Abstract
BACKGROUND
Chemotherapy has not had significant impact on survival for patients with metastatic melanoma. Bortezomib was shown to have additive/synergistic effect with a number of chemotherapeutic agents including paclitaxel and platinum. A phase I trial of this 3-drug combination reported that 6 of 28 patients treated with bortezomib followed by paclitaxel and carboplatin had a partial response (including 2 of 5 patients with metastatic melanoma).
METHODS
We conducted a 2-stage phase II clinical trial to assess the anti-tumor activity of this 3-agent combination in patients with metastatic melanoma who had received at most one prior chemotherapy for metastatic disease. Treatment included bortezomib 1.3 mg/m2 IV on days 1, 4, and 8, and paclitaxel 175 mg/m2 and carboplatin AUC 6 on day 2 of a 21 day cycle. The primary endpoint of this trial was tumor response rate.
RESULTS
Seventeen eligible patients were enrolled. A median of 4 cycles were administered (range 1-7). Three patients discontinued treatment due to persistent grade 4 neutropenia with grade 3 leukopenia (two patients) or grade 4 pulmonary embolism (one patient). Grade ≥ 3 toxicities included neutropenia (71%), leukopenia (41%), thrombocytopenia (29%), and arthralgia (12%). Two partial responses were observed (TRR 11.8%). Four patients had stable disease > 12 weeks. Median progression free survival (PFS) was 3.2 months and median survival 7.0 months.
CONCLUSIONS
Due to insufficient clinical efficacy, this trial did not proceed to second stage accrual. The combination of paclitaxel, carboplatin, and bortezomib demonstrated limited clinical benefit and was associated with significant toxicity.
Keywords: Taxol, carboplatin, bortezomib, metastatic melanoma
INTRODUCTION
The limited effectiveness of current therapeutic approaches in patients with metastatic melanoma underscores the importance for the development of novel agents. Bortezomib (PS-341) is a small, cell-permeable molecule that specifically and selectively inhibits proteosomes (1,2). As a single agent administered twice weekly for two of every three weeks at a dose of 1.5 mg/m2, bortezomib did not demonstrate significant clinical activity in patients with metastatic melanoma (no objective responses; median time to progression of 1.5 months) (3). However, recent preclinical studies demonstrated that bortezomib has additive/synergistic effects when combined with a number of chemotherapeutic agents including paclitaxel and platinum (4). A phase I trial of bortezomib in combination with carboplatin and paclitaxel in 33 patients with advanced solid tumors, including five metastatic melanoma, was reported by our group (5). Two different administration schedules based on a 21 day treatment cycle were tested. On “Schedule A” paclitaxel and carboplatin were given on day 1 followed by bortezomib on days 2, 5, and 8; on “Schedule B” bortezomib was given on days 1, 4, and 8 with paclitaxel and carboplatin on day 2. Thrombocytopenia was the dose limiting toxicity (DLT) for both schedules. The proportion of patients who developed severe neutropenia, anemia, and thrombocytopenia was similar among the treatment scheduled but 19 (68%) of the 28 patients on Schedule B were treated at dose levels higher than the maximally tolerated dose (MTD) of Schedule A. Also, 6 of the 28 treated with Schedule B had a partial response [malignant melanoma (2), NSCLC (3), and gynecologic cancer (1)] and only 1 of the 25 patients treated with Schedule A had a partial response (malignant melanoma). Given these findings, the recommended phase II dose schedule was Schedule B with bortezomib administered at 1.3 mg/m2, paclitaxel 175 mg/m2, and carboplatin AUC = 6.
Materials and methods
Patient eligibility criteria
Eligible patients were 18 years of age or older with histologic confirmation of malignant melanoma with manifestations of metastatic disease. Patients were required to have measurable disease as defined by the RECIST criteria, an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, a life expectancy > four months, and adequate organ function defined as follows: absolute granulocyte count ≥ 1500/mm3, platelet count ≥ 100,000/mm3, hemoglobin ≥ 9.0 gm/dL, creatinine ≤ 1.5 times the upper limit of normal (ULN), aspartate aminotransferase ≤ 3 times ULN, total bilirubin ≤ 1.5 mg/dL, and a urine dipstick for proteinuria of 1+ with a 24-hour urine protein < 500 mg of proteinuria/24 hours. These patients were enrolled from 2005-2007 at 7 separate cancer centers participating in our Phase 2 Cancer Consortium.
Exclusion criteria included: known CNS metastases, >1 prior chemotherapy regimen, prior carboplatin, paclitaxel, or bortezomib, history of allergic reactions attributed to compounds of similar chemical or biologic composition to the study agents, grade 2+ peripheral neuropathy, uncontrolled or current infection, symptomatic congestive heart failure, unstable angina pectoris or cardiac arrhythmia, > two prior immunotherapy regimens, and ≤ four weeks since radiotherapy, chemotherapy, immunotherapy, or surgery, ≤ eight weeks since monoclonal antibody therapy. Pregnant or nursing women were not eligible, and men or women of child bearing potential were required to practice appropriate contraception. All patients provided written informed consent in accordance with institutional review board requirements.
Treatment Administration and Evaluation
A treatment cycle was 21 days in length. Patients received bortezomib on days 1, 4, and 8, at a dose of 1.3 mg/m2; paclitaxel (PAC) was administered as a 3-hour intravenous infusion at a dose of 175 mg/m2 on day 2; and carboplatin (CBDCA) was administered as a 30-minute intravenous infusion at a dose of AUC 6 (Calvert's calculation) on day 2 as well. Standard intravenous “pre-medications” for PAC and CBDCA were administered prior to chemotherapy on day 2 (ranitidine 50 mg or cimetidine 300 mg, dexamethasone 20 mg, and diphenhydramine 50 mg). Patients continued to receive treatment according to protocol specifications if there was no evidence of excessive toxicity or progression of disease, initiation of non-protocol treatment, or patient request to discontinue therapy. Toxicities (based on NCI-CTAE version 3.0 grading criteria) that prompted treatment discontinuation included grade 3+ neuromotor, neurosensory, or neurologic pain adverse events. A maximum of two dose reductions were allowed for excessive toxicity. The first dose reduction resulted in a target AUC of 4 for CBDCA, PAC at 150 mg/m2, and bortezomib at 1.0 mg/m2. The second resulted in a target AUC of 3 for CBDCA, PAC at 100 mg/m2, and bortezomib at 0.7 mg/m2. If treatment was held due to unacceptable toxicity more than three weeks, study treatment was not to be restarted.
Treatment was to be held on day 1 of a cycle for ANC < 1500/mm3 or platelet count < 100,000/mm3 until counts arose above these levels and a grade 3+ non-hematologic non-neurologic toxicities until severity returned to preregistration levels or ≤ grade 1. A grade 2 neurologic toxicity required omitting bortezomib until grade ≤ 1 and then administering it at a dose reduced by one level. Adverse events reported during cycle weeks 1-3 requiring bortezomib to be omitted for the rest of the cycle and then all three agents to be administered at doses reduced by one level on subsequent cycles included: febrile neutropenia, grade 3+ vomiting not controlled by medication, ANC < 1500/mm3, and platelet count < 100,000/mm3. Other grade 3+ non-hematologic toxicities required only PAC and CBDCA to be administered at doses reduced by one level.
Not more than 14 days prior to registration, patients underwent a complete physical exam including a brief neurological exam, tumor evaluation, complete blood counts (WBC, ANC, hemoglobin, and platelets), serum chemistries (creatinine, total and direct bilirubin, albumin, glucose, calcium, AST, LDH, K, and Na), and urinalysis (proteinuria and UPC ratio). Physical exams, complete blood counts, serum chemistries, and toxicity evaluations were repeated at the completion of each cycle of treatment until treatment was discontinued. Tumors were evaluated using the RECIST criteria every six weeks during treatment.
Trial Design and Statistical Methods
The primary endpoint of this trial was the tumor response rate (TRR) defined as the number of eligible patients whose disease met the RECIST criteria for complete (CR) or partial (PR) response on 2 consecutive evaluations at least 6 weeks apart divided by the number of eligible patients enrolled onto the trial. A Simon two-stage phase II clinical trial design was chosen to test whether the true TRR was at most 15% against the alternative that the true TRR was at least 35% with significance level set at 0.10 and the likelihood of detecting that the TTR is greater than 15% when it is at least 35% set at 90%. Specifically, the first stage was to enroll 19 eligible patients and if at least 4 of these 19 patients had a confirmed tumor response, a second stage of enrollment would be opened for an additional 14 eligible patients. If at least 8 patients of the 33 eligible patients enrolled had a confirmed tumor response without excessive toxicity, the regimen would be considered for further testing in this patient population.
A 90% binomial confidence interval for the TRR was constructed. Toxicities were graded using the NCI-CAE version 3.0. Progression-free survival was defined as the time from registration to disease progression or death without documentation of progression. Survival time was defined as the time from registration to death due to any cause. Time to event distributions were estimated using the method of Kaplan and Meier.
RESULTS
Patient characteristics
Nineteen patients were entered on stage I of this trial between November 2005 and May 2007. One patient canceled participation after signing a consent form but prior to receiving any study treatment. One patient was found to be ineligible as the patient had a proteinuria of 1+ but a 24-hour urine protein was not taken. The criteria to open enrollment to stage II of this trial were not met and, as such, the trial was permanently closed to accrual. The following is a report of our findings among these 17 eligible patients (Table 1). The median age at enrollment was 59 years (range: 39-74 years). Nine patients (52.9%) had no prior systemic therapy for metastatic melanoma.
TABLE 1.
| Patient Characteristics | n = 17 |
|---|---|
| Median age (range) | 59 years (39-74) |
| Male | 12 (70.6%) |
| Prior systemic therapies | |
| None | 9 (52.9%) |
| Adjuvant setting only | 3 (17.6%) |
| Sargramostim | 1 |
| Temozolomide | 1 |
| Dacarbazine, Cisplatin, Velban, Interleukin-2, Interferon α | 1 |
| Metastatic setting only | 3 (17.6%) |
| Temozolomide | 1 |
| Everolimus | 1 |
| Interleukin-2 | 1 |
| Both adjuvant and metastatic settings | 2 (11.8%) |
| Interferon α, then Interleukin-2 | 1 |
| Interferon α, then Sargramostim | 1 |
| ECOG performance status | |
| 0 | 9 (52.9%) |
| 1 | 8 (47.1%) |
| Pre-existing signs and symptoms | |
| Grade 1 neurosensory | 1 (5.9%) |
| Grade 1 musculoskeletal pain | 3 (17.6%) |
| Grade 1 arthralgia | 3 (17.6%) |
| Grade 2 arthralgia | 3 (17.6%) |
Clinical outcomes
The median number of cycles administered was 4 cycles (total 62 cycles, range 1-7). Bortezomib was omitted on day 4 of 2 cycles (3.2%) and on day 8 of 15 cycles (24.2%). Severe (grade ≥ 3) hematologic toxicities led to dose reductions for 6 patients. Two patients discontinued treatment due to persistent grade 4 neutropenia with grade 3 leukopenia requiring a third dose reduction. One patient, after four cycles of treatment, developed a grade 4 pulmonary embolism and discontinued treatment. The most common severe (≥ grade 3) toxicities reported as possibly, probably, or definitely related to treatment included neutropenia (71%), leukopenia (41%), thrombocytopenia (29%), and arthralgia (12%). Grade 2 neurosensory toxicity was seen in 1 (6.0%) patient (Table 2). At the time of this report, all 17 patients have discontinued treatment. The reasons include disease progression (13 patients), refusal (1 patient), and excessive toxicity (3 patients).
TABLE 2.
Most Common Severe Toxicities
| Toxicity | Any Grade | Severe |
|---|---|---|
| Neutropenia | 82% | 71% |
| Leukopenia | 82% | 41% |
| Thrombocytopenia | 82% | 29% |
| Arthralgia | 29% | 12% |
Among the 17 patients enrolled, there were two (11.8%; 90% CI: 2.1-32.6%) patients with a confirmed PR. Both of these patients had had prior chemotherapy +/- immunologic therapy. Four (23.5%) patients had disease that remained stable for at least 12 weeks (completed at least 4 cycles of treatment with stable disease)--1 patient had no prior systemic therapy, 1 patient had had chemotherapy, and the other 2 patients had had immunologic therapy.
At last contact, 3 patients were alive with progression of disease and 14 patients have died of disease. The median progression-free survival time was 3.2 months and the median survival time was 7.0 months.
DISCUSSION
Approximately 62,480 new cases of melanoma and 8,420 deaths attributable to melanoma occurred in the United States in 2008 (6). For those patients with metastatic disease, the prognosis is very poor. The median survival of patients with metastatic melanoma ranges between two and eight months according to the site and the number of metastases. The estimated five year survival rate is less than 5-10% (7). With visceral metastases, the two year survival is only about 1-2% (8,9). Treatment options for patients with metastatic melanoma are limited. Surgery, when possible, should be recommended if complete removal of all visible metastases is achievable (10,11). It can provide quick and effective palliation and leads to long-term survival of more than five to ten years in some cases. Unfortunately, most patients with metastatic disease present with non-resectable disease and require systemic therapy. Current available systemic treatment approaches, including cytotoxic chemotherapy and immunotherapy, used either alone or in combination, have limited effectiveness. There is no regimen that could be considered as “standard of care” for metastatic melanoma. Dacarbazine is generally considered to be the most active single agent with a response rate of about 10-12% (12). However, the vast majority of responses are only partial and the median response duration is only four to six months. In addition, responses are rare in visceral sites (13). Although dacarbazine remains the only cytotoxic drug approved by the Food and Drug Administration for the treatment of patients with metastatic melanoma, there are no phase III trial data to support a survival benefit for dacarbazine relative to a “best supportive care” control (14). Temozolomide is an analog of dacarbazine which can be administered orally. It produces response rates of 13% which is similar to those obtained with dacarbazine in phase III studies (15). Fotemustine, which is not yet available in the U.S., gives a response rate of about 24% in metastatic melanoma (16). For all these drugs, complete response occurs in less than 10% of cases and the median duration of response is between four and six months without any survival advantage when compared with dacarbazine (17,18).
Bortezomib is an unconventional cytotoxic agent that was recently approved for the treatment of multiple myeloma (19) and is currently undergoing testing in solid tumor patients. It has a unique pattern of activity when screened against a panel of 60 human cancer cell lines including melanoma (20). Bortezomib is a modified dipeptidyl boronic acid derived from leucine and phenylalanine and is currently undergoing active investigation as a novel antineoplastic agent as summarized below (21,22). Bortezomib is a potent and reversible proteasome inhibitor (Ki-0.6 nM). It also inhibits the growth of cultured tumor cells by blocking cell division in the G2-M phase of the cell cycle, leading to cytotoxicity via apoptosis, and inhibits the degradation of the wild-type tumor suppressor protein p53, but not mutated forms of this protein. In addition, bortezomib stabilizes the CDK inhibitor p21 and inhibits the activation of NF-B by the stabilization of the inhibitor protein I B. Bortezomib inhibits NF-B dependent gene expression, as demonstrated by inhibition of cell surface adhesion molecule (CAM) transcription. As a consequence of the inhibition of CAM expression, bortezomib also inhibits the adhesion of tumor cells to endothelial cells. Overexpression of bc1-2, an anti-apoptotic effector protein, is inhibited by bortezomib, leading to cell death. As a single agent, bortezomib therapy was ineffective in patients with metastatic melanoma (3). However, it appeared to exhibit synergistic/additive anti-tumor activity with taxanes and platinum compounds leading to successful phase I testing. Thus our interest in combining bortezomib with paclitaxel/carboplatin for the treatment of metastatic melanoma.
The combination of paclitaxel and carboplatin is a relatively new development in metastatic melanoma despite being used in a broad range of solid tumors. Synergism between platinum and paclitaxel in pre-clinical studies has been established (23). To a limited extent, at the time of the design of our current trial, the combination of paclitaxel and carboplatin had already been tested in metastatic melanoma. Results of a phase II trial using paclitaxel (175 mg/m2) and carboplatin (AUC=7.5) administered every 21 days in patients with metastatic melanoma demonstrated that of 15 evaluable patients, 3 (20%) achieved a partial remission, 7 (47%) achieved stable disease, and 5 (33%) progressed (24). There were no complete responders. Eleven patients experienced grade 3 or 4 hematologic toxicity. The patients accrued on this study had good performance status (0-1) with 23% of patients exhibiting disease involving the skin, 40% lungs, 33% lymph nodes, 26% liver, and 13% other visceral organs. The treatment was deemed ineffective as first line therapy for patients with metastatic melanoma.
A second Phase II trial by the German Dermatologic Cooperative Oncology Group (DaCOG) was subsequently reported using weekly paclitaxel and carboplatin as second-line therapy in metastatic melanoma patients (25). Paclitaxel (at a dose of 80 mg/m2) and carboplatin (at a dose of 200 mg/m2) were given weekly for six weeks out of eight as a cycle. Of the sixteen patients who received this combination therapy, three patients (19%) had stable disease that lasted for sixteen weeks. The trial was discontinued because the response rate was considered too low to justify continuing the trial.
A retrospective report on the clinical use of the combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma suggested potential clinical benefit (26). Twenty-two of 31 reported patients received weekly paclitaxel 100 mg/m2 and carboplatin of an AUC 2; the remainder received paclitaxel 175-225 mg/m2 and carboplatin AUC 5. An objective partial response was noted in eight patients (26%) with an additional six patients (19%) noted to have stable disease. The median progression free survival was 3 months with a median overall survival of 7.8 months. The clinical benefit in 14 patients was thought to be clinically significant when the weekly regimen was used as second-line chemotherapy. The authors concluded that further investigation of the combination of paclitaxel and carboplatin in patients with metastatic melanoma was worth pursuing as the clinical outcomes data compared favorably to either dacarbazine or temozolomide therapy (18).
The greatest enthusiasm for the use of paclitaxel and carboplatin in the treatment of metastatic melanoma came after the presentation of the phase I/II clinical results of the combination of paclitaxel/carboplatin and sorafenib. The initial data suggested clinical response rates in the 50% range with acceptable toxicity (27). These data were encouraging enough to immediately enter development towards phase III clinical testing, suggesting that the combination of “small molecules” with the paclitaxel/carboplatin chemotherapy “backbone” was a reasonable approach in metastatic melanoma. Two phase III clinical trials randomizing sorafenib against placebo in combination with paclitaxel and carboplatin using a 21 day regimen were initiated in both chemotherapy naïve as well as previously treated patients, respectively. The results of the phase III trial in previously treated patients with metastatic melanoma were recently reported (28). Two-hundred-seventy patients were randomized to receive either the two-drug (paclitaxel/carboplatin) versus the three-drug regimen (paclitaxel/carboplatin 7.25 mg/m2/AUC=6 + sorafenib 400 mg b.i.d. days 2-19). This trial concluded that the addition of sorafenib to paclitaxel/carboplatin did not improve progression free survival or objective response rates as second-line therapy, failing to reproduce the original phase I/II data. Toxicities were tolerable. The same regimen is currently completing testing in chemotherapy naïve patients (E2603). In the least, the two cited phase III clinical trials have reinforced the notion of clinical utility of the combination of paclitaxel and carboplatin in the management of metastatic melanoma, now with phase III data comparing favorably with conventional dacarbazine or temozolomide results. We eagerly await the results of E3603.
In summary, bortezomib as a single agent or in combination with paclitaxel/carboplatin lacks sufficient clinical activity in patients with metastatic melanoma to warrant further investigation.
CONDENSED ABSTRACT.
Taxol, carboplatin, and bortezomib were given to patients with metastatic melanoma. This Phase II trial demonstrated limited clinical benefit and was associated with significant toxicity.
Figure 1.
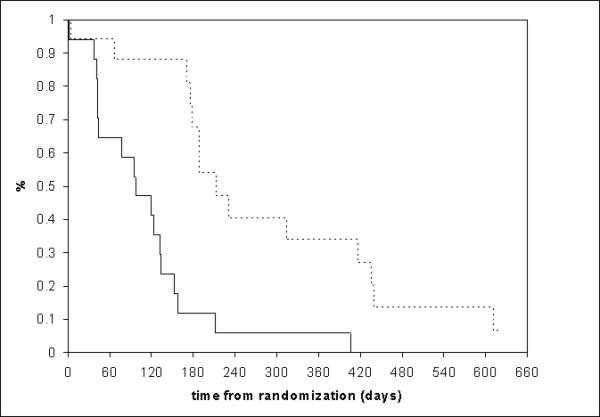
Survival distributions.
Acknowledgments
I would like to acknowledge Manuel Hidalgo, M.D., Ph.D., Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, Maryland, and Thomas Amatruda, III, M.D. Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, Minnesota, for contributing to the patient accrual, as well as Lisa Kottschade, R.N., C.N.P., Melanoma Study Group, Mayo Clinic and Mayo Foundation, Rochester, Minnesota, for kindly reviewing this manuscript. I would also like to acknowledge the P2C Protocol Development Office and clinical research associates involved in this study, but especially the help of Tamra Chomjak, Andrea Kukla, and Kalli Voll. I greatly appreciate the expert secretarial assistance of Carol Rodgers. In addition, I would like to thank Dr. Wright and his office at CTEP for their guidance and support of this trial.
Funded by: National Cancer Institute N01 CM62205 DCTD Supplied Investigational Agent: PS-341 (NSC: 681239, IND: 58443)
REFERENCES
- 1.Adams J. Preclinical and clinical evaluation of proteasome inhibitor PS-341 for the treatment of cancer. Curr Opin Chem Biol. 2002;6:493–500. doi: 10.1016/s1367-5931(02)00343-5. [DOI] [PubMed] [Google Scholar]
- 2.An WG, Hwang SG, Trepel JB, et al. Protease inhibitor-induced apoptosis: accumulation of wt p53, p21WAF1/CIP1, and induction of apoptosis are independent markers of proteasome inhibition. Leukemia. 2000;14:1276–1283. doi: 10.1038/sj.leu.2401812. [DOI] [PubMed] [Google Scholar]
- 3.Markovic SN, Geyer SM, Dawkins F, et al. A phase II study of bortezomib in the treatment of metastatic malignant melanoma. Cancer. 2005;103:2584–2589. doi: 10.1002/cncr.21108. [DOI] [PubMed] [Google Scholar]
- 4.Teicher BA, Ara G, Herbst R, et al. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res. 1999;5:2638–2645. [PubMed] [Google Scholar]
- 5.Ma C, Mandrekar SJ, Alberts SR, et al. A phase I and pharmacologic study of sequences of the proteaseome inhibitor, bortezomib (PS-341, Velcade), in combination with paclitaxel and carboplatin in patients with advanced malignancies. Cancer Chemother Pharmacol. 2007;59:207–215. doi: 10.1007/s00280-006-0259-9. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society . Cancer Facts & Figures 2008. American Cancer Society; Atlanta: 2008. [Google Scholar]
- 7.Barth A, Wanek A, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181:193–201. [PubMed] [Google Scholar]
- 8.Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–2751. doi: 10.1200/JCO.1999.17.9.2745. [DOI] [PubMed] [Google Scholar]
- 9.de Braud F, Khayat D, Kroon BB, et al. Malignant melanoma. Crit Rev Oncol Hematol. 2003;47:35–63. doi: 10.1016/s1040-8428(02)00077-x. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM. Palliative surgery for Stage IV melanoma: Is it a primary treatment (editorial)? Ann Surg Oncol. 1999;6:623–624. doi: 10.1007/s10434-999-0623-1. [DOI] [PubMed] [Google Scholar]
- 11.Urist MM. Pigment Cell. P. H. Rumke; Kargar, Basel: 1990. The role of surgery in the management of advanced melanoma. Therapy of advanced melanoma. pp. 1–13. [Google Scholar]
- 12.Atkins MB. Molecular Diagnosis, Prevention and Therapy of Melanoma. J. K. Kirkwood; New York: 1997. The role of cytotoxic chemotherapeutic agents either alone or in combination with biologic response modifiers. p. 219. [Google Scholar]
- 13.Bellett RE, Mastangelo MJ, Laucius JF, et al. Randomized prospective trial of DTIC alone versus BCNU plus vincristine in the treatment of metastatic malignant melanoma. Cancer Treat Rep. 1976;60:595–600. [PubMed] [Google Scholar]
- 14.Crosby T, Fish R, Coles B, et al. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev (2) 2000:CD001215. doi: 10.1002/14651858.CD001215. [DOI] [PubMed] [Google Scholar]
- 15.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 16.Jacquillat C, Khayat D, Banzet P, et al. Final report of the French multicenter phase II study of the nitrosourea fotemustine in 153 evaluable patients with disseminated malignant melanoma including patients with cerebral metastases. Cancer. 1990;66:1873–1878. doi: 10.1002/1097-0142(19901101)66:9<1873::aid-cncr2820660904>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22:1118–1125. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 18.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 19.Kane RC, Bross PF, Farrell AT, et al. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein JN, Myers TG, O'Connor PM, et al. An information-intensive approach to the molecular pharmacology of cancer. Science. 1997;275:343–349. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- 21.Amiri KI, Horton LW, LaFleur BJ, et al. Augmenting chemosensitivity of malignant melanoma tumors via proteasome inhibition. Implication for bortezomib (Velcade, PS-341) as a therapeutic agent for malignant melanoma. Cancer Res. 2004;64:4912–4918. doi: 10.1158/0008-5472.CAN-04-0673. [DOI] [PubMed] [Google Scholar]
- 22.Voorhees PM, Orlowski RZ. The proteasome and proteasome inhibitors in cancer therapy. Ann Rev Pharmacol Toxicol. 2006;46:189–213. doi: 10.1146/annurev.pharmtox.46.120604.141300. [DOI] [PubMed] [Google Scholar]
- 23.Jekunen AP, Christen RD, Shalinsky DR, et al. Synergistic interaction between cisplatin and taxol in human ovarian carcinoma cells in vitro. Br J Cancer. 1994;69:299–306. doi: 10.1038/bjc.1994.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodi FS, Soiffer RJ, Clark J, et al. Phase II study of paclitaxel and carboplatin for malignant melanoma. Am J Clin Oncol. 2002;25:283–286. doi: 10.1097/00000421-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Zimpfer-Rechner C, Hofmann U, Figi R, et al. Randomized phase II study of weekly paclitaxel versus paclitaxel and carboplatin as second-line therapy in disseminated melanoma: a multicentre trial of the Dermatologic Co-operative Oncology Group (DeCOG). Melanoma Res. 2003;13:531–536. doi: 10.1097/00008390-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Rao RD, Holtan SG, Ingle JN, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006;106:375–382. doi: 10.1002/cncr.21611. [DOI] [PubMed] [Google Scholar]
- 27.Flaherty KT, Bose M, Schuchter L, et al. Phase I/II trial of BAY 43-9006, carboplatin and paclitaxel demonstrates preliminary antitumor activity in the expansion cohort of patients with metastatic melanoma. J Clin Oncol. 2004;22(14S):7507. [Google Scholar]
- 28.Agarwala SS, Keilholz U, Hogg D, et al. Randomized phase III study of paclitaxel plus carboplatin with or without sorafenib as second-line treatment in patients with advanced melanoma. J Clin Oncol. 2007;25(18S):8510. [Google Scholar]


