Highlights
► A case of metastatic uterine papillary serous cancer with Her-2 gene amplification. ► Was treated with a Her-2 targeted agent and achieved durable remission. ► Her-2 targeting should be considered alone or in combination with chemotherapy.
Keywords: Endometrial cancer, Her-2, Targeted therapy, Papillary serous carcinoma
Introduction
Endometrial cancer is the most common cancer of the female reproductive system accounting for 6% of all cancers in women. Uterine papillary serous carcinoma (UPSC) and clear cell carcinoma account for less than 15% of all endometrial cancers but are biologically aggressive tumors. Papillary serous carcinoma is thought to develop from endometrial surface epithelium and presents histologically as complex papillary structures with marked atypia. They are more likely to present with deep myometrial invasion, lymph node involvement, and distant metastatic spread at presentation, compared to the more common endometrioid cancers.
Here we describe a case of uterine papillary serous carcinoma with recurrent metastatic disease to her lungs. Her tumor was positive for Her-2 gene amplification and she was offered treatment with Her-2 targeted therapy in an ongoing clinical trial. She demonstrated an excellent durable response lasting more than two years following her initial diagnosis of metastatic disease.
Case history
Patient is a 63 year-old woman, gravida 2, para 1, without significant past medical history, who was found to have suspicious cells on a routine Pap smear in 2006. A transvaginal ultrasound showed an endometrial cyst and hysteroscopy revealed a polyp-like structure that was removed. Pathology showed a cystic endometrial polyp with focal surface papillary serous carcinoma. The endometrial and endocervical curettings also showed fragments of serous papillary carcinoma. Staging PET/CT scan showed an enlarged endometrial cavity with no definite mass or suspicious FDG uptake. There was no evidence of distant metastatic disease. She underwent surgical staging comprising total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic/paraaortic lymph node dissection and omentectomy in September 2006. Intraoperatively, there was no evidence of peritoneal carcinomatosis or suspicious lymphadenopathy. The final pathology revealed a 4 mm grade 3 residual papillary serous carcinoma without myometrial invasion. There was no evidence of lympho-vascular invasion. The harvested pelvic and periaortic lymph nodes and pelvic washings were negative for malignancy. She was surgically staged FIGO stage IA and underwent adjuvant chemotherapy consisting of 6 cycles of Carboplatin (AUC 6) and Paclitaxel (175 mg/m2) given once every 3 weeks, as well as vaginal brachytherapy. She completed chemotherapy in February 2007 and a follow up PET/CT in July 2007 revealed no evidence of disease.
She remained asymptomatic and continued with routine surveillance until December 2008 when a repeat PET/CT showed new pulmonary nodules. There was an 8 × 12 mm right middle lobe nodule that was hypermetabolic with SUV of 2.4; a 4 mm nodule in the left lower lobe; and a 6.5 mm subpleural nodule in the lingula that had focal hypermatobolic activity with maximal SUV of 2.3. There was no evidence of local recurrence and no other sites of distant disease. A CT guided lung biopsy confirmed the metastatic adenocarcinoma consistent with papillary serous carcinoma of endometrial origin (Fig. 1). She subsequently went on to receive four more cycles of chemotherapy with Carboplatin and Paclitaxel with complete resolution of the pulmonary nodules and no new evidence of metastatic disease. The chemotherapy was held after that secondary to grade 4 anemia and she was followed clinically.
Fig. 1.
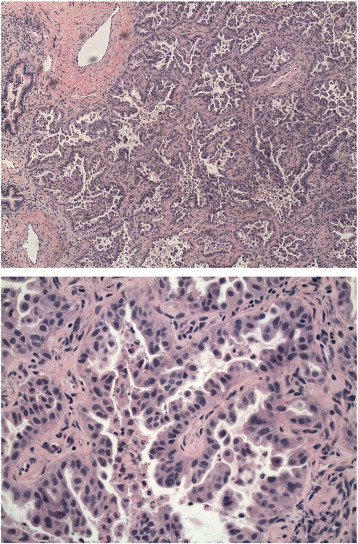
Lung biopsy showing adenocarcinoma with morphology and immunohistochemical staining consistent with patient's previous papillary serous endometrial carcinoma.
However, repeat scans in October 2009 showed new pulmonary nodules consistent with recurrent disease. Her lung biopsy specimen was found to have Her-2 gene amplification by FISH (fluorescence in situ hybridization). As such, she was enrolled in a single arm phase II clinical trial evaluating the role of the irreversible ErbB Family Blocker afatinib (BIBW 2992, Boehringer Ingelheim) in a variety of tumors overexpressing EGFR or with Her-2 gene amplification. She was started on a 50 mg daily dose and a follow up scan after 6 weeks showed complete response with resolution of the pulmonary nodules. Her treatment was complicated by grade 3 diarrhea, grade 2 rash, and grade 1 mucositis, requiring a decrease in dose to 40 mg daily. Her follow up scans at 12 weeks and at 6 months continued to show complete remission. However, she was taken off the drug in July 2010, after 8 months of treatment, secondary to recurrent grade 3 weight loss.
A routine follow up scan in November 2010 revealed a single subcentimeter nodule in the middle lobe of right lung. She underwent right VATS (video assisted thoracoscopic surgery) with wedge resection of the pulmonary nodule, with pathology confirming a 9 mm focus of metastatic serous carcinoma. There was no evidence of any other sites of metastatic disease and her subsequent scans confirmed complete remission. However, in June 2011, she was found to have recurrent lung lesions and was started on lapatinib, considering her excellent response to previous Her-2 targeted therapy. Unfortunately, she had progressive disease including development of brain metastases in December 2011. She received whole brain radiation therapy and was subsequently started on further palliative systemic chemotherapy.
Discussion
Uterine papillary serous carcinoma (UPSC) and clear cell carcinoma are biologically aggressive tumors. A SEER database report of 1473 UPSC diagnosed between 1988 and 2001 showed that more than half of patients presented with stage III/IV disease at presentation. The 5-year disease specific survival was much lower compared to grade 3 endometrioid tumors (55% vs. 77% respectively). The standard of care is complete surgical staging with total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO), complete pelvic/paraaortic dissection, omentectomy, and assessment of the peritoneal cavity including pelvic and diaphragmatic cytology. The amount of residual disease after surgery is a strong predictor of outcome. Most of the data on adjuvant therapy comes from retrospective analysis suggesting a benefit in terms of progression free and overall survival.
Her-2 belongs to the family of epidermal growth factor receptors and consists of a transmembrane cell surface receptor with tyrosine kinase activity. Her-2 expression has been found to be a poor prognostic factor in several cancers including breast and ovarian, however, there is only limited data in endometrial cancers. The frequency of Her-2 overexpression in UPSC is variably reported between 17 and 50% of the patients. A case series of 30 UPSC patients by Santin et al. showed that Her-2 amplification by FISH was found in 47% of patients including 4 patients with heterogeneous expression of gene amplification (Santin et al., 2005). A significantly shorter disease specific survival was found in patients overexpressing the Her-2 gene (17% vs. 84% at 4 years). A difference in Her-2 expression was also thought to be related to poor prognosis in African-American compared to Caucasian women. Similarly, a study of 68 patients with UPSC showed Her-2/neu overexpression in 18% of patients but out of these only 2 patients showed Her-2 gene amplification by FISH (Slomovitz et al., 2004). Her-2/neu overexpression was associated with a significantly shorter overall survival (p = .008). Grushko et al. looked at 234 patients of endometrial cancer enrolled in GOG 177 trial (Grushko et al., 2008). 104/234 (47%) patients were found to have 2 +/3 + Her-2 overexpression by IHC. Papillary serous carcinomas were more likely to express Her-2 compared to non-serous tumors (61% vs. 41%), including a higher proportion of patients presenting with strong (3 +) overexpression (26% vs. 18%). Her-2 gene amplification by FISH was however found to be present only in 21% of the UPSC specimens (compared to 11% of non-serous patients), with concordance rate of 60% between IHC and FISH. Other studies have reported higher concordance rate in UPSC patients, however, the number of patients in these studies were small.
The data on Her-2 targeting in endometrial cancer is limited and mostly based upon case reports. Villella et al. examined the Her-2 gene status in patients diagnosed with UPSC between 1999 and 2001 and found 5/19 (26%) patients to be positive for Her-2 overexpression by IHC (Villella et al., 2006). Two of these patients were treated with trastuzumab. A stage IIIC patient had stable disease for 3 months and a patient with stage IV disease had complete response for 6 months on trastuzumab therapy. Similarly, Santin et al. reported good clinical response with single agent trastuzumab in two patients; one with recurrent grade 3 endometrioid adenocarcinoma treated with chemotherapy plus trastuzumab and the other with stage IIIC UPSC who received upfront single agent trastuzumab (Santin et al., 2008). In contrast, a recent GOG study did not demonstrate any significant activity using single agent trastuzumab in 33 patients with Her-2 positive endometrial cancer (Fleming et al., 2010). Out of these, 11 patients had serous carcinoma, though FISH was positive in 8 of these patients.
Thus, the role of Her-2 targeting in endometrial carcinoma is still uncertain. Afatinib is a novel, potent, small-molecule tyrosine kinase inhibitor which irreversibly and selectively targets the ErbB family of receptors including EGFR and Her-2. For instance, afatinib treatment inhibits in vitro growth of the trastuzumab-resistant SUM 190 cell line, which overexpresses Her-2 (Hickish et al., 2009). In vivo, it has anti-tumor activity in SUM 190 xenografts (Solca et al., 2010) as well as in human xenograft models known to depend on ErbB signaling (Solca et al., 2005). Afatinib has shown encouraging results in lung and breast cancer and is currently undergoing clinical trials in several cancers. The most common side effects of this drug include diarrhea, rash, stomatitis, fatigue and decreased appetite, requiring a dose reduction or even treatment discontinuation (Miller et al., 2012). There is enough evidence that anti Her-2 therapy might be more beneficial to patients with UPSC or high grade endometrioid tumors. It is also possible that it might be more effective when given in combination with chemotherapy as clinical trials have shown synergistic activity of trastuzumab with other chemotherapy agents in breast cancer. We do see some durable responses with anti Her-2 therapy in UPSC as seen in our patient. We need good prospective data including patients with serous or clear cell carcinoma or high grade endometrioid tumors to evaluate the efficacy of this targeted approach.
Conflict of interest statement
Dr. Sumit Talwar has no conflicts of interests in relation to this publication.
Dr. Seth Cohen received research grants from Boehringer Ingelheim Pharmaceuticals for the clinical research trial.
References
- Santin A.D., Bellone S., Van Stedum S., Bushen W., Palmieri M., Siegel E.R., De Las Casas L.E., Roman J.J., Burnett A., Pecorelli S. Amplification of c-erbB2 oncogene: a major prognostic indicator in uterine serous papillary carcinoma. Cancer. 2005;104(7):1391. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- Slomovitz B.M., Broaddus R.R., Burke T.W. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J. Clin. Oncol. 2004;22(15):3126–3132. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- Grushko T.A., Filiaci V.L., Mundt A.J., Ridderstråle K., Olopade O.I., Fleming G.F. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2008 Jan;108(1):3–9. doi: 10.1016/j.ygyno.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella J.A., Cohen S., Smith D.H. HER-2/neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. Int. J. Gynecol. Cancer. 2006 Sep–Oct;16(5):1897–1902. doi: 10.1111/j.1525-1438.2006.00664.x. [DOI] [PubMed] [Google Scholar]
- Santin A.D., Bellone S., Roman J.J., McKenney J.K., Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. Int. J. Gynaecol. Obstet. 2008 Aug;102(2):128–131. doi: 10.1016/j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Fleming G.F., Sill M.W., Darcy K.M., McMeekin D.S., Thigpen J.T., Adler L.M., Berek J.S., Chapman J.A., DiSilvestro P.A., Horowitz I.R., Fiorica J.V. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2010;116(1):15. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickish H., Wheatley D., Lin N. Poster presented at the San Antonio Breast Cancer Symposium, December 9–13, 2009. 2009. Use of BIBW 2992, a novel irreversible EGFR/HER1 and HER2 tyrosine kinase inhibitor to treat patients with HER2-positive metastatic breast cancer after failure of treatment with trastuzumab. [Google Scholar]
- Solca F., Adolf G.R., Jones H. Beyond trastuzumab: second-generation targeted therapies for HER2-positive breast cancer. In: TBC E., editor. Milestones in Drug Therapy. Springer; 2010. [Google Scholar]
- Solca F., Baum A., Guth B. Vol. 118. 2005. BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor for cancer therapy. (Proceedings, AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics). (Abstract A244) [Google Scholar]
- Miller V.A., Hirsh V., Cadranel J. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012 Mar 23;13(5):528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]


