heterogenous ventricular activation caused by ischemia and myocardial scars. In non-ischemic DCM, inflammation, myocyte necrosis and fibrosis27,28 lead to uncoordinated depolarization of viable myocyte groups surrounded by fibrotic tissue, which is manifested on the surface ECG as fQRS and eventually lead to mechanical dyssynchrony.
Tigen et al, were the first to investigate the relationship between fQRS and IVD in patients of non-ischemic DCM and narrow QRS interval (duration <120 ms).8 In this study, significant systolic dyssynchrony was defined as the maximum difference in sustained systolic velocities between any two LV segments of more than 100 ms. Using this criteria, fQRS on the surface ECG was found to detect IVD in 72.5% patients (n = 40) with sensitivity of 90.6% and positive predictive value of 72.5%. Subsequently, in a small study, Basaran et al9 reported that fQRS complexes on the surface ECG in patients of non-ischemic DCM were associated with significant IVD in 76% patients (n = 25) as assessed by the ‘Yu index’ (Ts-SD >32.6 ms). But due to lack of control group of patients without fQRS, they could not assess the sensitivity or PPV of fQRS on ECG to predict significant IVD as assessed by the Yu index.
The ‘Yu index’ has been shown to be an excellent predictor of response to CRT therapy defined by reverse remodeling (≥15% reduction in LV end-systolic volume). In patients with QRS duration >150 ms, the ‘Yu index’ has a sensitivity and specificity of 100% and 78%, respectively to predict reverse remodeling, while in patients with QRS duration of 120–150 ms the sensitivity is 83% and specificity is 86%.30 So we decided to use ‘Yu index’ to assess mechanical dyssynchrony in patients with fQRS and drug refractory symptomatic non-ischemic dilated cardiomyopathy as it may also serve as a predictor of response to CRT in this subgroup of patients with narrow QRS. Yu et al14 developed this twelve segment model using color coded TDI that integrates information from three apical views (four-chamber, two-chamber and apical long axis view). In contrast to the simple two segment (apical four-chamber view to detect basal septal-lateral delay) and four segment (basal segments of septal, lateral, inferior and anterior walls) approaches to detect IVD, this 12 segment model also includes the anterior septum and posterior walls which often have dyssynchrony. The mechanical dyssynchrony index is derived by calculating the standard deviation of the time to peak systolic velocity in the ejection phase in the 12 segments and a cut-off value of >32.6 ms has been derived to define significant IVD. We used the color coded TDI to assess IVD as this has been recommended as ‘the preferred approach’ by the American Society of Echocardiography Dyssynchrony Writing Group.29 To the best of our knowledge, this is the first study to show that fQRS on the surface ECG has a sensitivity of 84.78% and a PPV of 78% to detect significant IVD as defined by the ‘Yu index’.
We also found that fQRS complexes indicated either the most delayed segment or one of the dyssynchronous segment with a sensitivity and specificity of 93% and 90% respectively. This can guide the echocardiographer in localizing the dyssynchronous LV segment on TDI. Further it may also help in planning the site of LV lead position during CRT.
A randomized controlled trial, RethinQ31 investigated the role of CRT in HF patients with narrow QRS and echocardiographic evidence of IVD. In the absence of ECG evidence of electrical dyssynchrony, IVD per se failed to provide clinical benefits with CRT over medial therapy. As our study has shown that fQRS on the surface ECG is a marker of electrical dyssynchrony and can predict significant IVD as defined by the ‘Yu index’ in more than 80% patients, it may also serve as a predictor of response to CRT in this sub-group of HF patients with narrow QRS.
In our study, QRS duration was significantly greater in patients with fQRS complexes as compared to those without fragmentation, although both groups had QRS duration of <120 ms. As fragmentation along with widening of QRS, leads to electrical dyssynchrony, fQRS in patients with QRS <120 ms may be considered to have the same significance as wide QRS to predict the presence of electrical dyssynchrony leading to IVD and responsiveness to CRT in patients of non-ischemic DCM.
4.1. Study limitations
We used the ‘Yu index’ to assess IVD. The PROSPECT study32 has, however, shown that the 12 segment TDI model had inter-observer and intra-observer variability of 34% and 11% respectively, limiting the reproducibility of the technique. Studies with newer techniques like tissue synchronization, real time 3D echocardiography and speckle tracking for predicting IVD in patients with fragmented narrow QRS complexes, should be undertaken.
5. Conclusion
Fragmentation of QRS complex on ECG is an important predictor of electrical dyssynchrony resulting in significant IVD in patients with non-ischemic DCM and a narrow QRS interval. fQRS on the surface ECG is also helpful for localizing the dyssynchronous segment. In future, studies are needed to assess the beneficial effects of CRT in drug refractory, symptomatic HF patients with narrow fragmented QRS complexes and significant IVD.
Conflicts of interest
All authors have none to declare.
References
- 1.Epstein A.E., DiMarco J.P., Ellenbogen K.A. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Kashani A., Barold S.S. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46:2183–2192. doi: 10.1016/j.jacc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 3.Freudenberger R., Sikora J.A., Fisher M. Electrocardiogram and clinical characteristics of patients referred for cardiac transplantation: implications for pacing in heart failure. Clin Cardiol. 2004;27:151–153. doi: 10.1002/clc.4960270311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleeker G., Schalij M., Molhoek S. Frequency of left ventricular dyssynchrony in patients with heart failure and a narrow QRS complex. Am J Cardiol. 2005;95:140–142. doi: 10.1016/j.amjcard.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 5.Auricchio A., Yu C.M. Beyond the measurement of QRS complex toward mechanical dysynchrony: cardiac resynchronization therapy in heart failure patients with a normal QRS duration. Heart. 2004;90:479–481. doi: 10.1136/hrt.2003.024273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohi K., Suffoletto M., Murali S. Benefit of cardiac resynchronization therapy to a patient with a narrow QRS complex and ventricular dyssynchrony identified by tissue synchronization imaging. Eur J Echocardiogr. 2005;6:455–460. doi: 10.1016/j.euje.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Yu C.M., Chan Y.S., Zhang Q. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006;48:2251–2257. doi: 10.1016/j.jacc.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 8.Tigen K., Karaahmet T., Gurel E. The utility of fragmented QRS complexes to predict significant intraventricular dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Can J Cardiol. 2009;25(9):517–522. doi: 10.1016/s0828-282x(09)70137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basaran Y., Tigen K., Karaahmet T. Fragmented QRS Complex are associated with cardiac fibrosis and significant intraventricular systolic dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Echocardiography. 2011;28(1):62–68. doi: 10.1111/j.1540-8175.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 10.Das M., Khan B., Jacob S. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 11.Schiller N.B., Shah P.M., Crawford M. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of the Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 12.Shiota T., Jones M., Teien D.E. Evaluation of mitral regurgitation using a digitally determined colour Doppler flow convergence ‘centerline’ acceleration method: studies in an animal model with quantified mitral regurgitation. Circulation. 1994;89:2879–2886. doi: 10.1161/01.cir.89.6.2879. [DOI] [PubMed] [Google Scholar]
- 13.Gorcsan J., 3rd, Strum D.P., Mandarino W.A. Quantitative assessment of alterations in regional left ventricular contractibility with color-coded tissue Doppler echocardiography. Comparison with sonomicrometry and pressure volume relations. Circulation. 1997;95:2423–2433. doi: 10.1161/01.cir.95.10.2423. [DOI] [PubMed] [Google Scholar]
- 14.Yu C.M., Lin H., Zhang Q. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bax J.J., Marwick T.H., Molhoek S.G. Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol. 2003;92:1238–1240. doi: 10.1016/j.amjcard.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Yu C.M., Fung W.H., Lin H. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol. 2003;91:684–688. doi: 10.1016/s0002-9149(02)03404-5. [DOI] [PubMed] [Google Scholar]
- 17.Breithardt O.A., Stellbrink C., Kramer A.P. Echocardiographic quantification of left ventricular asynchrony predicts an acute hemodynamic benefit of cardiac resynchronization therapy. J Am Coll Cardiol. 2002;40:536–545. doi: 10.1016/s0735-1097(02)01987-3. [DOI] [PubMed] [Google Scholar]
- 18.Bordachar P., Lafitte S., Reuter S. Echocardiographic parameters of ventricular dyssynchrony validation in patients with heart failure using sequential biventricular pacing. J Am Coll Cardiol. 2007;44:2157–2165. doi: 10.1016/j.jacc.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 19.Bax J.J., Bleeker G.B., Marwick T.H. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Cleland J.G., Daubert J.C., Erdmann E. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 21.Bristow M.R., Saxon L.A., Boehmer J. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 22.Haghjoo M., Bagherzadeh A., Fazelifar A.F. Prevalence of mechanical dyssynchrony in heart failure patients with different QRS durations. Pacing Clin Electrophysiol. 2007;30:616–622. doi: 10.1111/j.1540-8159.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 23.Bleeker G.B., Holman E.R., Steendijk P. Cardiac resynchronization therapy in patients with a narrow QRS complex. J Am Coll Cardiol. 2006;48:2243–2250. doi: 10.1016/j.jacc.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 24.Achilli A., Sassara M., Ficili S. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and "narrow" QRS. J Am Coll Cardiol. 2003;42:2117–2124. doi: 10.1016/j.jacc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Takemoto Y., Hozumi T., Sugioka K. Beta-blocker therapy induces ventricular resynchronization in dilated cardiomyopathy with narrow QRS complex. J Am Coll Cardiol. 2007;49:778–783. doi: 10.1016/j.jacc.2006.05.081. [DOI] [PubMed] [Google Scholar]
- 26.Marcassa C., Campini R., Verna E. Assessment of cardiac asynchrony by radionuclide phase analysis: correlation with ventricular function in patients with narrow or prolonged QRS interval. Eur J Heart Fail. 2007;9:484–490. doi: 10.1016/j.ejheart.2007.01.002. 39. [DOI] [PubMed] [Google Scholar]
- 27.Timonen P., Magga J., Risteli J. Cytokines, interstitial collagen and ventricular remodelling in dilated cardiomyopathy. Int J Cardiol. 2008;124:293–300. doi: 10.1016/j.ijcard.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Marijianowski M.M., Teeling P., Mann J. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol. 1995;25:1263–1272. doi: 10.1016/0735-1097(94)00557-7. [DOI] [PubMed] [Google Scholar]
- 29.Gorcsan J., Abraham T., Agler D.A. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting–a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Yu C.M., Fung J.W., Chan C.K. Comparison of efficacy of reverse remodeling and clinical improvement for relatively narrow and wide QRS complexes after cardiac resynchronization therapy for heart failure. J Cardiovasc Electrophysiol. 2004;15:1058–1065. doi: 10.1046/j.1540-8167.2004.03648.x. [DOI] [PubMed] [Google Scholar]
- 31.Beshai J.F., Grimm R.A., Nagueh S.F. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–2471. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 32.Chung E.S., Leon A.R., Tavazzi L. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]


