Highlights
► An HPV 11 positive ICC was detected. ► Infection by other HR types was ruled out. ► The same genotype was found in a metastatic lesion.
Keywords: HPV 11, Invasive cervical cancer, Immunodefficient, HIV, Metastasis
Introduction
Women infected by the human immunodeficiency virus (HIV) also have a high prevalence of human papillomavirus (HPV) infections with the presence of multiple HPV types being typical (Levi et al., 2004). Furthermore, the incidence of cervical abnormalities is significantly higher than in immunocompetent hosts, with these lesions likely to be difficult to eradicate (Ellerbrock et al., 2000). Invasive cervical cancer (ICC) is considered one of the Acquired Immune Deficiency Syndrome (AIDS)-defining conditions (Bower et al., 2006). However, the true relationship between HIV infection and cervical cancer is controversial. It has been postulated that in the era of highly active antiretroviral therapy (HAART), longer survival would allow for the development of ICC, thus an increase in the incidence of ICC was expected to be observed. After more than 10 years of the introduction of HAART, this expected increase does not seem to be taking place (Massad et al., 2009). Persistent infection with one or more high risk HPV genotypes is a necessary cause for the development of ICC, with HPV 16 and 18 responsible for a large proportion of the cases, although with minor differences among geographical regions. HPV 11 presents a low-risk for cervical cancer, causing benign hyperproliferative lesions as genital warts and oral papillomatosis. Brown and co-workers detected high-risk HPV DNA on condylomata acuminata lesions from HIV immunosupressed and otherwise healthy patients, always in association to a low-risk HPV type, either HPV 6 or 11 (Brown et al., 1999). Conversely, low-risk HPV DNA types were found in high-grade lesions, but again in association with high-risk HPV types (Clifford et al., 2006), making it difficult to attribute causality to a specific genotype.
In a retrospective cross-sectional study, which analyzed 10,575 cases of ICC from the five continents in between 1949 and 2009, only 16 cases were described to harbor a single infection with a low risk HPV. Among them, only 2 were HPV-11 positive (De Sanjosé et al., 2010). A recent study carried out on HIV-infected ICC patients and HIV negative ICC cases from Kenya, found one case of a HPV-11 positive ICC patient in the context of a multiple infection, and, surprisingly, two cases in the HIV-negative group of ICCs containing HPV 11 and 40 as single infections (De Vuyst et al., 2008).
In this case report, we describe a case of metastatic ICC harboring HPV-11 occurring in an HIV-infected patient.
Case report
A 38-year-old HIV-infected female patient presented to the outpatient clinic of Instituto do Câncer do Estado de São Paulo (ICESP), Brazil, complaining of vaginal bleeding. Examination aided by a speculum revealed an exophytic lesion that was biopsied. The specimen was sent for histopathologic examination and for HPV genotyping (Virology Laboratory of Instituto de Medicina Tropical).
Cervical squamous cell carcinoma was diagnosed. Pelvic magnetic resonance imaging revealed a large cervical tumoral lesion invading the right parametrium and the posterior vaginal fornix. The patient underwent radiotherapy. At two months follow up, the patient complained of enlarged neck lymph nodes that were biopsied and also sent for histopathologic examination. Both primary and metastatic samples were diagnosed as squamous cell carcinoma. Palliative chemotherapy with cisplatin was performed. After four months, the patient developed acute bowel obstruction due to progression of the disease and died.
Methods
In situ HPV DNA detection was performed with biotinylated probes Y1411 HPV types 6/11 DNA Probe Mix and Y1443 GenPoint HPV, biotinylated DNA Probe targeting sequences of “high-risk” HPV genotypes 16, 18 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68. Signal amplification was developed with K620 (GenPoint, Dako, Carpinteria, CA, USA).
DNA was extracted from fresh tumor using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and HPV genotyping was carried out with the PapilloCheck Kit (Greiner Bio-One, Frickenhausen, Germany).
DNA was extracted from slices of the paraffin-embedded lymph nodes by the Genomic DNA Extraction kit (Real Biotech Inc., Taiwan) and submitted to HPV genotyping with the INNO-LiPA HPV Genotyping v2 (Innogenetics, Gent, Belgium), which employs PCR amplification followed by hybridization to solid-phase immobilized oligonucleotide probes. As it has been described that the absence of a basic amino acid motif at position 31 of the HPVs E6 proteins is strongly correlated to their p53 degradation activity (Fu et al., 2010) we reasoned whether this particular oncogenic HPV 11 isolate would bear a non-basic motif at this key position. An 789 bp E6/E7 amplicon was generated from fresh cervical tumor DNA by PCR amplification with the primers forward 5′ GGAGGGACCGAAAACGGTTCAACCGA 3′ and reverse 5′ TGGTGCGCAGATGGGACACACAA 3′. Purified PCR product was sequenced in an automated ABI Prism ABI 3100 Genetic Analyser (Applied Biosystems, California, USA).
Molecular findings
Fresh tumor DNA submitted to the Papillocheck, which targets the HPV E1 gene and paraffin-embedded metastatic lymph nodes tissue DNA tested by the Inno-Lipa HPV, targeting the HPV L1 gene, revealed the single presence of HPV-11. In contrast, “in-situ” hybridization did not depict any HPV type on the lymph nodes sections. Comparative analysis of the E6 sequence demonstrated a high similarity to an HPV-11 reference strain (GenBank Entry# FN907963). In the visual inspection of the E6/E7 alignment, a positively charged basic residue (K = lysine) in position 31 of E6 gene was observed (Fig. 1).
Fig. 1.
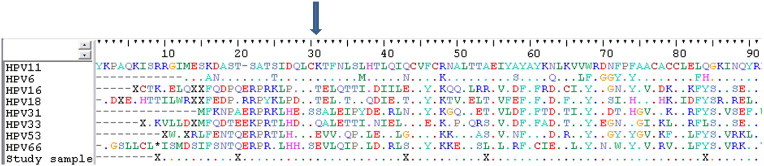
Alignment of HPV alpha E6 amino acid sequences, including HPV low risk types (HPV-6 and 11), HPV high risk types (HPV-16, 18, 31, 33 and 53) and this isolate. Region between position 33 and 47 represents a proposed E6-AP binding domain. Arrow points to the amino acid at position 31 which is associated to p53 degradation (ref. Massad et al., 2009). “.” Indicates identical residues to the HPV-11 E6 sequence (GenBank Entry # FN907963).
Discussion
This case report illustrates an ICC associated to a HPV-11 single infection, what is almost exclusively found in benign lesions. Little is known about the mechanism of HPV-11 associated carcinogenesis because of its rare occurrence. In this study, we tried to envisage a potential biochemical mechanism responsible for this enhanced transforming activity by a non-oncogenic type, i.e. E6 point mutation in position 31. We observed lysine, a positively charged residue, in the position 31 of E6 sequence, which displays weak p53 degradation activity. Host biological factors (Nicol et al., 2008) beyond CD4 cell-count, may have played an important role on this rare neoplasic occurrence.
Conflict of interest statement
No conflict of interest.
Acknowledgments
We would like to thank Prof. Paul Brennan, International Agency for Research on Cancer (IARC, Lyon-France) for grammar review and helpful suggestions in the management of this case report.
References
- Bower M., Mazhar D., Stebbing J. Should cervical cancer be an acquired immunodeficiency syndrome-defining cancer? J. Clin. Oncol. 2006;24:2417–2419. doi: 10.1200/JCO.2005.05.4908. [DOI] [PubMed] [Google Scholar]
- Brown D.R., Schrieder J.M., Bryan J.T., Stoler M.H., Fife K.H. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosupressed patents. J. Clin. Microbiol. 1999;37:3316–3322. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford G.M., Gonçalves M.A., Franceschi S. HPV and HIV Study Group. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS. 2006;20(18):2337–2344. doi: 10.1097/01.aids.0000253361.63578.14. [DOI] [PubMed] [Google Scholar]
- De Sanjosé S., Quint W.G.V., Alemany L., Geraets D.T., Klaustermeier J.E., Lloveras B. On behalf of the Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- De Vuyst H., Gichangi P., Estambale B., Njuguna E., Franceschi S., Temmerman M. Human papillomavirus types in women with invasive cervical carcinoma by HIV status in Kenya. Int. J. Cancer. 2008;122(1):244–246. doi: 10.1002/ijc.23045. [DOI] [PubMed] [Google Scholar]
- Ellerbrock T.V., Chiasson M.A., Bush T.J., Sun X.W., Sawo D., Brudney K. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283:1031–1037. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- Fu L., Van Doorslaer K., Chen Z., Ristriani T., Masson M., Travé G. Degradation of p53 of human alphapapillomavirus E6 proteins shows a stronger correlation with phylogeny than oncogenicity. PLoS One. 2010;5(9):e12816. doi: 10.1371/journal.pone.0012816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi J.E., Fernandes S., Tateno A.F., Motta E., Lima L.P., Eluf-Neto J. Presence of multiple human papillomavirus types in cervical samples from HIV-infected women. Gynecol. Oncol. 2004;92(1):226–232. doi: 10.1016/j.ygyno.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Massad L.S., Seaberg E.C., Watts D.H., Minkoff H., Levine A.M., Henry D., Colie C. Long-term incidence of cervical cancer in women with human immunodeficiency virus. Cancer. 2009;115:524–530. doi: 10.1002/cncr.24067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol A.F., Nuovo G.J., Salomão-Estevez A., Grinsztejn B., Tristão A., Russomano F. Immune factors involved in the cervical immune response in the HIV/HPV co-infection. J. Clin. Pathol. 2008;61(1):84–88. doi: 10.1136/jcp.2007.047290. Epub 2007 May 4. Erratum in: J Clin Pathol 2008;61(7):880. Grinsztein, B [corrected Grinsztejn, B]. [DOI] [PubMed] [Google Scholar]


