Abstract
Chest pain is one of the chief presenting complaints among patients attending Emergency department. The diagnosis of acute myocardial infarction may be a challenge. Various tools such as anamnesis, blood sample (with evaluation of markers of myocardial necrosis), ultrasound techniques and coronary computed tomography could be useful. However, the interpretation of electrocardiograms of these patients may be a real concern. The earliest manifestations of myocardial ischemia typically interest T waves and ST segment. Despite the high sensitivity, ST segment deviation has however poor specificity since it may be observed in many other cardiac and non-cardiac conditions. Therefore, when ST–T abnormalities are detected the physicians should take into account many other parameters (such as risk factors, symptoms and anamnesis) and all the other differential diagnoses. The aim of our review is to overview of the main conditions that may mimic a ST segment Elevation Myocardial Infarction (STEMI).
Keywords: Chest pain, ECG, ST segment, Myocardial infarction, Differential diagnosis
1. Introduction
Emergency physicians, frequently, deal with patients symptomatic for acute chest pain. In this setting, some aspects such as clinical features and biomarkers of myocardial necrosis may have an important role. In many cases, the accurate diagnosis of myocardial infarction (MI) may be, however, a real challenge. Indeed, the typical clinical presentation may be absent and a non-specific elevation of plasmatic levels of cardiac troponin I could be detectable. The twelve-lead electrocardiogram (ECG) is then an integral part of the diagnostic work up of patient with acute chest discomfort. It is the easiest and available instrument to confirm or exclude the diagnosis of MI and to decide the appropriate treatment strategy. The earliest manifestations of myocardial ischemia typically interest T waves and ST segment. It is possible to make diagnosis of acute ST segment Elevation Myocardial Infarction (STEMI) when, in a certain clinical context, a new ST segment elevation is detected in at least two continuous leads. In an ECG recorded at a paper speed of 25 mm/s and an amplification of 10 mm/mV, the ST segment elevation from the baseline should be measured 80 ms after the J point and is considered present if the deviation is ≥0.2 mV in men and ≥0.15 mV in women in V2–V3 leads (≥0.1 mV in other leads). Despite the high sensitivity, the ST segment deviation has, however, a poor specificity since it may be observed in many other conditions (such as left bundle branch block, hypertrophic cardiomyopathy or left ventricle aneurysm). Furthermore, the problem of equivocal electrocardiographic features is frequent in the departments of emergency care, especially in patients with hypertension or previous history of MI. A wrong diagnosis led patients to unnecessary (invasive or conservative) cares. Sharkey et al1 have previously reported the magnitude of the problem, observing that almost 11% of patients with suspected acute coronary syndrome receive unnecessary thrombolytic therapy. A recently published article has investigated the ability to recognize an MI of 15 trained cardiologists coming from various countries (North America, Israel and Europe). They were asked to evaluate some ECG with ST segment elevation (>0.1 mV) in at least two contiguous leads and to say if, in presence of symptoms, the modifications were consistent with an MI or related to other causes. Only eight (of 116) ECG were recorded in patients with a real documented STEMI, that was however diagnosed in a variable percentage (7.8% and 33%). Moreover, when ST segment elevation was related to “other causes”, the diagnostic orientation was frequently wrong. Our aim is to overview the main conditions that may electrographically mimic a STEMI.
2. ST segment
ST segment represents the interval between depolarization and repolarization of the ventricles. It can be evaluated by using as baseline reference both the PQ and the TP segments, which are both expression of the diastolic potentials. It is, however, correct to refer to the TP segment, since the PQ segment may be altered by atrial lesions (i.e. valvular diseases, acute pericarditis or atrial infarction). In limb leads, the ST segment is isoelectric in >75% of healthy adults. Instead, a variable grade of ST segment elevation in the precordial leads is usually detected. Usually, the magnitude of this deviation is related to the amplitude of QRS complex and is higher and more common in young adults of male gender.2 In non-pathological conditions, the elevation can reach even 0.3 mV in V2–V3, while is rarely more than 0.1 mV in the other precordial leads. In the MI, ST segment elevation is believed to represent a “current of injury” from damaged cells that are partially depolarized to the healthy myocardium. However, in animal models that have used patch clamps experiments, this current leads to a depression of the TQ segment (rather than to an ST elevation) for the inability of damaged myocardium to repolarize fully. The differences in human subjects are mainly related to the presence in the normal electrocardiograph of an alternated current amplifier, which compensates any shift of the TQ segment from the isoelectric baseline. Therefore, in presence of currents of injury, each compensation of the TQ segment induces a displacement of ST segment in a higher position and leads to an elevation. It is more difficult to explain the mechanisms leading to ST segment elevation in other conditions in which a current of injury is not present. The ST segment represents the phase two (plateau) of the action potential (AP): it is possible to suppose that any modification of AP may result in an ST segment deviation. In some non-ischemic conditions (such as the Brugada Syndrome), the speed of AP in the epicardial surface seems to be lower than in the endocardial one.
3. Natural variants
3.1. Early repolarization
The early repolarization, also known as benign early repolarization (BER), which is considered a non-pathological condition, is observed in almost 1% of general population and in almost 48% of patients referred to an emergency point of care for chest pain.3 The electrophysiological basis is not clear. Possible mechanisms include “hypervagotonia”,4 “asthenic habitus”,5 and an early start of the repolarization in the epicardial surface before the end of depolarization in the entire myocardium.6
In BER, the ECG shows an elevated ST segment that presents a concave slope in more than one lead. A notch (or a little delay) in the terminal portion of the QRS complex, associated with the presence of high and concordant T waves, is also detectable.7 The elevation is usually < 0.2 mV (80–90% of the cases) but in some patients it can reach even 0.5 mV. Only in 2% of the cases, an elevation >0.5 mV is found. The highest elevation is usually observed in precordial leads (from V2 to V5); if it is confined to limb leads a different diagnosis should be evaluated. The aspect of T waves (which are typically tall, peaked and concordant) may resemble that one detected in acute myocardial infarction (AMI), especially in the case of posterior involvement. Sometimes to distinguish early repolarization from other pathological conditions, such as pericarditis, may be challenging. However, in patients with acute pericarditis, at least at the beginning, there is a frequent depression of PR segment, especially in V6 with a specular elevation in aVR, not present in BER. Moreover, ST segment elevation is usually more pronounced and prone for dynamic changes in pericarditis than in BER8.
3.2. Left ventricular hypertrophy and hypertrophic cardiomyopathy
Left ventricular hypertrophy is a very common condition that is associated with ST segment elevation. Usually, as in left bundle branch block, the repolarization pattern is discordant to the QRS. The elevation is more commonly observed in leads V2–V3 and is usually <0.3 mV with minor abnormalities in V4–V6.9 Hypertrophic cardiomyopathy is a primitive myocardial disease, which is characterized by the hypertrophy of the wall (especially of the septum) in the absence of overload mismatch. Usually it may be suspected by using ECG and then confirmed with ultrasounds technique. When a septal hypertrophy is present, the ECG shows high voltage of R waves in antero-lateral leads associated to Q waves in anterior and inferior leads. The T waves, usually being very deep and inverted in V2–V4, may resemble a non-Q AMI. Rarely, a real ST elevation might be seen (Fig. 1).
Fig. 1.
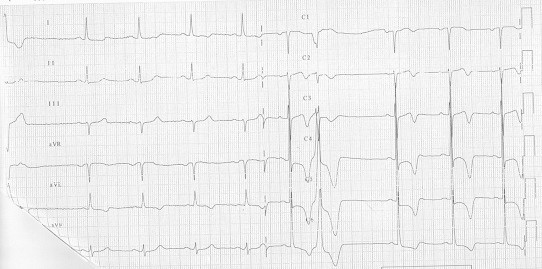
Hypertrophic cardiomyopathy.
3.3. Left bundle branch block
In patients with left bundle branch block (LBBB) ST–T abnormalities are common, making it difficult to assess the presence of myocardial ischemia. In this condition ST segment and T–waves are usually discordant with the QRS, since they are directed in opposite directions. A concordant ST segment shift should always be referred to a myocardial lesion and considered as strongly indicative of MI. In the opposite, a discordant ST segment displacement may be indicative of a myocardial lesion when specific standard criteria are exceeded. In example, discordant elevation of the J point ≥0.5 mV in V1–V2 is strongly suggestive of MI in the presence of LBBB.10 However, the same ST changes have been observed in 7.3% of a population of 124 patients with LBBB without MI.11 In the same group, a direct correlation between QRS amplitude and entity of the ST segment elevation was found. Moreover, in LBBB, the QRS/T ratio appears to be more predictive than the amplitude of ST elevation. When this ratio is near to or less than 1, the probability that the repolarization abnormality is related to an MI is high.12
4. Artifacts
4.1. Leads malpositioning
Usually, during stress test and in emergency point of care, even the peripheral electrodes are positioned on the chest (Mason-Likar disposition). Sometimes, this kind of disposition may alter the Einthoven Triangle leading to possible alterations of the ST segment.13
4.2. Electrical cardioversion
During electrical cardioversions or the adminisration of a DC shock by an implantable cardioverter defibrillator, a transient ST segment elevation is rarely observed.14,15 A consistent ST elevation for more than 2 min may indicate a myocardial lesion (Fig. 2).
Fig. 2.
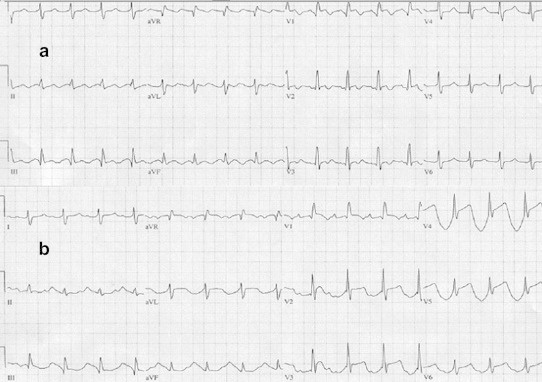
Atrial Flutter 2:1(a) after a DC shock (b).
5. Cardiovascular diseases
5.1. Pericarditis
Pericardium (from the Greek περι: around, καρδιον: heart) is a double layered membrane containing the heart and roots of the great vessels. The space between the two layers is filled with pericardium fluid, which protects the heart from any kind of external injury. Although pericardium is electrically inactive, its infection and/or inflammation may affect the external part of epicardium and cause significant ECG modifications, whose evolutions can be subdivided into four phases.16 The first ECG modifications usually affect the ST segment and are characterized by a concave elevation in those leads with a positive QRS, as the ST segment owns a vector with a positive angle (ranging between +30° and +60°) concordant with QRS vector. These abnormalities are evident in almost all leads, as pericarditis generally affects the whole pericardium. According to the ST vector, the elevation is higher in DII, while is lower (or almost absent) in DI or aVL.16 At the same time, the contemporary depression of the PR segment is the expression of an atrial lesion (Fig. 3).17 Such modifications may resemble acute ischemia, but in this case ST elevation is seen only in contiguous leads (regional lead group such as anterior, inferior or lateral/apical) with a frequent depression in the reciprocal leads.18
Fig. 3.
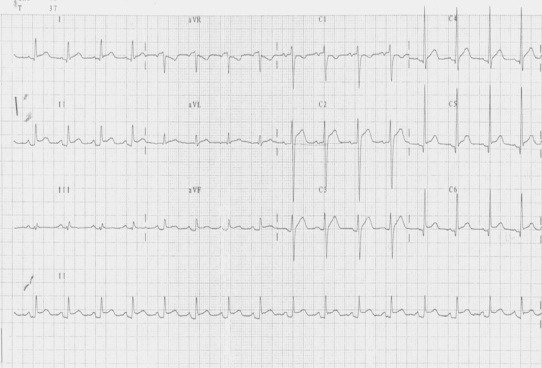
Acute pericarditis.
5.2. Myocarditis
It is clinically and pathologically defined as the inflammation of the myocardium, whose classification, diagnosis and treatment continue to prompt considerable debate. Clinical manifestations range from subclinical disease (asymptomatic ECG abnormalities) to fulminant heart failure presenting as a new onset cardiomyopathy. Myocarditis masquerading as an acute coronary syndrome has been well described.19 It should be considered in young patients symptomatic for acute, ischemic-like chest pain associated with ECG abnormalities and global (rather than segmental) left ventricular dysfunction on echocardiography. The ECG changes, despite angiographically documented absence of coronary vessels involvement, vary from ST segment upsloping in contiguous leads, T waves inversions, widespread ST segment depression and pathological Q waves (Fig. 4).20
Fig. 4.
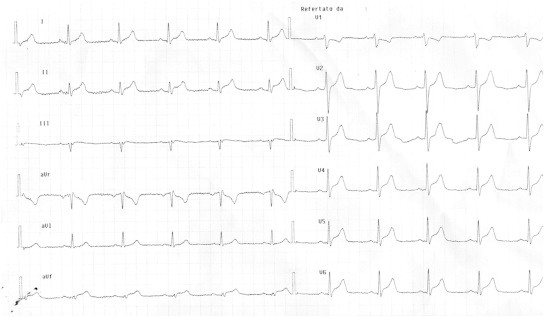
ECG of a young patient presenting with acute ischemic-like chest pain and normal coronary arteries. The magnetic resonance confirmed the presence of myocarditis.
5.3. Aortic dissection
Aortic dissection may sometimes be associated with an ST segment elevation due to the involvement of the ostia of coronary arteries, to cardiogenic shock after tamponade or to pre-existing coronary artery disease.21 In other cases, it is possible to observe a phenomenon called “wandering ST”, which is a marker of the disease progression and is characterized by the transient elevation of the ST segment in some leads that reappears in other leads.22
5.4. Prinzmetal's angina
Originally described in 1959 by Prinzmetal, it is more prevalent in smokers of male gender (74%) and is characterized by sudden episodes of chest pain at rest due to reversible coronary spasm. The spasm may occasionally be induced by emotional stresses or efforts. It is associated to the elevation of ST segment. Episodes are usually brief and rapidly interrupted by administration of nitrates, but they can be occasionally complicated by syncope, life-threatening arrhythmias or also sudden death. The modifications of ST segment, although transient, are the same of STEMI, since they result from transmural ischemia (Figs. 5 and 6).23
Fig. 5.
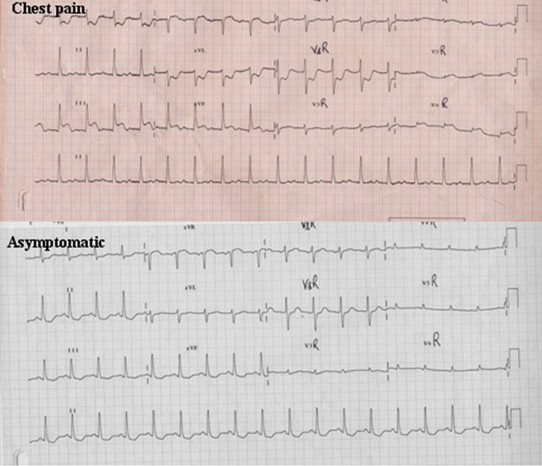
Transient elevation of ST segment in inferior leads in patients symptomatic for chest pain and histamine fish poisoning.
Fig. 6.
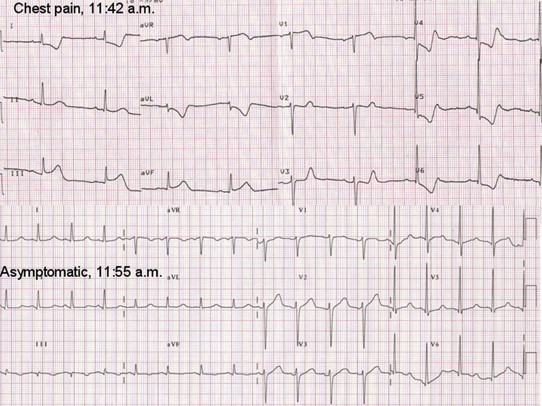
Transient elevation of ST segment in inferior leads in patients symptomatic for chest pain who was going to be transferred in the surgery room for an orthopedic intervention.
5.5. Takotsubo Cardiomyopathy
Described for the first time in Japan in 1991, the Apical Ballooning Syndrome is a cardiomyopathy characterized by an acute extensive and reversible akinesis of the medium-apical portion of the left ventricle in the absence of obstructive stenosis of epicardial coronary arteries. The term “Takotsubo” refers to the shape of left ventricle that resembles the octopus traps that Japanese fishermen use to catch octopus. Since the clinical features may mimic an acute coronary syndrome, the differential diagnosis is crucial in selecting the proper therapeutic strategy, especially in the acute phase. The twelve-leads ECG is not specific and may present ST segment elevation, Q waves, QT prolongation and asymmetric inverted T waves.24 In patients with Takotsubo Cardiomyopathy (TC), the ST segment usually has a lower maximal elevation that involves a greater number of leads without reciprocal depression. In other words, when present, the ST segment elevation is extensive and diffuse, extending beyond the perfusion territory of any single coronary artery (Fig. 7). Abnormal Q waves may be detectable, but they usually disappear within 30 days. The temporary presence of Q waves is mainly due to necrosis of Purkinje cell and not to an irreversible myocardial damage as shown from the absence of late enhancement at cardiac magnetic resonance.25 Kosuge et al26 have recently presented an interesting analysis comparing the admission ECG (within 6 h from the onset of symptoms) of patients with TC and first anterior AMI. In TC, the ST segment elevation most frequently occurred in – aVR (inverted aVR; +30°), while was rare in V1. Lead – aVR bridges the gap between lead I (0°) and lead II (60°) and directly faces the apical and infero-lateral regions of left ventricle. The perfusion territory of left anterior descending artery (LAD) usually does not extend to these regions and the prevalence of ST segment shipment in aVR in the setting of an anterior AMI is low. In the opposite, in TC the diffuse ST segment movement (especially in aVR) is thought to reflect the extensive wall-motion abnormalities centered around the apex. Moreover, lead V1 face the right ventricular anterior or paraseptal regions, which are both rarely involved in TC. The authors concluded that the elevation of ST segment in aVR (i.e., depression in aVR) combined with no (or less) elevation in V1 may allow to distinguish the TC from anterior AMI, irrespective of the occlusion site (proximal or distal to the first septal branch) of LAD.
Fig. 7.
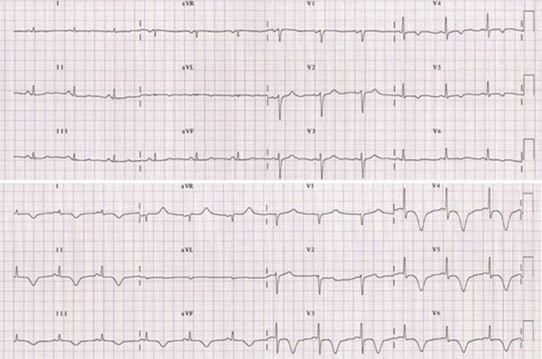
ECG at admission (up) and at discharge (down) of patients with Takotsubo cardiomyopathy.
5.6. Brugada Syndrome and arrhytmogenic right ventricular cardiomyopathy/dysplasia
Osher and Wolff, for the first time in 1953, described a persistent ST elevation in precordial leads without ischemia or any other possible explanation.27 In 1992, Brugada et al described a syndrome which was characterized by right bundle branch block, ST segment elevation in leads V1–V3 and sudden cardiac death in subjects <65 years.28 Originally, three different patterns of Brugada syndrome were described. However only the type I that is charecterized by a right bundle branch block pattern, ST segment elavation with coving can be confused with MI. Usually, a clear interruption between the r’ deflection of the QRS complex and ST segment is not present and the ST segment shows a deep deflection, which may represent a certain gradient between the epicardium and endocardium. An accurate analysis of the ST and J point is needed to distinguish Brugada syndrome from MI. In type I Brugada syndrome, the maximum ST elevation is detected in v1 or V2 and the J point is at a high point of elevation before descending rapidly to the nadir of a negative T wave (Fig. 8). In MI, the J point elevation is usually maximum in V3 and the displacement is not so fast. The so called type II and III patterns present an ST elevation with a superior concavity which simplifies in most cases the differentiation with acute STEMI.29
Fig. 8.
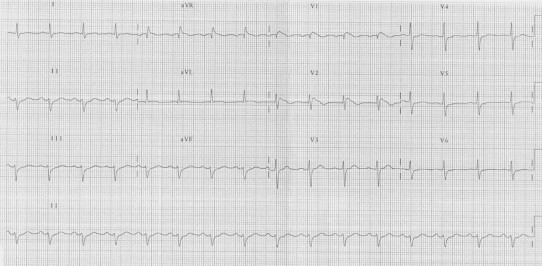
Brugada syndrome (type I).
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is a genetically determined heart muscle disorder that is characterized by the fibro-fatty replacement of right ventricle myocardium in the so called “triangle of dysplasia”. The disease has a variable expression but even in its concealed form individuals are often asymptomatic but may be at risk of sudden cardiac death especially during exertion. The (major and minor) criteria for the diagnosis, which are based on structural, histological, ECG, arrhythmic and familial features, have been recently revised.30 Notably, in subjects with ARVC/D, there is often an elevation of ST segment which is similar to that detectable in Brugada syndrome and represent a challenge in differentiating between a real Brugada syndrome or an acute ischemia (Fig. 9).31
Fig. 9.
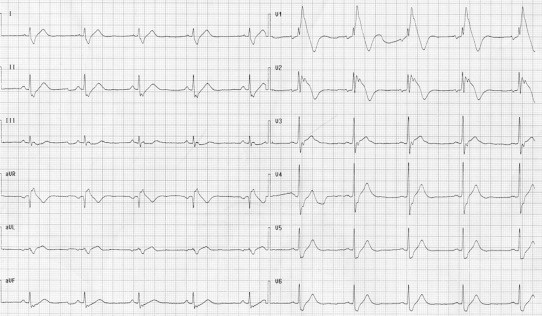
Arrhythmogenic right ventricular cardiomyopathy/dysplasia.
6. Pulmonary diseases
6.1. Pulmonary thromboembolism
The Pulmonary thromboembolism (PTE) is a blockage of the main artery of the lung (or one of its branches) usually due to the embolization of substance from deep vein thrombosis. The obstruction of the blood flow through the lungs leads to an increased pressure overload in the right chambers. Major PTE results whenever the combination of embolism size and underlying cardiopulmonary status interact to produce hemodynamic instability. The presence of hypotension, shock or cardiac arrest defines a threefold to sevenfold increase in mortality, with a majority of deaths occurring within 1 h of presentation.32 Furthermore, recent evidence indicates that the presence of right ventricular dysfunction identifies a subgroup of “normotensive” patients with a much more guarded prognosis who may benefit from more intensive therapy with thrombolytic agents.33As the result of pressure overload in the right ventricle, the ECG usually shows tachycardia/atrial fibrillation, right bundle branch block and/or the pattern S1Q3T3, which is considered suggestive, even if not specific (Fig. 10). Occasionally, an ST segment elevation in right precordial leads is also possible.34 The evidence of Q waves in DIII and aVF (but not in DII) without ST elevation in the same leads may allow to differentiate a PTE from an MI.
Fig. 10.
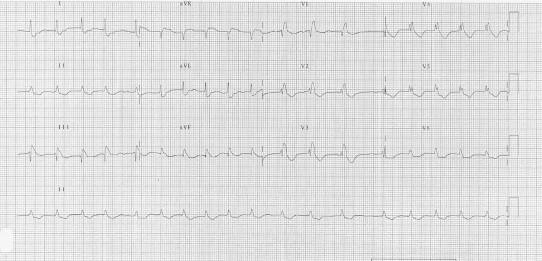
Pulmonary thromboembolism.
6.2. Pneumothorax
Pneumothorax (PNX) is an abnormal collection of air or gas in the pleural space that separates the chest wall form the lung and may interfere with normal breathing. The first association between a spontaneous PNX and ECG modifications, i.e. ST elevation in precordial leads suggestive of anterior myocardial ischemia, was made in 1979 by Slay et al. According to these authors, a hypertensive PNX is able to induce a hypotensive state with a resulting reduction of coronary blood flow.35 The consequent myocardial ischemia, and the related ECG changes, are however resolved without MI. Interestingly, ECG abnormalities may be different in relation to the site (left or right sided) of PNX. An abnormal axis deviation is more common in the left sided PNX. Moreover, it has also been associated with a reduction of the amplitude of QRS complex and, sometimes, with QRS alternation. Instead, changes in the morphologies of QRS complex (in particular new right bundle branch block) and T waves (inversion) appear more commonly in right sided PNX. The scientific literature reports some cases of patients (especially older and with previous coronary artery disease) with a PNX (especially hypertensive PNX) who initially received a wrong diagnosis of MI and, sometime, were also treated with thrombolytic therapy or underwent invasive coronary.36
6.3. Atelectasis and pulmonary metastases
Atelectasis (from Greek τελης: incomplete; κτασισ is: extension) is the collapse of lung tissue and results in reduced (or absent) gas exchange and oxygen absorption to healthy tissue. The ECG with ST segment elevation usually shows development of a deep S wave in lead I and a new Q wave in lead III. The T waves are, however, normal and the classic “SIQ3T3 pattern” observed in patients with PTE is not present.37 Nevertheless, atelectasis may led to hypoxemic vasoconstriction of the pulmonary circulation, which in combination with diversion of cardiac output to right lung led to increase in pulmonary arterial pressure. Pulmonary hypertension may be able to overload the right ventricle and results in ST segment changes in inferior leads. Pulmonary metastases are another cause of ST segment alterations in patients without cardiac artery diseases.
7. Gastrointestinal diseases
7.1. Acute pancreatitis
Although the differentiation of acute pancreatitis from an acute coronary syndrome is rarely a challenge, an accurate analysis of serial ECG may be helpful in presence of overlapping signs and symptoms. Pancreatitis itself may indeed produce minor transient ECG changes that frequently involve T waves (inversions) or ST segment (depression).38 However, sometimes typical changes, such as ST segment elevation without underlying cardiac pathology39 may be recorded. The metabolic abnormalities, such as hypocalcemia, hypernatremia, hypokalemia, hypomagnesemia and insulin-induced hypoglycemia40 that often characterize the acute pancreatitis, may be involved in the genesis of these electrocardiographic features. Other possible mechanisms could also cause the ST segment elevation and in particular coronary vasospasm,41 exacerbation of prior coronary artery disease or formation of thrombus in the coronary arteries due to the increased platelet adhesiveness or to coagulopathy induced by pancreatic enzyme.42
7.2. Acute cholecystitis
Acute cholecystitis is another acute abdominal condition, which can produce an ST segment elevation that mimics AMI. Although these ECG changes have been related in humans with surgery,43 the underlying pathophysiological mechanism remains unclear. Gallbladder distension is able to increase heart rate, arterial blood pressure and plasma renin levels.44 Studies in animal models have suggested its significant role on coronary blood flow.45
8. Other conditions
8.1. Hyperkalemia
Potassium is one of the most important electrolytes of specific and non-specific myocardial tissue. Consequently, significant variations of its plasmatic levels may have dramatic effects on electric activities and cause arrhythmias. Hyperkalemia induces an ST elevation in right precordial leads that resembles an AMI (Fig. 11).46 Indeed, the increase of potassium leads to a decrease in the duration of the AP with a gradual increase toward less negative values. These events can cause sharp tall T waves, reduction in the amplitude of P waves, prolongation of the PQ interval, enlargement of QRS complex and the ST segment elevation.47 Such modifications (especially the ST elevation with tall peaked T waves) can resemble an MI.
Fig. 11.
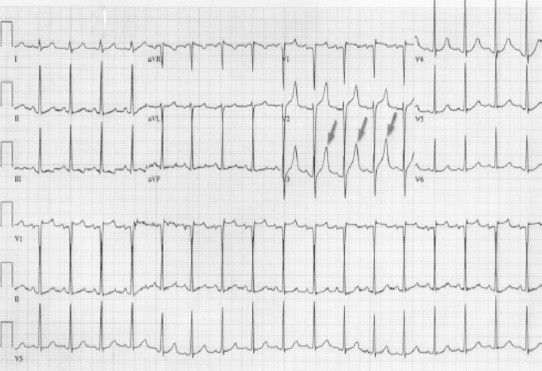
Hyperkalemia.
8.2. Drug induced ST segment elevation
Many drugs may have cardiac effects through various mechanisms. Some drugs can have a “direct” effect by stimulating cardiac receptors or by inducing anatomic damage or inflammation. Some others may “indirectly” interfere with the activity of autonomous nervous system. A lot of cardiac side effects and ECG modification can be associated with drug consumption. When an ST segment elevation (or an ECG modification) without a clear clinical setting is detected, the presence of a drug interaction should be always excluded. For example, clozapine, which intensively acts on nervous system, may cause hypotension, tachycardia, ST displacement (depression and/or elevation) (Fig. 12) and myocarditis. In such cases, clozapine should be no longer administrated.48,49
Fig. 12.
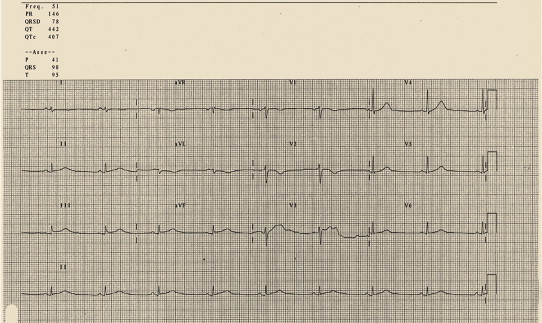
Elevation of ST segment during treatment with clozapine.
8.3. Hemorrhagic cerebrovascular disease
ECG changes are usually observed in patients with acute cerebrovascular pathology such as strokes or subarachnoid hemorrhages. Subarachnoid hemorrhage is generally due to the rupture of an aneurism and is characterized by headache, rushing, nausea and loss of consciousness. The sympathetic neuronal activation, which is consequent to the increase of intracranial pressure, seems to play an important pathogenic role. The ECG modifications may sometimes resemble an MI and lead to wrong treatments, potentially dangerous for the clinical conditions.
The most common abnormalities are prolongation of QT interval, inversion of T waves and appearance of abnormal U waves. Some patients have QRS complex of high voltages that are not related to the hypertrophy of myocardial walls.50 The literature has reported cases of ST segment elevation in absence of coronary artery disease; they are probably related to the catecholamine effect on myocytes or to spasm of the epicardial coronary arteries, which at least may even cause a real myocardial ischemia. This situation may be also seen in pheochromocytoma, cocaine abuse or emotional stress.51 Sometimes the subarachnoid hemorrhage may also induce an ST segment elevation without the release of markers of myocardial necrosis nor anatomical necrosis, as confirmed by the autopsy (Fig. 13).52
Fig. 13.
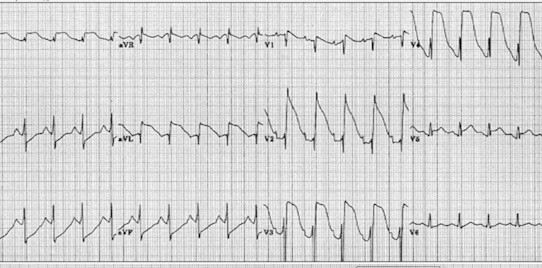
ECG during acute subarachnoid hemorrhage.
9. Conclusion
Chest pain is one of the chief presenting complaints among patients attending Emergency department. Guidelines recommend that patients with suggestive symptoms of myocardial ischemia and ST elevations in more than two contiguous leads should receive immediate reperfusion therapy. Furthermore, novel strategies (such as pre-hospital wireless electrocardiographic transmission) aimed at reduce door-to-balloon time are dependent on the accurate interpretation of ECG by trained readers.53 As the ECG is a simple, noninvasive tool in the diagnosis and management of coronary artery disease, all emergency physicians must be proficient in the interpretation of ECG during evaluation of patients symptomatic for chest pain. However, sometimes the fast diagnosis of MI may be challenging. Indeed, the typical clinical presentation may be absent in 15% of the cases and the ST segment deviation, despite the high sensibility, has a poor specificity since it may be observed in many other conditions. In this setting, the evaluation of a misleading condition is prudent. In other words, in a certain clinical context, when equivocal ECG abnormalities are detected, other differential diagnosis should be taken into account in order to avoid unnecessary (and sometimes dangerous) therapies.
Conflicts of interest
All authors have none to declare.
References
- 1.Sharkey S.W., Berger C.R., Brunette D.D., Henry T.D. Impact of the electrocardiogram on the delivery of thrombolytic therapy for acute myocardial infarction. Am J Cardiol. 1994;73:550–553. doi: 10.1016/0002-9149(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 2.Green L.S., Lux R.L., Haws C.W., Williams R.R., Hunt S.C., Burgess M.J. Effect of age, sex, and body habitus on QRS and ST-T potential maps of 1100 normal subjects. Circulation. 1985;71:244. doi: 10.1161/01.cir.71.2.244. [DOI] [PubMed] [Google Scholar]
- 3.Mehta M.C., Jain A.C. Early repolarization on scalar electrocardiogram. Am J Med Sci. 1995;309:305–311. doi: 10.1097/00000441-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Vacanti L.J. Thoracic pain and early repolarization syndrome at the cardiologic emergency unit. Arq Bras Cardiol. 1996;67:335–338. [PubMed] [Google Scholar]
- 5.Fenichell N.N. A long term study of concave RS-T elevation—anormal variant of the electrocardiogram. Angiology. 1962;13:360–366. doi: 10.1177/000331976201300804. [DOI] [PubMed] [Google Scholar]
- 6.Grant R.P., Estes E.H., Doyle J.T. Spatial vector electrocardiography: the clinical characteristics of ST and T vectors. Circulation. 1951;3:182–197. doi: 10.1161/01.cir.3.2.182. [DOI] [PubMed] [Google Scholar]
- 7.Wasserburger R.M., AIt W.J., Lloyd C. The normal RS-T segment elevation variant. Am J Cardiol. 1961;8:184–192. [Google Scholar]
- 8.Glinzton L.E., Laks M.M. The differential diagnosis of acute pericarditis from the normal variant: new electrocardiographic criteria. Circulation. 1982;65:1004–1009. doi: 10.1161/01.cir.65.5.1004. [DOI] [PubMed] [Google Scholar]
- 9.Smith S.W., Zvosec D.L., Henry T.D., Sharkey S.W. 1st ed. Lippincott Williams & Wilkins; 2002. The ECG in Acute MI: An Evidence-Based Manual of Reperfusion Therapy. [Google Scholar]
- 10.Sgarbossa E.B., Pinski S.L., Barbagelata A. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) investigators. N Engl J Med. 1996;334:931. doi: 10.1056/NEJM199602223340801. [DOI] [PubMed] [Google Scholar]
- 11.Madias J.E., Sinha A., Ashtiani R., Argawal H., Win M., Narayan V.K. A critique of the new ST-segment criteria for the diagnosis of acute myocardial infarction in patients with left bundle-branch block. ClinCardiol. 2001;24:652–655. doi: 10.1002/clc.4960241004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oreto G. L'elettrocardiogramma: un mosaico a 12 tessere. Centro Scientifico Editore. 2009 166e168. [Google Scholar]
- 13.Toosi M.S., Sochansk M.T. False ST elevation in a modified 12-lead surface Electrocardiogram. J Electrocardiol. 2008;41:197–201. doi: 10.1016/j.jelectrocard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Kok L.C., Mitchell M.A., Haines D.E., Mounsey J.P., DiMarco J.P. Transient ST elevation after transthoracic cardioversion in patients with hemodynamically unstable ventricular tachyarrhythmia. Am J Cardiol. 2000;85:878–881. doi: 10.1016/s0002-9149(99)00886-3. A9. [DOI] [PubMed] [Google Scholar]
- 15.Gurevitz O., Lipchenca I., Yaacoby E. ST-segment deviation following implantable cardioverter shock: incidence, timing and clinical significance. PACE. 2002;25:1429–1432. doi: 10.1046/j.1460-9592.2002.01429.x. [DOI] [PubMed] [Google Scholar]
- 16.Spodick D.H. Electrocardiogram in acute pericarditis. Distributions of morphologic and axial changes by stages. Am J Cardiol. 1974;33:470–474. doi: 10.1016/0002-9149(74)90603-1. [DOI] [PubMed] [Google Scholar]
- 17.Spodick D.H. Grune& Stratton; New York: 1959. Acute Pericarditis; pp. 17–30. [Google Scholar]
- 18.Costantini M., Tritto C., Licci E. Myocarditis with ST-elevation myocardial infarction presentation in young man. A case series of 11 patients. Int J Cardiol. 2005;101:157–158. doi: 10.1016/j.ijcard.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Dec J.W., Jr., Wakdman H., Southern J., Fallon J.T., Hutter A.M., Jr., Palacios I. Viral myocarditis mimicking acute myocardial infarction. J Am CollCardiol. 1992;20:85–89. doi: 10.1016/0735-1097(92)90141-9. [DOI] [PubMed] [Google Scholar]
- 20.Sarda L., Colin P., Boccara F. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol. 2001;37:786–792. doi: 10.1016/s0735-1097(00)01201-8. [DOI] [PubMed] [Google Scholar]
- 21.Hirata K. Electrocardiographic abnormalities in patients with acute aortic dissection. Am J Cardiol. 1995;76:1207–1212. doi: 10.1016/s0002-9149(99)80342-7. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T., Fukumoto Y., Mohri M., Inokuchi K., Hirakawa Y., Takeshita A. Wandering ST-segment elevation. Circulation. 2003;108:e102–e103. doi: 10.1161/01.CIR.0000091886.24045.84. [DOI] [PubMed] [Google Scholar]
- 23.Wang K., Asinger R.W., Marriott H.J. ST segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003;349:2128–2135. doi: 10.1056/NEJMra022580. [DOI] [PubMed] [Google Scholar]
- 24.Hansen Peter Riis. Takotsubo cardiomyopathy: an under-recognized myocardial Syndrome. Eur J Intern Med. 2007;18:561–565. doi: 10.1016/j.ejim.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Sharkey S.W., Lesser J.R., Menon M., Parpart M., Maron M.S., Maron B.J. Spectrum and significance of electrocardiographic patterns, troponin levels, and thrombolysis in myocardial infarction frame count in patients with stress (Tako-tsubo) cardiomyopathy and comparison to those in patients with ST elevation anterior wall myocardial infarction. Am J Cardiol. 2008;101:1723–1728. doi: 10.1016/j.amjcard.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 26.Kosuge M., Ebina T., Hibi K. ST segment depression in lead aVR combined with no ST segment elevation in lead V1 differentiates Takotsubo cardiomyopathy from anterior acute myocardial infarction. J Am CollCardiol. 2012;59:E423. [Google Scholar]
- 27.Osher H.L., Wolf L. Electrocardiographic pattern simulating acute myocardial injury. Am J Med Sci. 1953;226:541–556. [PubMed] [Google Scholar]
- 28.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. J AmCollCardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 29.Oreto G. L'Elettrocardiogramma: un mosaico a 12 tessere. Centro Scientifico Editore. 2009 207e222.1361. [Google Scholar]
- 30.Marcus F.I., McKenna W.J., Sherril D. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corrado D., Basso C., Buja G., Nava A., Rossi L., Thiene G. Right bundle branch block, right precordial ST-segment elevation and sudden death in young people. Circulation. 2001;103:710–717. doi: 10.1161/01.cir.103.5.710. [DOI] [PubMed] [Google Scholar]
- 32.Wood K.E. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877–905. doi: 10.1378/chest.121.3.877. [DOI] [PubMed] [Google Scholar]
- 33.Kreit J.W. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest. 2004;125:1539–1545. doi: 10.1378/chest.125.4.1539. [DOI] [PubMed] [Google Scholar]
- 34.Kosuge M., Kimura K., Ishikawa T. Electrocardiographic differentiation between acute pulmonary embolism and acute coronary syndromes on the basis of negative T waves. Am J Cardiol. 2007;99:817–821. doi: 10.1016/j.amjcard.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 35.Slay R.D., Slay L.E., Luehrs J.G. Transient ST elevation associated with tension pneumothorax. JACEP. 1979;8:16–18. doi: 10.1016/s0361-1124(79)80442-6. [DOI] [PubMed] [Google Scholar]
- 36.Brocke-Utne Botz G. Are electrocardiographic changes the first sign of an impending peri-opertaive pneumothorax? Anaesthesia. 1992;47:1057–1059. doi: 10.1111/j.1365-2044.1992.tb04203.x. [DOI] [PubMed] [Google Scholar]
- 37.Goddard R., Scofield R.H. Right pneumothorax with the SIQ3T3 electrocardiogram pattern usually associated with pulmonary embolus. Am J Emerg Med. 1997;15:310–312. doi: 10.1016/s0735-6757(97)90023-1. [DOI] [PubMed] [Google Scholar]
- 38.Cohen M.H., Rotsztain A., Bowen P.J. Electrocardiographic changes in acute pancreatitis resembling acute myocardial infarction. Am Heart J. 1971;82:672–677. doi: 10.1016/0002-8703(71)90337-1. [DOI] [PubMed] [Google Scholar]
- 39.Baurlein T.C., Stobe L.H.O. Acute pancreatitis simulating myocardial infarction with characteristic electrocardiographic changes. Gastroenterology. 1954;27:861–864. [PubMed] [Google Scholar]
- 40.Leak D., Starr P. The mechanism of arrhythmias during insulin induced hypoglycemia. Am Heart J. 1962;63:688–691. doi: 10.1016/0002-8703(62)90014-5. [DOI] [PubMed] [Google Scholar]
- 41.Terradellas J.B., Bellot J.F., Saris A.B. Acute and transient ST segment elevation during bacterial shock in seven patients without apparent heart disease. Chest. 1982;81:444–448. doi: 10.1378/chest.81.4.444. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman J.S., Taylor A., Wright I.S. The effect of intravenous trypsin administration on the electrocardiogram in the rabbit. Circulation. 1954;10:338–342. doi: 10.1161/01.cir.10.3.338. [DOI] [PubMed] [Google Scholar]
- 43.Brettwlesser E.R. Electrocardiographic observation in chronic cholecystitis before and after surgery. Am J Med Sci. 1947;213:598–602. doi: 10.1097/00000441-194705000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Molinari C., Grossini E., Mary D.A., Vacca G. Effect of distension of the gallbladder on plasma renin activity in anesthetized pigs. Circulation. 2000;101:2539–2545. doi: 10.1161/01.cir.101.21.2539. [DOI] [PubMed] [Google Scholar]
- 45.Vacca G., Battaglia A., Grossini E., Mary D.A., Molinari C. Reflex coronary vasoconstriction caused by gallbladder distension in anesthetized pigs. Circulation. 1996;94:2201–2209. doi: 10.1161/01.cir.94.9.2201. [DOI] [PubMed] [Google Scholar]
- 46.Sims D.B., Sperling L.S. ST-segment elevation resulting from hyperkalemia. Circulation. 2005;111:295–296. doi: 10.1161/01.CIR.0000165127.41028.D1. [DOI] [PubMed] [Google Scholar]
- 47.Levine H.D., Wanzer S.H., Merril J.P. Dialyzable currents of injury in potassium intoxication resembling acute myocardial infarction and pericarditis. Circulation. 1956;13:29–36. doi: 10.1161/01.cir.13.1.29. [DOI] [PubMed] [Google Scholar]
- 48.Collins E.J., Lalonde P., Jones B.D. Clozapine in the treatment of refractory schizophrenia: Canadian policies and clinical guidelines. Can J Psychiatry. 1992;37:482–488. doi: 10.1177/070674379203700704. [DOI] [PubMed] [Google Scholar]
- 49.James K., Louis L. ST-segment elevations without myocardial infarction in patient on clozapine. Amer J Emerg Med. 1996;14:111–112. doi: 10.1016/S0735-6757(96)90036-4. [DOI] [PubMed] [Google Scholar]
- 50.Sommargren C.E., Zaroff J.G., Banki N., Drew B.J. Electrocardiographic repolarization abnormalities in subarachnoid hemorrhage. J Electrocardiol. 2002;35 doi: 10.1054/jelc.2002.37187. [DOI] [PubMed] [Google Scholar]
- 51.Hurst J.W. Electrocardiographic changes intracranial hemorrhage mimicking myocardial infarction. N Engl J Med. 2003;349:1874–1875. doi: 10.1056/NEJM200311063491922. [DOI] [PubMed] [Google Scholar]
- 52.Cropp G.J., Manning G.W. Electrocardiographic changes simulating myocardial ischemia and infarction associated with spontaneous intracranial hemorrhage. Circulation. 1960;22:25–38. doi: 10.1161/01.cir.22.1.25. [DOI] [PubMed] [Google Scholar]
- 53.Steg G., James S.K., Atar D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]


