Abstract
Background
Hepatic venous outflow obstruction (HVOO) can have acute or chronic presentation. In the chronic variety of inferior vena cava (IVC) obstruction, endovascular management with balloon angioplasty and stent implantation has emerged as a feasible, safe alternative to surgery which has high incidence of mortality and morbidity.
Aims and objectives
To study the feasibility and long-term follow-up of endovascular management of chronic IVC obstruction.
Methods
We studied 12 cases of HVOO who underwent endovascular management (balloon dilatation ± stenting). In most of the cases, the cause of obstruction was not obvious, but one case had metastatic hepatic nodules compressing on IVC. Diagnosis was established by clinical examination, venous Doppler and was confirmed by venography and/or computed tomography (CT) angiography. Cases underwent balloon dilatation and/or stenting.
Results
Out of 12 cases, six had membranous obstruction (four complete and two incomplete), five cases had segmental stenosis and one case had tumour compression. The lesion was crossed with either guide wire or Brockenbrough needle with Mullins sheath assembly and balloon dilatation was done with Inoue or Mansfield balloon. Seven cases underwent balloon dilatation alone while five cases underwent stenting. There was procedural success in all cases with reduction of gradient by 84%, disappearance of collaterals and clinical improvement. During the follow-up of 13 years, one case had restenosis, which was managed by stenting.
Conclusion
Endovascular management of IVC obstruction is safe with good long-term patency rates.
Keywords: Balloon dilatation, IVC obstruction, IVC stenting, IVC venography, Restenosis
Introduction
Inferior vena cava (IVC) obstruction can result from thrombosis secondary to hypercoagulable disorders, extrinsic compression by tumours, infective phlebitis, inflammation, trauma, surgery, or in many number of cases, idiopathic.1,2 In longstanding cases, it results in swelling of extremities, pain, venous ulceration and impaired liver and renal functions. The course of the disease can be rapidly fatal, or at times it may be confused with other causes of cirrhosis and portal hypertension. This form of chronic IVC obstruction is common in developing countries.1
Morphologically, IVC obstruction can be segmental, diffuse, discrete complete membranous or incomplete membranous obstruction. Discrete membranous obstruction now is believed to be a sequel of thrombosis and subsequent partial recanalisation.1
Diagnosis can be established by clinical examination, colour Doppler sonography, contrast-enhanced computed tomography (CECT), magnetic resonance imaging (MRI), and venography.
In the past, surgical created shunts were the options for managing hepatic venous outflow obstruction (HVOO). It had high mortality and morbidity, as the patients were very sick and surgery was involving thoracoabdominal approach in a highly congested patient.4,14 The percutaneous management has emerged as a very promising modality of management of HVOO. Balloon dilatation with or without stenting of hepatic veins (HV) and/or IVC has been reported earlier by various authors.15,16 The feasibility and long-term patency of IVC stenting is not very clear, especially in this part of the world, where membranous obstruction is commoner than in the West.
The purpose of this study is to evaluate the clinical presentation, diagnosis and modalities of endovascular management of IVC obstruction.
Materials and methods
From 1996–2009, we studied 12 cases of HVOO who underwent endovascular management.
In our study, only chronic occlusion of IVC is considered which is defined as symptoms and objective imaging evidence of occlusion >3 months.
Age of presentation was 28–55 years (mean 35 years). There were seven males and five females.
In most of the cases, the causes of IVC obstruction were not known, except in one, which had metastatic carcinoma (adenocarcinoma stomach) with multiple secondary nodules on the liver with IVC compression.
Baseline characteristics and clinical presentation is given in Table 1 and Figures 1 and 2.
Table 1.
Baseline characteristics of patients with chronic inferior vena cava obstruction.
| Age | 35 ± 6.1 yr |
| Sex (male/female) | 7/5 |
| Oedema of lower extremities | 12/12 cases |
| Abdominal swelling and ascites | 12/12 cases |
| Prominent veins on abdomen and back | 12/12 cases |
| Hepatomegaly | 10/12 cases |
| Hepatomegaly with nodules on liver (metastatic nodules from adenocarcinoma of stomach) | 1/12 cases |
| Splenomegaly | 4/12 cases |
| Impaired hepatic function | 10/12 cases |
Figure 1.
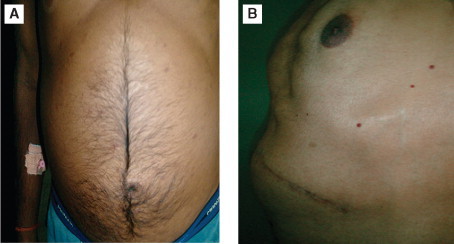
A case of membranous obstruction of inferior vena cava presenting with ascites and spider nevi on the abdominal wall.
Figure 2.
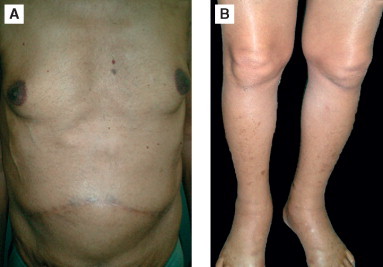
Inferior vena cava compression by metastatic nodules-note the nodular appearance of liver (A) and oedema feet (B).
Diagnosis was established by clinical examination, ultrasound Doppler study and confirmed by angiography and/or spiral CT angiography.
The details of the procedure, risks and benefits were explained to all patients and a written consent was obtained. The procedure was done under local anaesthesia with mild sedation whenever needed (either fentanyl citrate or midazolam hydrochloride).
Seven cases had acute-on-chronic obstruction of IVC and were treated with 24-hour infusion of either streptokinase or urokinase (100,000 U/hr), before taking up the procedure.
Angiographic profile of 12 cases who underwent balloon angioplasty with or without stenting is as follows.
-
1.
Membranous (complete) obstruction—four cases.
-
2.
Membranous (incomplete) obstruction—two cases.
-
3.
Segmental obstruction—five cases.
-
4.
External compression by tumour—one case.
All the cases had IVC obstruction; there was no involvement of HVs in our series. Out of 12 cases, six cases underwent balloon dilatation alone whereas six cases underwent stenting of IVC. One case who underwent balloon dilatation alone, presented with restenosis twice and treated with stent implantation.
Procedural details
In all cases, IVC obstruction was confirmed by angiography. In two cases of incomplete membranous obstruction of IVC (MOVC), the lesion was crossed with guide (0.032” Terumo) wire and angioplasty using Inoue balloon was done with good result.
In four cases with complete membranous obstruction, the lesion was crossed with Brockenbrough needle (straightened)-Mullins sheath assembly which was exchanged to steel-coiled guide wire. Then, the IVC obstruction was dottered using the septal dilator (used to dilate the interatrial septum during mitral valvuloplasty). The lesions were dilated with Inoue balloon (18–22 cc volume) and achieved good result in all cases (Figure 3).
Figure 3.
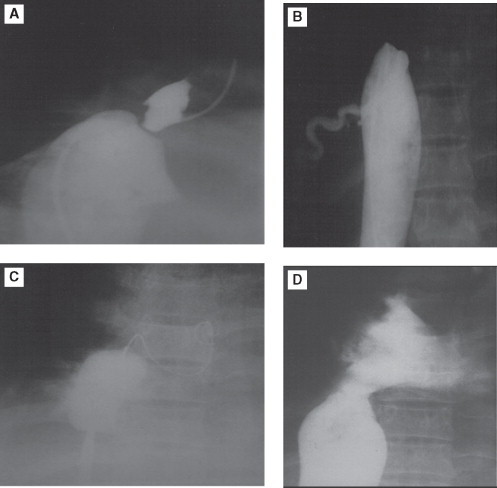
Image reveals complete obstruction of IVC simultaneous contrast injection from upper and lower limb approach revealing membranous obstruction (A and B). Lesion crossed with Brockenbrough needle puncture/coiled guide wire through Mullins sheath in right atrial. (C) MVOC dilated with Inoue balloon (C). Final post balloon angioplasty image (D) reveals no residual obstruction. IVC: inferior vena cava, MOVC: membranous obstruction of IVC.
Segmental IVC obstruction was crossed with guide wire (Terumo 0.035” 135 cm/7 F multipurpose catheter assembly). In few cases, lesions were dottered with septal dilator and then balloon angioplasty was done using Mansfield balloon or Inoue balloon. One case underwent balloon angioplasty alone and five cases of segmental IVC obstruction were stented primarily (a total of nine stents were used, seven–self-expanding, two–balloon mounted) (Figure 4).
Figure 4.
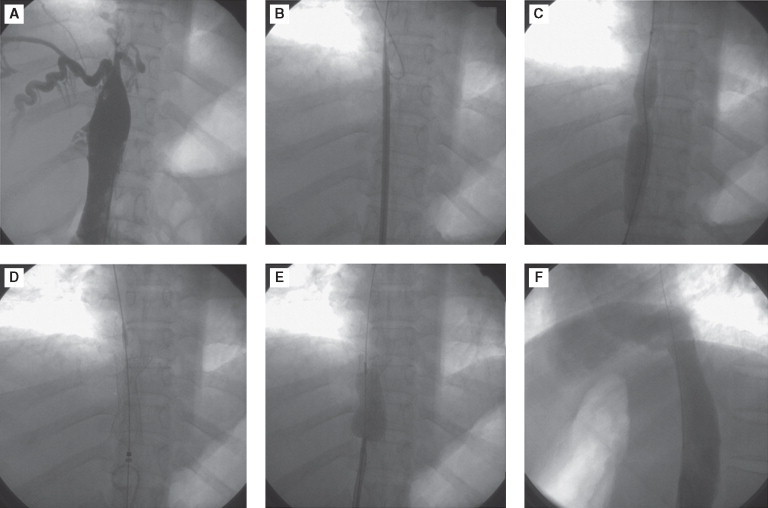
Image shows segmental occlusion (A), which was crossed with guide wire (B) and predilated (C). It was stented (D) and post dilated (E). Note immediate disappearance of collaterals (F), which were abundant initially.
The case who had undergone balloon dilatation alone for segmental IVC obstruction (in March 1995) came with restenosis 7 months after the index procedure. He was treated with repeat angioplasty and stent implantation. While deploying (from femoral vein), self-expanding wall stent (18 mm × 80 mm) slipped proximally and got deployed in the IVC across the renal veins. The second stent (balloon crimped Palmaz Schatz) also missed the intended site (hepatic segment of IVC close to RA-IVC junction) of deployment and had to be deployed in the hepatic segment of IVC proximal to the HV entry. A wall stent (third stent 18 mm × 40 mm) was deployed accurately at the site of the lesion. The same patient presented with re-restenosis at the proximal part of the third stent and adjacent IVC, 8 years later, which was dilated with balloon and stented successfully (Palmaz Schatz stent). Subsequently, after 5 years follow-up, he is asymptomatic with patent IVC.
One patient who had tumour compression of IVC (hepatic tumour metastasis from adenocarcinoma stomach), palliation was done by stenting the IVC with self-expandable Wall stent (22 mm × 70 mm) (Figure 5). It was post dilated with Inoue balloon. Two months later, he died of hepatocellular failure (due to extensive metastasis), but remained free of IVC obstruction till death.
Figure 5.
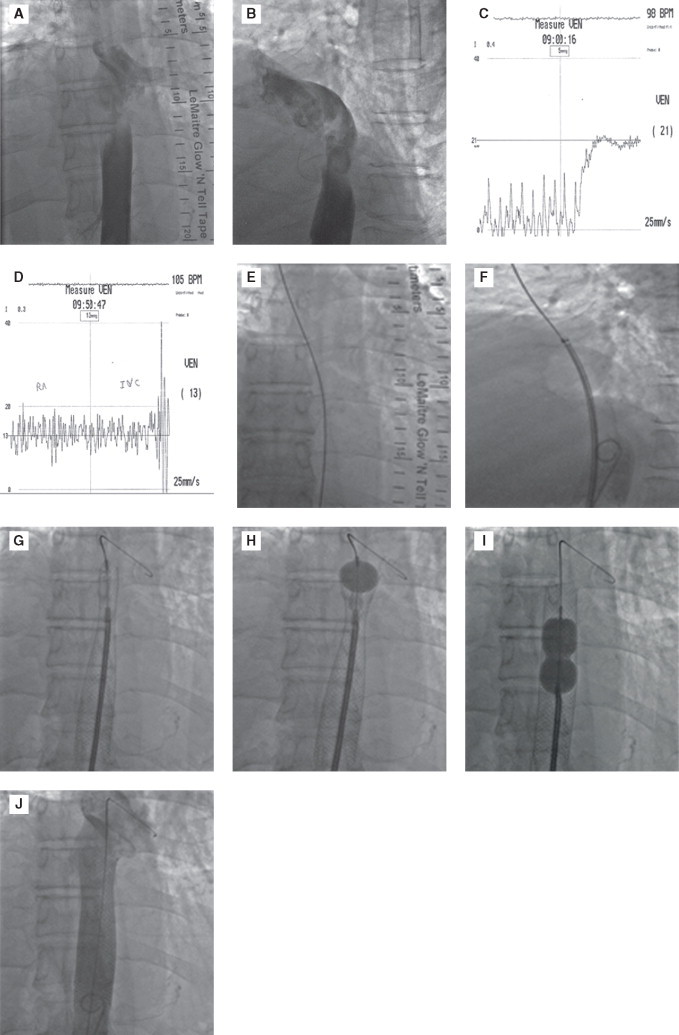
Image reveals obstruction to inferior vena cava (IVC) (haepatic portion-A and B). There is significant gradient between IVC and right atrial, across the obstruction which was relieved following stenting (C and D). Guide wire is passed across the obstruction (E), stent positioned (F), deployed (G), post dilated with Inoue balloon (H and I). At the end, no residual obstruction (J).
Procedural success was defined as venographic evidence of rapid flow through created lumina and disappearance of collaterals and absence of significant gradients across the level of obstruction.
All patients received anticoagulation with unfractionated heparin during the procedure and oral anticoagulation so as to maintain international normalised ration (INR) of 2–3 indefinitely.
Follow-up was done at 1st month, 3rd month and every 6 months subsequently. A thorough clinical examination was done in each visit apart from ultrasound Doppler, liver function test every 6 months.
Results
The procedure was successful in all 12 cases. There was no gradient across the lesion in the cases that underwent stenting for segmental IVC obstruction. Gradients decreased by 84% (22 ± 5 mmHg to 4 ± 3 mmHg) in MOVC who underwent isolated balloon angioplasty. The IVC venogram revealed IVC obstruction was adequately dilated (%<30% residual lesion in isolated balloon angioplasty in MVOC, no residual lesion in the stented group). Antegrade runoff was normal in all cases. Collaterals disappeared in all cases immediately. Oedema, ascites and hepatomegaly gradually decreased over 1 week. Hepatic impairment improved in most cases by 1 month, it persisted in two cases.
Stents were placed in seven cases (five cases during the first procedure and two cases during follow-up, as they had restenosis). Details of the procedure are given in Table 2.
Table 2.
Results of endovascular management of inferior vena cava obstruction.
| Patient no. | Type of obstruction | Endovascular management | Stenting | Technical success |
|---|---|---|---|---|
| 1 | Complete membranous | Balloon dilatation only | None | Yes |
| 2 | Complete membranous | Balloon dilatation only | None | Yes |
| 3 | Segmental | Initial balloon dilatation, restenosis, one stent placement which got displaced, needed two stents. Re-restenosis, balloon dilatation and stented |
|
Yes |
| 4 | Segmental | Balloon dilatation+stenting | 18 × 60 mm self-expanding wall stent | Yes |
| 5 | Complete membranous | Balloon dilatation only | None | Yes |
| 6 | Incomplete membranous | Dilatation with Inoue balloon | None | Yes |
| 7 | Segmental | Balloon dilatation+stenting | 14 × 50 mm precise self-expanding stent | Yes |
| 8 | Tumour compression | Stenting post dilatation with Inoue balloon | 22 × 70 mm wall stent | Yes |
| 9 | Segmental | Dilatation with Inoue balloon | None | Yes |
| 10 | Complete membranous | Balloon dilatation only | None | Yes |
| 11 | Segmental | Balloon dilatation+stenting | 14 × 80 mm precise and 14 × 60 mm precise | Yes |
| 12 | Incomplete membranous | Balloon dilatation only | None | Yes |
Late results and follow-up
Follow-up varies from 15 months to 14 years. One patient presented with restenosis 8 months after balloon angioplasty which was treated with stent implantation and 8 years later, he presented with re-restenosis at the proximal end of stent and adjacent IVC (Figure 6) which was subsequently dilated and stented. Fourteen years follow-up of this patient revealed patent stents.
Figure 6.
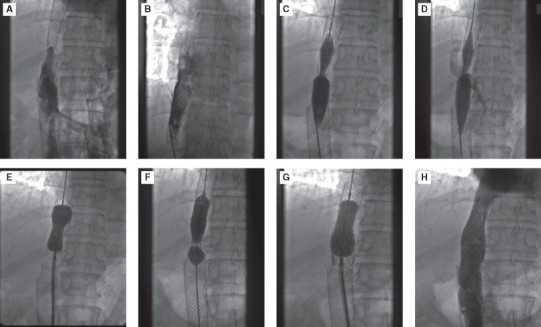
Image reveals restenosis at the proximal end and adjacent part of the stent (A). Lesion was crossed (B) and predilated (C), lesion did not yield but balloon gave away (D). It was then predilated with Inoue balloon (E) and stented with Palmaz Schatz stent, mounted on Tyshak balloon (F). Post dilated with Inoue balloon subsequently (G). End result was excellent with patent Inferior vena cava (H).
Two cases died because of hepatocellular failure (1 and 3 months after the procedure), unrelated to the procedure. Both patients had patent IVC till death.
The other cases (both membranous and segmental IVC obstruction), have maintained patent IVC, on follow-up, by ultrasound Doppler studies.
Discussion
In 1845, Budd described three cases of HV thrombosis (HVT) due to infectious phlebitis.
Chiari described in 1899 three additional cases of HV occlusion due to phlebitis, IVC involvement was present in one of the cases. Since Pleasants’ literature review in 1911 of 296 cases, the Budd-Chiari syndrome (now termed as HVOO) has included both HV and IVC obstruction/stenosis.1
Okuda in 2001 emphasised that the classic HVOO also called HVT and MOVC, also called primary thrombosis of the IVC, are epidemiologically, pathologically, and clinically different and should be treated as two different clinical entities.1,2
The HVT and MOVC have a different onset, clinical manifestations and natural history. Whereas HVT is a severe disease with an acute onset, MOVC presents as a mild disease at the onset, which can eventually turn into a fibrous occlusion of the IVC.
According to Pant and Punamia,17 HVOO can be identified into following categories:
Type A: IVC obstruction.
Type B: Pure HV obstruction with short segment stenosis.
Type C: Combined short segment HV and IVC occlusion.
Type D: Extensive intrahepatic occlusion of HVs with no identifiable main HV.
These can be differentiated on the basis of cross-sectional imaging and catheter angiography. Type A or isolated IVC obstruction is common in the Indian subcontinent. Our study is restricted to this form of HVOO.
Membrane like obstruction commonly found angiographically, has been found to consist of organised old thrombus arranged in layers of different ages. The clinical onset is mild and causes liver damage by congestion. A distinct pattern of subcutaneous venous collaterals typically develops together with retroperitoneal collaterals through the ascending lumbar and the iliolumbar veins into the hemiazygos and azygos veins. This type of the disease is more common in Asia and Africa.
The HVT has an acute onset with severe liver damage often requiring urgent portocaval decompression and might even require liver transplantation depending on the extent of venous involvement. The collateral venous pathways are similar to those seen in portal hypertension. This type of the disease is more common in Western countries. In our study, we did not come across hepatic venous involvement either in isolation or in combination with IVC obstruction.
The diagnosis of HV outflow block is established by the clinical history and physical examination. Clinically, it is recognised by hepatomegaly, portal hypertension, impaired liver function, and formation of communicating channel, and oedema or ulcer in the lower extremities. The clinical signs of ascites, abdominal pain, and hepatomegaly are the typical triad of HVOO.
The best imaging methods are ultrasound, computed tomography (CT) and MRI.
Once the diagnosis is established, inferior vena cavography is needed to guide endovascular techniques.
Goal of treatment is to give a long-term patency of IVC with minimal mortality and morbidity. In case of tumour compression, it is restricted to palliation to relieve symptoms.
Treatment of IVC obstruction includes surgical (direct visualisation of obstruction and removal or venous bypass grafts) or interventional (balloon dilatation ± stenting).6–12 Surgery though successful, carries higher risk of complications in patients who already had impaired liver function.3–5 Mortality in surgical group is higher–33–40% (Kimura et al. & Iwaragi et al.).4
Gloviczki et al.18 recommended spiral venous grafts or polytetrafluoroethylene (PTFE) grafts to bypass the obstruction. In their series, 43% needed additional surgeries later and only 29% of cases maintained graft patency at 2 years. Seven out of 11 cases had PTFE patent graft at 9 months.
Angioplasty using balloon is a technique developed in the early 1990s which carries higher chances of success with minimal complications. Results of angioplasty are good in cases of discrete membranous obstruction, but disappointing in cases of segmental obstruction.19,20 This can be attributable to venous recoil, low flow states and thrombogenecity of IVC. Stents have revolutionised the endovascular management and to maintain long-term patency of IVC.21–24 There is a paucity of studies utilising stents in IVC obstruction especially in chronic setup. Most of the studies were conducted in acute form of the disease.21
Different balloons were used by different interventionists. In our study, we used Inoue balloon in many cases. Use of Inoue balloon was popularised by Yang et al. in 1992, it carries clear advantages13 which can be summarised as follows:
-
1.
Adult IVC measures 20–22 mm, thus single Inoue balloon is ideal to dilate. Balloon to IVC ratio should be 1:1–1.1:1.
-
2.
Balloon diameter is ideal for dilating membrane, as the waist of the balloon locks the centre of the membrane and dilates.
-
3.
Lower inflation pressure is required (1–2 atm), for a shorter period of time (3 seconds).
-
4.
Rubber-Nylon micromesh of IB has stronger build, thus ideal to dilate tough membrane.
-
5.
Balloon size can be altered, thus it can be tailor made, suiting each type of lesion.
We were successful in re-establishing the patency of IVC in all 12 cases. During the long-term follow-up, 11/12 cases maintained patency, the one case that earlier had undergone stenting was re-intervened and additional stent placement was possible.
All the cases received anticoagulation for an indefinite period. We did not come across any case of stent thrombosis.
The procedure was safe and well tolerated in all our cases with no evidence of pulmonary embolisation, bleeding, infection, or haematoma formation. However, we recommend use of thrombolytic therapy for 24 hours, before undertaking intervention, in cases where acute-on-chronic thrombosis is suspected. This is to prevent pulmonary embolisation.
Current status of stenting
The decision of stenting the IVC is often individualised. However, it is particularly useful in the following cases.
-
1.
Long segment of obstruction or multiple segments of obstruction.
-
2.
Restenosis after balloon angioplasty.
-
3.
Enlarged caudate lobe of liver or nodular compression on IVC (e.g. haepatoma).
Conclusion
Balloon angioplasty is efficacious in relieving IVC obstruction with minimal morbidity. Inoue balloon is ideal to dilate membranous obstruction. Stents should be used whenever there is residual obstruction after balloon angioplasty, segmental obstruction, restenosis following balloon angioplasty and extrinsic compression of IVC. Follow-up results are favourable for balloon angioplasty ± stenting with minimal re-stenosis rates.
References
- 1.Okuda H, Yamagata H, Obata H. Epidemiological and clinical features of Budd-Chiari syndrome in Japan. J Hepatol. 1995;22:1–9. doi: 10.1016/0168-8278(95)80252-5. [DOI] [PubMed] [Google Scholar]
- 2.Okuda K. Budd Chiari syndrome. J Gastroenterol Hepatol. 2001;16:1179–1183. doi: 10.1046/j.1440-1746.2001.02577.x. [DOI] [PubMed] [Google Scholar]
- 3.Kohli V, Pande GK, Dev V. Management of hepatic venous outflow obstruction. Lancet. 1993;18:718–722. doi: 10.1016/0140-6736(93)91712-u. [DOI] [PubMed] [Google Scholar]
- 4.Slakey DP, Klein AS, Venbrux AC. Budd-Chiari syndrome: current management options. Ann Surg. 2001;233:522–527. doi: 10.1097/00000658-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh V, Sinha SK, Nain CK. Budd-Chiari syndrome: our experience of 71 patients. J Gastroenterol Hepatol. 2000;15:550–554. doi: 10.1046/j.1440-1746.2000.02157.x. [DOI] [PubMed] [Google Scholar]
- 6.Kovalik EC, Newman GE, Suhocki P. Correction of central venous stenosis: use of angioplasty and vascular Wallstents. Kidney Int. 1991;45:1177–1181. doi: 10.1038/ki.1994.156. [DOI] [PubMed] [Google Scholar]
- 7.De BK, Biswas PK, Sen S. Management of the Budd-Chiari syndrome by balloon cavoplasty. Indian J Gastroenterol. 2001;20:151–154. [PubMed] [Google Scholar]
- 8.McCarthy PM, van Heerden JA, Adson MA. The Budd-Chiari syndrome. Medical and surgical management of 30 patients. Arch Surg. 1985;120:657–662. doi: 10.1001/archsurg.1985.01390300007001. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZG. Management of Budd-Chiari syndrome: experience from 430 cases. Asian J Surg. 1996;19:23–30. [Google Scholar]
- 10.Orloff MJ, Daily PO, Orloff SL. A 27-year experience with surgical treatment of Budd-Chiari syndrome. Ann Surg. 2000;232:340–352. doi: 10.1097/00000658-200009000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao T, Liu C, Liu C. Interventional endovascular treatment of Budd-Chiari syndrome with long-term follow-up. Swiss Med Wkly. 2005;135:318–326. doi: 10.4414/smw.2005.10947. [DOI] [PubMed] [Google Scholar]
- 12.Pisani-Ceretti A, Intra M, Prestipino F. Surgical and radio-logic treatment of primary Budd-Chiari syndrome. World J Surg. 1998;22:48–54. doi: 10.1007/s002689900348. [DOI] [PubMed] [Google Scholar]
- 13.Martin LG, Henderson JM, Millikan WJ. Angioplasty for long-term treatment of patients with Budd-Chiari syndrome. AJR. 1990;154:1007–1010. doi: 10.2214/ajr.154.5.2138842. [DOI] [PubMed] [Google Scholar]
- 14.Panis Y, Belghiti J, Valla D. Porto systemic shunts in Budd Chiari syndrome: long term survival and factors affecting shunt patency in 25 patients in Western countries. Surgery. 1994;115:276–281. [PubMed] [Google Scholar]
- 15.Carrasco CH, Charnsangavej C, Wright KC. Use of the Gianturco self-expanding stent in stenoses of the superior and inferior venae cavae. J Vasc Interv Radiol. 1992;3:409–419. doi: 10.1016/s1051-0443(92)72054-5. [DOI] [PubMed] [Google Scholar]
- 16.Furui S, Sawada S, Irie T. Hepatic inferior vena cava obstruction: treatment of two types with Gianturco expandable metallic stents. Radiology. 1990;176:665–670. doi: 10.1148/radiology.176.3.2143840. [DOI] [PubMed] [Google Scholar]
- 17.Rochan Pant, Punamiya SJ. Budd Chiari syndrome: Interventional Therapy. Bombay Hospital Journal Volume 44(4), October, 2002. Available at http://www.bhj.org/journal/2002_4404_oct/therap_614.htm
- 18.Gloviczki, Patel K, David R. Femorocaval bypass with femoral crossover bypass for iliofemoral and caval occlusion. J Vasc Surg. 1997;26:989–993. doi: 10.1016/s0741-5214(97)70011-9. [DOI] [PubMed] [Google Scholar]
- 19.Davidson CJ, Newman GE, Sheik KH. Mechanism of angioplasty in hemodialysis fistula stenosis evaluated by intravascular ultrasound. Kidney Int. 1991;40:91–95. doi: 10.1038/ki.1991.185. [DOI] [PubMed] [Google Scholar]
- 20.Kovalik EC, Newman GE, Suhocki P. Correction of central venous stenosis: use of angioplasty and vascular wall stents. Kidney Int. 1991;45:1177–1181. doi: 10.1038/ki.1994.156. [DOI] [PubMed] [Google Scholar]
- 21.Gillams A, Dick R, Platts A, Irving D, Hobbs K. Dilatation of the inferior vena cava using an expandable metal stent in Budd-Chiari syndrome. J Hepatol. 1991;13:149–151. doi: 10.1016/0168-8278(91)90808-o. [DOI] [PubMed] [Google Scholar]
- 22.Yang XL, Cheng TO, Chen CR. Successful treatment by percutaneous balloon angioplasty of Budd-Chiari syndrome caused by membranous obstruction of inferior vena cava: 8-year follow-up study. J Am Coll Cardiol. 1996;28:1720–1724. doi: 10.1016/S0735-1097(96)00385-3. [DOI] [PubMed] [Google Scholar]
- 23.Park J, Chung J, Han J, Han M. Interventional management of benign obstruction of the hepatic inferior vena cava. JVIR. 1994;5:403–409. doi: 10.1016/s1051-0443(94)71515-3. [DOI] [PubMed] [Google Scholar]
- 24.Kaul U, Agarwal R, Jain P, Sharma S, Wasir HS. Management of idiopathic obstruction of the hepatic and suprahepatic inferior vena cava with a self-expanding metallic stent. Cathet Cardiovasc Diag. 1996;39:252–257. doi: 10.1002/(SICI)1097-0304(199611)39:3<252::AID-CCD9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]


