Abstract
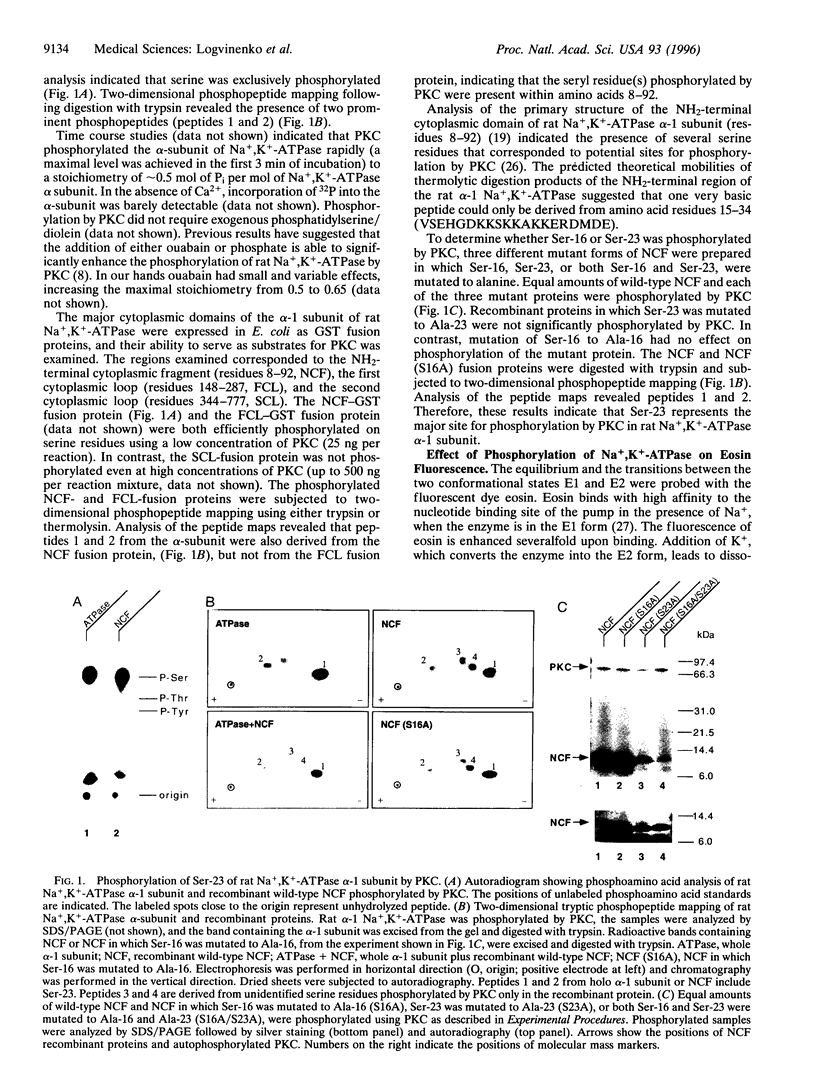
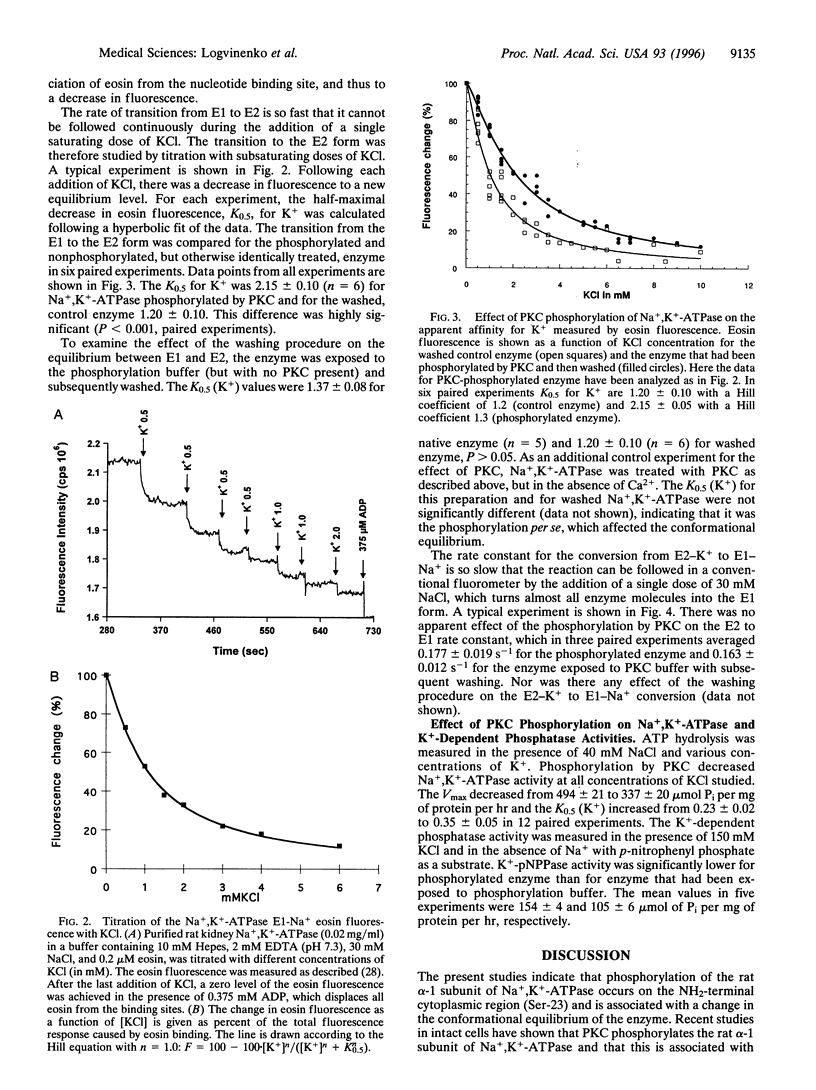
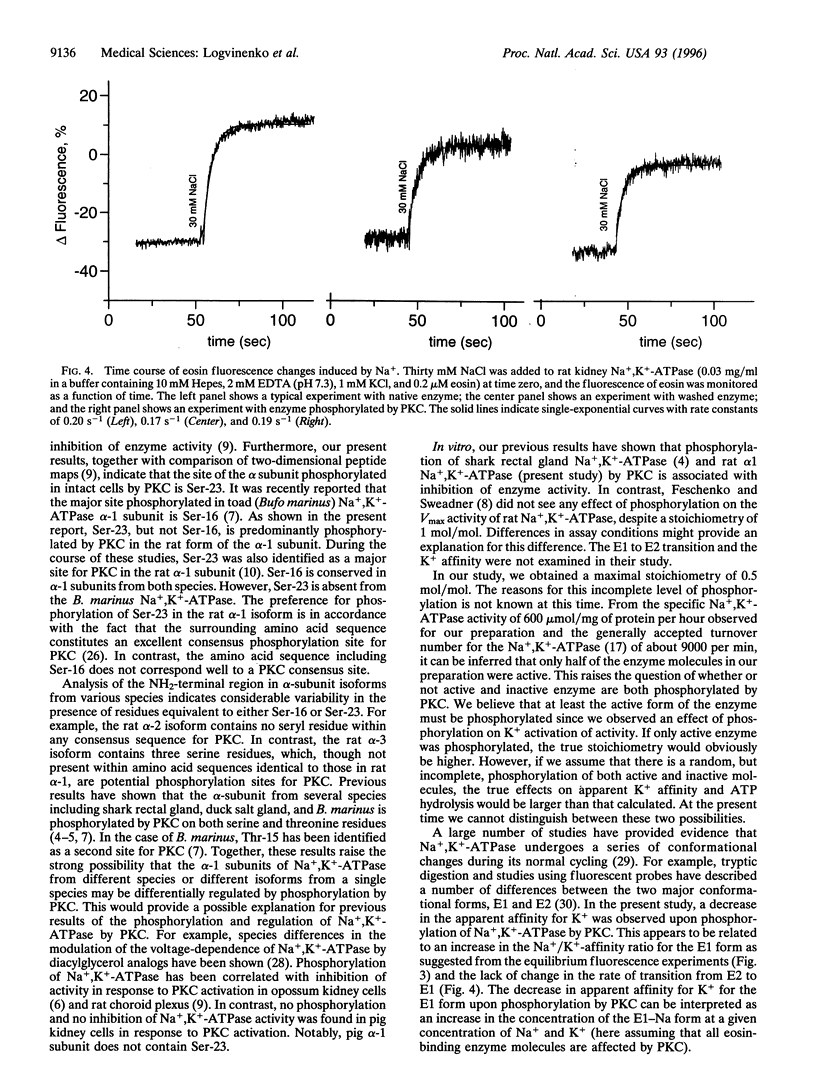
Phosphorylation of the alpha-1 subunit of rat Na+,K(+)-ATPase by protein kinase C has been shown previously to decrease the activity of the enzyme in vitro. We have now undertaken an investigation of the mechanism by which this inhibition occurs. Analysis of the phosphorylation of recombinant glutathione S-transferase fusion proteins containing putative cytoplasmic domains of the protein, site-directed mutagenesis, and two-dimensional peptide mapping indicated that protein kinase C phosphorylated the alpha-1 subunit of the rat Na+,K(+)-ATPase within the extreme NH2-terminal domain, on serine-23. The phosphorylation of this residue resulted in a shift in the equilibrium toward the E1 form, as measured by eosin fluorescence studies, and this was associated with a decrease in the apparent K+ affinity of the enzyme, as measured by ATPase activity assays. The rate of transition from E2 to E1 was apparently unaffected by phosphorylation by protein kinase C. These results, together with previous studies that examined the effects of tryptic digestion of Na+,K(+)-ATPase, suggest that the NH2-terminal domain of the alpha-1 subunit, including serine-23, is involved in regulating the activity of the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A., Holtbäck U., Syrén M. L., Svensson L. B., Fryckstedt J., Greengard P. Activation/deactivation of renal Na+,K(+)-ATPase: a final common pathway for regulation of natriuresis. FASEB J. 1994 Apr 1;8(6):436–439. doi: 10.1096/fasebj.8.6.8168694. [DOI] [PubMed] [Google Scholar]
- Beguin P., Beggah A. T., Chibalin A. V., Burgener-Kairuz P., Jaisser F., Mathews P. M., Rossier B. C., Cotecchia S., Geering K. Phosphorylation of the Na,K-ATPase alpha-subunit by protein kinase A and C in vitro and in intact cells. Identification of a novel motif for PKC-mediated phosphorylation. J Biol Chem. 1994 Sep 30;269(39):24437–24445. [PubMed] [Google Scholar]
- Bertorello A. M., Aperia A., Walaas S. I., Nairn A. C., Greengard P. Phosphorylation of the catalytic subunit of Na+,K(+)-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11359–11362. doi: 10.1073/pnas.88.24.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celsi G., Nishi A., Akusjärvi G., Aperia A. Abundance of Na(+)-K(+)-ATPase mRNA is regulated by glucocorticoid hormones in infant rat kidneys. Am J Physiol. 1991 Feb;260(2 Pt 2):F192–F197. doi: 10.1152/ajprenal.1991.260.2.F192. [DOI] [PubMed] [Google Scholar]
- Chibalin A. V., Vasilets L. A., Hennekes H., Pralong D., Geering K. Phosphorylation of Na,K-ATPase alpha-subunits in microsomes and in homogenates of Xenopus oocytes resulting from the stimulation of protein kinase A and protein kinase C. J Biol Chem. 1992 Nov 5;267(31):22378–22384. [PubMed] [Google Scholar]
- Daly S. E., Lane L. K., Blostein R. Functional consequences of amino-terminal diversity of the catalytic subunit of the Na,K-ATPase. J Biol Chem. 1994 Sep 30;269(39):23944–23948. [PubMed] [Google Scholar]
- Esmann M. ATPase and phosphatase activity of Na+,K+-ATPase: molar and specific activity, protein determination. Methods Enzymol. 1988;156:105–115. doi: 10.1016/0076-6879(88)56013-5. [DOI] [PubMed] [Google Scholar]
- Esmann M. Influence of Na+ on conformational states in membrane-bound renal Na,K-ATPase. Biochemistry. 1994 Jul 19;33(28):8558–8565. doi: 10.1021/bi00194a022. [DOI] [PubMed] [Google Scholar]
- Ewart H. S., Klip A. Hormonal regulation of the Na(+)-K(+)-ATPase: mechanisms underlying rapid and sustained changes in pump activity. Am J Physiol. 1995 Aug;269(2 Pt 1):C295–C311. doi: 10.1152/ajpcell.1995.269.2.C295. [DOI] [PubMed] [Google Scholar]
- Feschenko M. S., Sweadner K. J. Conformation-dependent phosphorylation of Na,K-ATPase by protein kinase A and protein kinase C. J Biol Chem. 1994 Dec 2;269(48):30436–30444. [PubMed] [Google Scholar]
- Feschenko M. S., Sweadner K. J. Structural basis for species-specific differences in the phosphorylation of Na,K-ATPase by protein kinase C. J Biol Chem. 1995 Jun 9;270(23):14072–14077. doi: 10.1074/jbc.270.23.14072. [DOI] [PubMed] [Google Scholar]
- Fisone G., Cheng S. X., Nairn A. C., Czernik A. J., Hemmings H. C., Jr, Hög J. O., Bertorello A. M., Kaiser R., Bergman T., Jörnvall H. Identification of the phosphorylation site for cAMP-dependent protein kinase on Na+,K(+)-ATPase and effects of site-directed mutagenesis. J Biol Chem. 1994 Mar 25;269(12):9368–9373. [PubMed] [Google Scholar]
- Fisone G., Snyder G. L., Fryckstedt J., Caplan M. J., Aperia A., Greengard P. Na+,K(+)-ATPase in the choroid plexus. Regulation by serotonin/protein kinase C pathway. J Biol Chem. 1995 Feb 10;270(6):2427–2430. doi: 10.1074/jbc.270.6.2427. [DOI] [PubMed] [Google Scholar]
- Good L., Nazar R. N. An improved thermal cycle for two-step PCR-based targeted mutagenesis. Nucleic Acids Res. 1992 Sep 25;20(18):4934–4934. doi: 10.1093/nar/20.18.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Ishii T., Lemas M. V., Takeyasu K. Na(+)-, ouabain-, Ca(2+)-, and thapsigargin-sensitive ATPase activity expressed in chimeras between the calcium and the sodium pump alpha subunits. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6103–6107. doi: 10.1073/pnas.91.13.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ plus K+ )-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim Biophys Acta. 1974 Jul 12;356(1):36–52. doi: 10.1016/0005-2736(74)90292-2. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L., Karlish S. J. Defective conformational response in a selectively trypsinized (Na+ + K+)-ATPase studied with tryptophan fluorescence. Biochim Biophys Acta. 1980 Apr 10;597(2):305–317. doi: 10.1016/0005-2736(80)90108-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Middleton J. P., Khan W. A., Collinsworth G., Hannun Y. A., Medford R. M. Heterogeneity of protein kinase C-mediated rapid regulation of Na/K-ATPase in kidney epithelial cells. J Biol Chem. 1993 Jul 25;268(21):15958–15964. [PubMed] [Google Scholar]
- Munzer J. S., Daly S. E., Jewell-Motz E. A., Lingrel J. B., Blostein R. Tissue- and isoform-specific kinetic behavior of the Na,K-ATPase. J Biol Chem. 1994 Jun 17;269(24):16668–16676. [PubMed] [Google Scholar]
- Nairn A. C., Greengard P. Purification and characterization of Ca2+/calmodulin-dependent protein kinase I from bovine brain. J Biol Chem. 1987 May 25;262(15):7273–7281. [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Robinson J. D., Pratap P. R. Indicators of conformational changes in the Na+/K(+)-ATPase and their interpretation. Biochim Biophys Acta. 1993 Jun 8;1154(1):83–104. doi: 10.1016/0304-4157(93)90018-j. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. Eosin as a fluorescence probe for measurement of conformational states of Na+,K+-ATPase. Methods Enzymol. 1988;156:278–281. doi: 10.1016/0076-6879(88)56028-7. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. The Na,K-ATPase. J Bioenerg Biomembr. 1992 Jun;24(3):249–261. doi: 10.1007/BF00768846. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Vasilets L. A., Omay H. S., Ohta T., Noguchi S., Kawamura M., Schwarz W. Stimulation of the Na+/K+ pump by external [K+] is regulated by voltage-dependent gating. J Biol Chem. 1991 Sep 5;266(25):16285–16288. [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Isolation and characterization of two distinct forms of protein kinase C. J Biol Chem. 1987 Apr 5;262(10):4836–4843. [PubMed] [Google Scholar]




