Abstract
The aberrant transcription factors associated with many human malignancies function by deregulation of tumorigenic pathways. However, identification of these pathways has come slowly. Virtually all cases of Ewing’s Sarcoma and peripheral Primitive Neuroectodermal Tumor (PNET) are associated with aberrant transcription factors which fuse amino-terminal EWS with the DNA binding moiety of an ETS transcription factor (FLI-1 in 90% of cases). Attempts to identify the downstream targets of these chimeras in the Ewing Family Tumors (EFT) on the basis of differential gene regulation have produced little association with tumor biology. As an alternative approach, we have used highly efficient retroviral systems to biologically screen cDNA derived from cells transformed by EWS/FLI-1. We have identified the recently described PDGF-C as target of EWS/ETS transcriptional deregulation. This transcriptional deregulation is specific to EWS/FLI. PDGF-C possesses substantial biologic activity in vitro and in vivo. It is expressed in EFT cell lines and in primary tumors. Within these EFT cell lines, PDGF-C expression is dependent upon EWS/FLI activity. These results suggest that PDGF-C may be a significant mediator of EWS/FLI driven oncogenesis.
Keywords: Ewing’s Sarcoma, Ewing Family Tumors, PDGF-C, EWS/FLI
Introduction
As we enter what has been termed the post-genomic era (Vukmirovic & Tilghman, 2000), even greater emphasis will be placed on the functional relationships between genes. Nowhere is this emphasis more relevant than in the large group of human malignancies which harbor aberrant transcription factors (Rabbitts, 1994). While aberrant transcription factors are presumed to function through the deregulation of tumorigenic pathways, the identification of these pathways has come slowly (Vogt et al., 1999).
The pediatric solid tumors Ewing’s Sarcoma and peripheral Primitive Neuroectodermal Tumor (PNET), the Ewing Family Tumors (EFT), are an excellent example of the present problems in functional genomics. Virtually all EFT’s are associated with oncogenic chimeric proteins which fuse amino-terminal EWS with the DNA binding moiety of an ETS transcription factor (FLI-1 in 90% of cases) (Delattre et al., 1992; May & Denny, 1997). These chimeras function as transcription factors which are biochemically distinct from either parent gene (May et al., 1993b). Attempts to identify the downstream targets of these chimeras on the basis of differential gene regulation have produced targets with little apparent association with tumor biology (Arvand et al., 1998; Braun et al., 1995; Lawlor et al., 1998; Thompson et al., 1996; Welford et al., 1998). Clearly, if we are to understand the important role that aberrant transcription factors play in EFT and other human malignancies, further systematic identification of downstream pathways is necessary.
One current approach is to utilize microarray technology to produce and analyze the expression profiles of large numbers of tumors until patterns emerge (Golub et al., 1999). Results to date suggest that substantial biologic variability will be an obstacle to this approach (Perou et al., 2000). As an alternative approach, we have used highly efficient retroviral systems to biologically screen for transforming genes which are directly or indirectly transcriptionally deregulated as a consequence of the EWS/FLI aberrant transcription factor. With this approach, we have identified the recently described PDGF-C (Li et al., 2000) as target of EWS/ETS transcriptional deregulation. PDGF-C is upregulated by several variant EWS/ETS transcription factors. Like EWS/FLI, PDGF-C demonstrates substantial in vitro transformation and in vivo tumorigenic activity in a model system. PDGF-C is also expressed in EFT cell lines and in primary tumors. Finally, PDGF-C expression is dependent upon EWS/FLI activity in EFT cell lines. These results suggest that PDGF-C may be a significant mediator of EWS/FLI driven transformation and oncogenesis. Additionally, our isolation of PDGF-C further demonstrates the utility of phenotypic expression cloning in unraveling complex biologic processes.
Results
An Expression Cloning Strategy Detects A Novel EWS/FLI Target Gene
In order to isolate transforming genes which are differentially regulated by EWS/FLI, we employed a retroviral-based expression system (Whitehead et al., 1995). The phenotype employed was EWS/FLI induced transformation of NIH3T3 cells (May et al., 1993a). cDNA was isolated from two polyclonal NIH3T3 populations — retrovirally transduced with either EWS/FLI or empty retroviral vector. These lines were passaged and selected in parallel. In order to avoid repeated isolation of EWS/FLI, we employed a comparably active (data not shown) EWS/FLI construct with a NotI site at the chimeric junction. After cDNA synthesis, NotI digestion was used to inactivate EWS/FLI cDNA’s. Adapted cDNA was ligated into a retroviral vector and packaged into retroviral cDNA stocks. NIH3T3 cells were transduced with retroviral cDNA stocks derived from each cell population. Each cDNA transduced population was then plated in soft agar, using conditions in which EWS/FLI readily induces formation of transformed foci. After incubation, foci were found only in the cells transduced with EWS/FLI cDNA. Inserts from each focus were recovered by PCR. To screen out false positives, recovered inserts from each primary focus were subcloned into a secondary retroviral vector and the resulting retrovirus was used to transduce fresh NIH3T3 cells. Ultimately, two reproducibly transforming cDNAs were recovered intact. One, an EWS/FLI-Not I cDNA, was retrovirally derived and the result of incomplete digestion. This result validates our assay by demonstrating that a cDNA with transforming potential can be carried through such a multi-step procedure. The other transforming cDNA was found to be identical to the recently described PDGF-C (Li et al., 2000). All functional units of PDGF-C were present including N-terminal leader sequence, central CUB domain (Kristiansen et al., 1999), and C-terminal cystine-knot region homologous to the platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) growth factor family. Taken together, these structural features strongly suggest that the PDGF-C is a secreted growth factor with the ability to transform NIH3T3 cells.
PDGF-C Expression is Specifically Induced by Biologically Significant EWS fusion Proteins
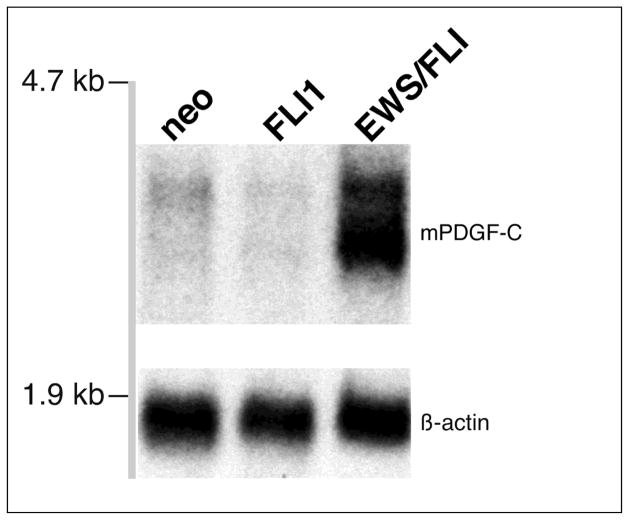
Isolates acting downstream of the EWS/FLI oncogenic transcription factor would be expected to have increased expression in EWS/FLI transformed cells. However, it is possible that constitutively expressed genes unaffected by EWS/FLI might transform in this assay solely due to high expression levels conferred by the retroviral system. Figure 1 demonstrates that PDGF-C transcript levels are markedly upregulated in EWS/FLI transformed NIH3T3 cells.
Figure 1. PDGF-C is induced in response to EWS/FLI expression in NIH 3T3 cells.
Abundant PDGF-C expression is detected by northern blot analysis of total RNA from polyclonal NIH 3T3 cells stably expressing the EWS/FLI fusion gene from a retroviral vector. Only low-levels of PDGF-C are apparent in NIH 3T3 cells stably expressing either empty retroviral vector (neo) or the native FLI-1 gene. Probes used are as indicated.
This demonstrates that, directly or indirectly, PDGF-C is upregulated as a consequence of EWS/FLI expression and that phenotypic expression cloning can be used to isolate differentially expressed genes.
PDGF-C is specifically induced by EWS/FLI and other related fusion genes
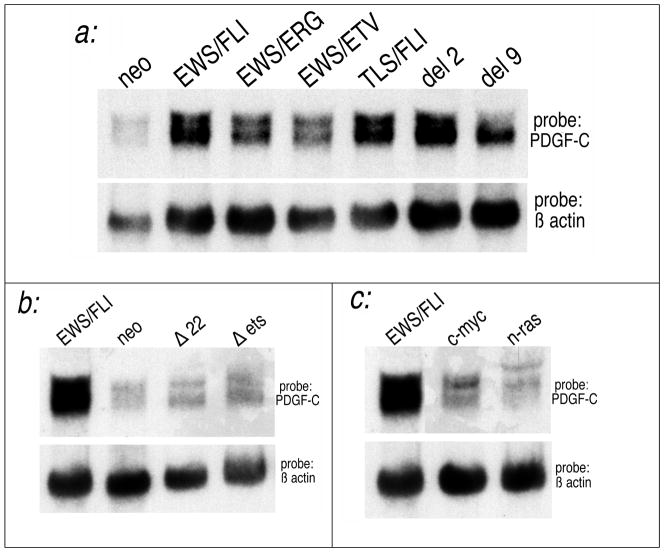
The variant EWS/ETS chimeras all produce clinically indistinguishable disease and similar in vitro effects (Thompson et al., 1999). One would therefore expect a valid target of EWS/FLI also to be upregulated by these other EWS/ETS chimeras (Im et al., 2000). We found that PDGF-C was also induced by EWS/ETS variant chimeras (Figure 2a) including (i) EWS/ERG, found in 5% of ES/PNET’s (Sorensen et al., 1994), (ii) EWS/ETV, a less common variant (Jeon et al., 1995), and (iii) TLS/FLI, a transforming artificial chimera that substitutes EWS with its homologue TLS/FUS(May et al., 1997). Additionally, two attenuated mutants of EWS/FLI, del 2 (Δ12–82) and del 9 (Δ82–246) (Lessnick et al., 1995), were also found to upregulate PDGF-C.
Fig. 2. PDGF-C is specifically induced by EWS/FLI and other related fusion genes.
Polylclonal NIH 3T3 cellular populations were made by transduction with retroviral vectors expressing the constructs indicated. (a) Fusion genes structurally similar to EWS/FLI induce PDGF-C. These include EWS/ERG and EWS/ETV which occur in a minority of EFT’s, TLS/FLI which is artificial chimera of similar in vitro activity, and attenuated EWS deletion mutants del 2 and del 9. (b) PDGF-C not induced by EWS/FLI mutants which abolish essential transactivation (Δ22) and DNA binding domains (Δets). (c) PDGF-C is not effectively induced by unrelated transforming oncogenes such as c-myc and n-ras even though these also transform NIH3T3 cells. Murine PDGF-C was used as the probe. Comparable levels of each chimera or transforming oncogene were demonstrated on Northern Blot (data not shown).
Each of these EWS/ETS chimeras is associated with either EFT’s or possesses substantial in vitro transforming activity. The finding that each chimera similarly upregulates PDGF-C suggests a link between PDGF-C and EWS/FLI transformation and tumorigenesis.
Because of the invariant structural features of EWS/ETS chimeras (Zucman et al., 1993) and their distinctive transactivation potential (May et al., 1993b), it is presumed that biologically relevant targets of EWS/FLI will depend on transcriptional activation by EWS/FLI and not some non-transcriptional mechanism. Figure 2b demonstrates that PDGF-C is not upregulated by EWS/FLI constructs which abrogate its essential DNA binding (Δets) and transactivation (Δ22) properties (May et al., 1993a). This suggests that PDGF-C upregulation depends on EWS/FLI transcriptional activation, though that dependence may be direct or indirect.
Targets which are uniquely upregulated by EWS/FLI are more likely to be intimately involved in EFT development than targets upregulated in cells transformed by unrelated means. NIH3T3 cells are also transformed by oncogenes unrelated to EWS/FLI, including c-myc and n-ras (May et al., 1997). Even though these agents produce phenotypes similar to EWS/FLI, they do not induce PDGF-C as does EWS/FLI (Figure 2c). This further suggests that PDGF-C is unique to EWS/FLI transformation and is not simply a non-specific consequence of altered growth properties.
PDGF-C is a More Potent In Vitro and In Vivo Transforming Agent Than EWS/FLI
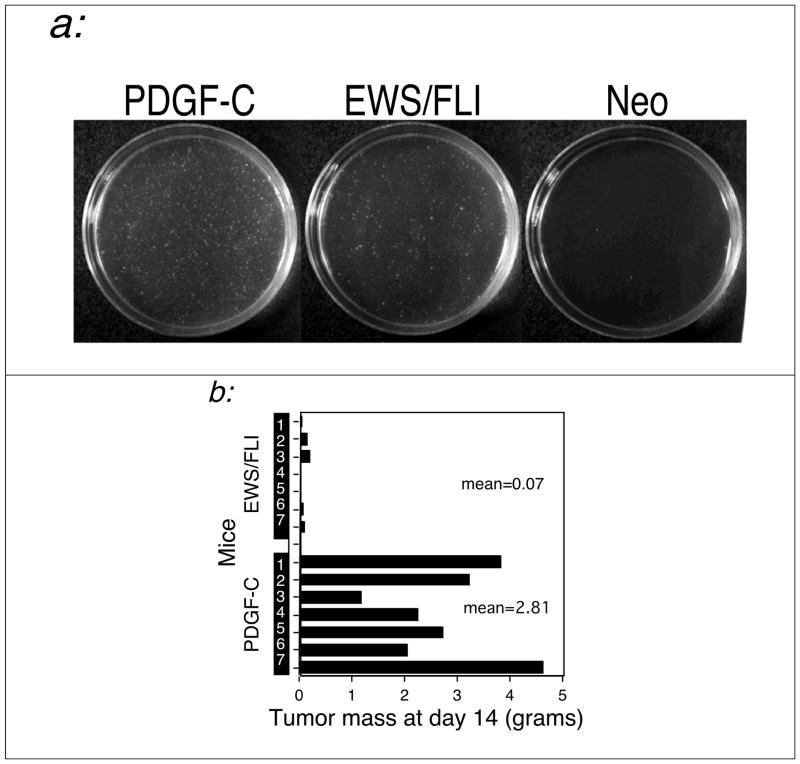
Other candidate EWS/FLI downstream effectors have shown attenuated phenotypes when compared to EWS/FLI. This outcome suggests a contributory but secondary role in mediating EWS/FLI phenotype (May et al., 1997). To assess the relative contribution PDGF-C makes to the EWS/FLI phenotype, we compared the phenotypes induced by PDGF-C and EWS/FLI under identical circumstances. Both were expressed in untransformed NIH3T3 cells at comparably high levels (data not shown) and phenotypes were measured by soft agar colony formation and by tumor formation in SCID mice. As shown in Figure 3, PDGF-C is more efficient than EWS/FLI at both inducing anchorage independent growth and at inducing tumor formation in immunodeficient mice.
Fig. 3. PDGF-C is a more potent transforming agent than EWS/FLI.
Polyclonal NIH 3T3 cellular populations stably expressing either PDGF-C, EWS/FLI or vector alone (neo) were developed and assayed for transformation both (a) in vitro and (b) in vivo. (a). Specific quantities of cells expressing each construct were plated in soft agar as described in Methods. The EWS/FLI and neo plates shown are at a 10-fold greater cell density than that of the PDGF-C plate. As can be seen, PDGF-C expression induces a much greater degree of anchorage-independent growth than does EWS/FLI or vector alone. This experiment was performed three times, and a typical result is shown. (b). 1 × 106 cells expressing each construct were injected into nude mice. After two weeks, the average resulting tumor mass was markedly higher in mice injected with PDGF-C-expressing cells (2.81 grams) than in those injected with cells expressing EWS/FLI (0.07 grams). Cells expressing vector alone resulted in no detectable tumor formation at this time point (data not shown).
These data indicate that PDGF-C has potent biologic activity and the efficiency of its induction may be a rate-determining factor in EWS/FLI transformation. Furthermore they show how the supraphysiologic expression levels seen in retroviral expression cloning can facilitate the phenotypic isolation of downstream effectors.
PDGF-C is Expressed in a High Percentage of ES/PNET Cell Lines and Primary Tumors
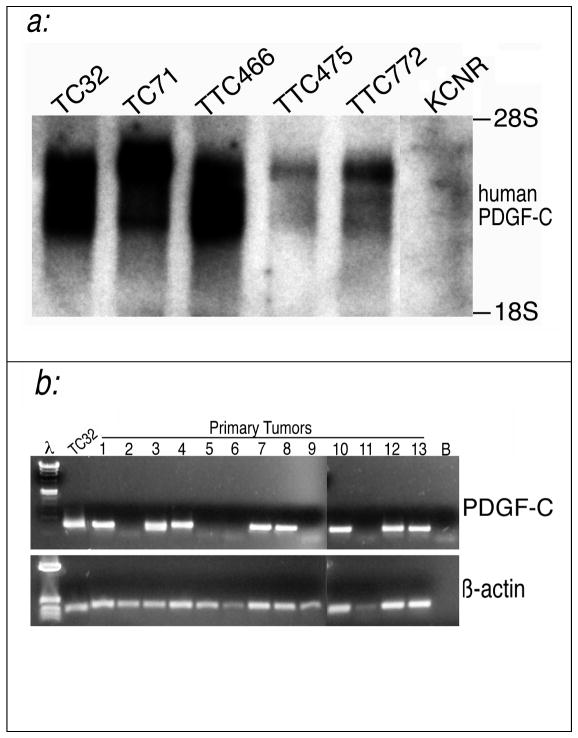
While PDGF-C appears to be a potent mediator of EWS/FLI phenotype in a model cell line, this does not indicate a role in EWS/FLI associated human tumors. We therefore evaluated several EFT cell lines for PDGF-C expression (Figure 4a). We found PDGF-C transcript in all lines evaluated and not in a neuroblastoma cell line (KCNR).
Fig. 4. PDGF-C is expressed in several EFT cell lines and primary tumor specimens.
(a) A northern blot containing total RNA from a variety of EFT-derived cancer cell lines was probed with human PDGF-C. PDGF-C transcripts were detected in all cell lines regardless of whether they contained an EWS/FLI (TC32, TC-71, TTC-475. and TTC-772) or an EWS/ERG (TTC-466) fusion. PDGF-C expression was absent in the KCNR cell line derived from an unrelated tumor type (neuroblastoma) that lacks an EWS-containing fusion. (b) PDGF-C is expressed in a large proportion of EFT primary tumors. Nested RT-PCR with primers specific to a 400-bp N-terminal region of human PDGF-C was performed on thirteen EFT primary tumor specimens, designated by tumor bank accession numbers, and the PNET cell line TC32. The expected fragment was detected in roughly 60% of the tumor specimens analyzed. RT-PCR on β-actin was used as a control for RNA integrity. λ designates lambda HindIII marker. The marker used β-actin for was pBR327 HinfI. B designates a buffer blank lane.
A similar Northern Blot of cell lines representing eight other unrelated tumor types demonstrated expression in only two of eight (data not shown). This indicates that PDGF-C is not indiscriminately upregulated in any passaged tumor cell line.
Since cell line expression patterns can vary with passage, we also assessed expression in primary EFT specimens. As Figure 4b demonstrates, PDGF-C transcript could be demonstrated in over 60% of the primary tumors assessed. These data are consistent with a role for this biologically potent protein in a substantial number of EFT’s. Further correlation with tumor clinical characteristics and with the type of EWS/ETS chimera are indicated.
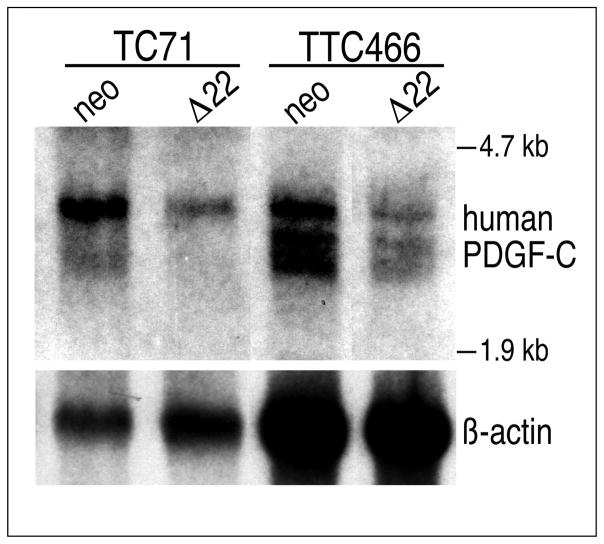
PDGF-C Expression is Dependent on EWS/FLI
Expression of PDGF-C in tumor derived tissue suggests but does not establish it as acting downstream of EWS/FLI in EFT cells. To address this issue, we employed an EWS/FLI dominant negative construct. EWS/FLI constructs which delete the amino terminal EWS transactivation domain have been shown to inhibit EFT cell line tumorigenesis (Kovar et al., 1996) and EWS/FLI in vitro transformation (Thompson et al., 1999) through dominant negative inhibition. We introduced a construct of similar activity (data not shown) into two EFT cell lines. We used an IRES containing retroviral vector and continuous selection to ensure continued expression of the inhibitory construct. Pure polyclonal cell populations were selected and were assessed for PDGF-C expression. Figure 5 shows that dominant negative EWS/FLI resulted in lower levels of PDGF-C transcript.
Fig. 5. PDGF-C expression in EFT cell lines is dependent on EWS/FLI activity.
A northern blot containing total RNA from polyclonal, continuously selected populations of TC71 and TTC466 cells stably expressing either Δ22, a dominant negative EWS/FLI, or vector alone was probed with human PDGF-C. In both cell lines, blocking EWS/FLI activity with Δ22 decreases PDGF-C transcript levels. TC71 expresses EWS/FLI and TTC466 expresses EWS/ERG. Abundant levels of Δ22 transcript were detected (data not shown).
These data demonstrate that, while PDGF-C expression may not be a universal feature of EFT’s, its expression depends of the activity of the EWS/FLI transcription factor in tumor derived cells. It therefore represents a biologically relevant gene acting downstream of EWS/FLI.
Discussion
PDGF-C is a biologically potent secreted growth factor that is specifically induced by EWS/FLI in vitro. Its expression can be demonstrated in a majority of EFT cell lines and primary tumors. Expression in EFT cell lines appears to be EWS/FLI dependent. These data support PDGF-C as a potent mediator of the EWS/FLI oncogenic phenotype in both in vitro systems and in human tumors.
PDGF-C has been demonstrated to be a secreted growth factor with homology to PDGF and other members of the PDGF/VEGF family (Li et al., 2000). A wealth of evidence has implicated members of this family in the pathogenesis of a variety of tumor systems (Heldin & Westermark, 1991). This is the first report to link PDGF-C with a malignancy.
We presume PDGF-C induces transformation by closing an autocrine loop within the cell, a mechanism that is utilized by other oncogenic members of this family (Keating & Williams, 1988; Leveen et al., 1994). PDGF-C may also act in vivo to enhance interactions between the tumor and surrounding stromal cells analogous to its predicted role in kidney development (Ding et al., 2000; Li et al., 2000).
The ability of PDGF-C to efficiently transform NIH 3T3 cells is quite surprising given the recent demonstration of its binding to the α but not the β form of the PDGF receptor (PDGFR) (Li et al., 2000). Several studies have shown that PDGF-A, which is also thought to signal exclusively through the αPDGFR, acts only weakly to transform NIH 3T3 cells (Beckmann et al., 1988; Heldin et al., 1992). Several possibilities exist to explain these disparate outcomes of signaling through the same receptor. The dynamics of the PDGF-C/αPDGFR and PDGF-A/αPDGFR interactions may differ such that the former leads to increased or sustained levels of activated receptors. Alternatively, PDGF-C may signal through other receptors in addition to the α PDGFR.
As with the other PDGF/VEGF family members, PDGF-C seems to also play an important role in development. PDGF-C expression is dynamically regulated during development (Ding et al., 2000) and it appears to play a role in the developing kidney, lung and other organs including the neural crest (Ding et al., 2000; Li et al., 2000). At least in the kidney, it appears that PDGF-C is able to direct mesenchymal proliferation when secreted by adjacent epithelium. This is of interest since EFT’s may arise from neural crest or primitive mesenchymal cells (de Alava & Gerald, 2000), although no definitive cell of origin has yet been defined.
The presence of a CUB domain is unique to PDGF-C among the PDGF/VEGF family. The CUB domain has been recently shown to have an inhibitory function which is abrogated by extracellular proteolytic cleavage (Li et al., 2000). The CUB domain may also mediate protein-protein interactions or interactions between PDGF-C and elements of the extracellular matrix as similar domains have been shown to do in a number of developmentally regulated proteins (Li et al., 2000). It remains to be determined if the cleaved CUB domain has biologic activity or if it serves to recruit or interact with a secondary receptor in some contexts. Further experimental and clinical evidence will be required to more firmly establish the importance of PDGF-C to human tumorigenesis.
Further understanding of the biology of EFT’s depends on the identification of biologically relevant targets of aberrant EWS/ETS chimeric transcription factors. The same can be said of a wide variety of tumor systems which harbor aberrant oncogenic transcription factors. However, instances linking oncogenic transcriptional deregulation with biologically significant effectors have been relatively few (He et al., 1998; Tetsu & McCormick, 1999). Approaches such as differential display, RDA/SSH, SAGE, or microarrays have been widely employed to identify potential targets of oncogenic deregulation. The introduction of DNA-chip based screens further facilitates such approaches and makes them more comprehensive. However, the tumor to tumor variation thus far encountered (Perou et al., 2000) suggests that substantially more data must be analyzed for functional patterns to emerge. In EFT’s, the most compelling target yet identified was found through a candidate gene approach based upon knowledge of promoter binding sites (Hahm et al., 1999) and not by a broader systematic screen. We have taken a contrasting approach that employs biologic selection to broadly screen and identify targets of phenotypic significance. Genes identified with this approach are no more likely to be direct targets than those identified by any of these alternate methods. However, the biologic activity assured by our approach provides higher likelihood of target involvement in processes driving tumor development.
These data demonstrate that a primary oncogenic protein such as EWS/FLI can be effectively used to identify other oncogenic proteins that are activated downstream. Drawbacks to this approach have included the phenotypic leakiness seen in mammalian systems (Hannon et al., 1999). In fact, we found a high incidence of false positives in our experiment. Any future strategies must account for this phenotypic variability with adequate provision for secondary screening. Recently described vector systems (Hannon et al., 1999) address this issue and have already shown promising results (Hudson et al., 1999; Maestro et al., 1999). In the face of the complexity of oncogenic transcriptional deregulation, approaches such as ours can be a valuable complement to expression profiling in unraveling the complex biologic processes involved in tumor development.
Materials and Methods
Expression cloning
An EWS/FLI fusion containing a Not I site within its breakpoint region was initially constructed by PCR. Oligonucleotide primers (sequence available on request) were used to amplify the EWS and Fli-1 domains respectively and to link each to an in frame NotI site. PCR products were cut with NotI and flanking restriction endonucleases and ligated into a cloning vector. The insert was subcloned into the retroviral expression vector (SRαMSVtkneo-ΔHindIII) and replication-deficient retroviral stocks were created from this construct using 293T cells and methods previously described (Muller et al., 1991). These viral stocks were then used to transduce early passage NIH 3T3 cells. After a suitable expansion and G418 selection, poly A+ RNA was collected from each cellular population and oligo-dT primed cDNA libraries were created according to the manufacturers protocols (Gibco BRL Superscript II Kit). Adapted cDNA from each population was digested with Not I and then cloned into pCTV1B, a retroviral vector designed specifically for expression cloning provided by Dr. Robert Kay. The subsequent library amplification and retroviral production steps were performed as previously described (Foster et al., 1999; Whitehead et al., 1995). Retroviral pools representing 3×106 bacterial clones from each library were used to transduce 3×106 early passage NIH 3T3 cells. Two days after transduction, the cells were plated in soft agar (see below) 30 plates/library, 1.5×105 cells/plate in 10 cm2 plates. After two weeks, colonies were recovered and expanded. Secondary screening procedures via PCR amplification, subcloning cDNA inserts and replating in soft agar were performed as previously described (Whitehead et al., 1995).
Soft agar transformation assay
SRαMSVtkneoΔHindIII containing full length PDGF-C, EWS/FLI, or lacking insert were used to create retrovirus stocks as previously described (May et al., 1993a). These viruses were then used to transduce equivalent populations of early passage NIH 3T3 cells. After transduction, the cells were selected in G418 for at least one week. Polyclonal, selected populations were plated in soft agar at either 5,000 or 50,000 cells per 6-cm-diameter plate and at either 10 or 20% half calf serum/half fetal calf serum in Iscove’s medium as previously described. Agar plates were photographed approximately 2 weeks after plating.
Tumorigenesis assay
The same cellular populations used in the aforementioned soft agar assay were also injected subcutaneously into nude mice. Seven mice per population were injected at a single site with 1 × 106 cells in 0.5 cc DMEM/5% calf serum. The mice were inspected for two weeks. At this time point, the mice were euthanized and the resultant tumors excised and weighed. PDGF-C and EWS/FLI transcript was demonstrated in Northern blots made from tumor samples.
Screening of cDNA library for Human PDGF-C
Human PDGF-C was obtained from a human TC32 library using Image Clone 307186 (Research Genetics) as a probe. Methods for library construction and screening are as previously described (Thompson et al., 1996).
RT-PCR of tumor samples
Five micrograms of total RNA from each tumor specimen was used to create a random hexamer-primed cDNA following the Superscript RT II protocol (Gibco). Ten percent of the cDNA synthesis reactions from each specimen were then amplified by two-stage nested PCR using PDGF-C-specific primers (1st stage: 5′-cggtcttggtatggagattagt, 3′-cttctaagtccaactgccatct; 2nd stage: 5′-tggatacaacttacgtttgatgaa, 3′-cgaataaggtcttccaaggtact). Cycling parameters for the first stage-1 cycle × 94° for 5 min.; 30 cycles × 94° for 40 sec., 58° for 40 sec. and 72° for 1 min.; 72° for 7 min. Four percent of the first stage reactions was used as the template in a second PCR using the same parameters but with the second set of nested primers. Single-stage PCR was also performed in parallel on the cDNA populations with β-actin-specific primers (5′-tcacccacactgtgcccatctacga, 3′-cagcggaaccgctcattgccaatgg) to control for RNA integrity. Cycling parameters were identical to the first stage of the aforementioned PCR.
Dominant negative EWS/FLI
A dominant negative EWS/FLI construct tagged with an amino terminal FLAG epitope was made using methods similar to those previously published (Thompson et al., 1999). It was cloned into the retroviral expression vector pLXIN (Clontech) and used to generate amphotrophic retroviral stock using the PT67 packaging line (Clontech). To confirm biologic activity of our construct, NIH3T3 cells expressing the dominant negative were used to superinfect either NIH3T3 cells or the same cells transduced and polyclonally selected with EWS/FLI (hygromycin resistant). These cells were then selected in G418 to obtain comparable polyclonal populations. When comparison was made to the starting population of EWS/FLI-hygromycin cells passaged in parallel, those superinfected with the dominant negative yielded smaller colonies.
Tumor cell lines were infected with the same retrovirus and selected continuously under G418 to maintain expression of the dominant negative in pure polyclonal cell populations. Expression of PDGF-C and of the dominant negative construct was confirmed by northern blot.
Northern Blots
Total RNA was obtained using either acid phenol extraction (Trizol, Gibco/BRL) or by column purification (Qiagen Rneasy). Electrophoresis, transfer, and hybridization are as previously described.
Acknowledgments
W.A.M. is supported with funding from the American Cancer Society (RPG-99-096-01), the Concern Foundation and The Wendy Will Case Cancer Fund. The authors wish to acknowledge Dr. Robert Kay for providing vectors and technical assistance, Dr. Timothy Triche for providing EFT cell lines, Dr. Kevin Shannon for providing N-ras clones, Dr. Mike Ruppert for technical assistance, and Dr. Randy Wada for critical review of the manuscript.
References
- Arvand A, Bastians H, Welford SM, Thompson AD, Ruderman JV, Denny CT. Oncogene. 1998;17:2039–45. doi: 10.1038/sj.onc.1202129. [DOI] [PubMed] [Google Scholar]
- Beckmann MP, Betsholtz C, Heldin CH, Westermark B, Di Marco E, Di Fiore PP, Robbins KC, Aaronson SA. Science. 1988;241:1346–9. doi: 10.1126/science.2842868. [DOI] [PubMed] [Google Scholar]
- Braun BS, Frieden R, Lessnick SL, May WA, Denny CT. Mol Cell Biol. 1995;15:4623–30. doi: 10.1128/mcb.15.8.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alava E, Gerald WL. J Clin Oncol. 2000;18:204–13. doi: 10.1200/JCO.2000.18.1.204. [DOI] [PubMed] [Google Scholar]
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G, Aurias A, Thomas G. Nature. 1992;359:162–5. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- Ding H, Wu X, Kim I, Tam PPL, Koh GY, Nagy A. Mechanisms of Development. 2000;96:209–13. doi: 10.1016/s0925-4773(00)00425-1. [DOI] [PubMed] [Google Scholar]
- Foster KW, Ren S, Louro ID, Lobo-Ruppert SM, McKie-Bell P, Grizzle W, Hayes MR, Broker TR, Chow LT, Ruppert JM. Cell Growth & Differentiation. 1999;10:423–34. [PubMed] [Google Scholar]
- Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES. Science. 1999;286:531–7. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- Hahm KB, Cho K, Lee C, Im YH, Chang J, Choi SG, Sorensen PH, Thiele CJ, Kim SJ. Nat Genet. 1999;23:222–7. doi: 10.1038/13854. [DOI] [PubMed] [Google Scholar]
- Hannon GJ, Sun P, Carnero A, Xie LY, Maestro R, Conklin DS, Beach D. Science. 1999;283:1129–30. doi: 10.1126/science.283.5405.1129. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Ostman A, Eriksson A, Siegbahn A, Claesson-Welsh L, Westermark B. Kidney Int. 1992;41:571–4. doi: 10.1038/ki.1992.84. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Crit Rev Oncog. 1991;2:109–24. [PubMed] [Google Scholar]
- Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. J Exp Med. 1999;190:1375–82. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im YH, Kim HT, Lee C, Poulin D, Welford S, Sorensen PH, Denny CT, Kim SJ. Cancer Res. 2000;60:1536–40. [PubMed] [Google Scholar]
- Jeon IS, Davis JN, Braun BS, Sublett JE, Roussel MF, Denny CT, Shapiro DN. Oncogene. 1995;10:1229–34. [PubMed] [Google Scholar]
- Keating MT, Williams LT. Science. 1988;239:914–6. doi: 10.1126/science.2829358. [DOI] [PubMed] [Google Scholar]
- Kovar H, Aryee DN, Jug G, Henockl C, Schemper M, Delattre O, Thomas G, Gadner H. Cell Growth & Differentiation. 1996;7:429–37. [PubMed] [Google Scholar]
- Kristiansen M, Kozyraki R, Jacobsen C, Nexo E, Verroust PJ, Moestrup SK. Journal of Biological Chemistry. 1999;274:20540–4. doi: 10.1074/jbc.274.29.20540. [DOI] [PubMed] [Google Scholar]
- Lawlor ER, Lim JF, Tao W, Poremba C, Chow CJ, Kalousek IV, Kovar H, MacDonald TJ, Sorensen PH. Cancer Res. 1998;58:2469–76. [PubMed] [Google Scholar]
- Lessnick SL, Braun BS, Denny CT, May WA. Oncogene. 1995;10:423–31. [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Genes & Development. 1994;8:1875–87. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U. Nat Cell Biol. 2000;2:302–9. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach DH, Hannon GJ. Genes Dev. 1999;13:2207–17. doi: 10.1101/gad.13.17.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May WA, Arvand A, Thompson AD, Braun BS, Wright M, Denny CT. Nat Genet. 1997;17:495–7. doi: 10.1038/ng1297-495. [DOI] [PubMed] [Google Scholar]
- May WA, Denny CT. Curr Top Microbiol Immunol. 1997;220:143–50. [PubMed] [Google Scholar]
- May WA, Gishizky ML, Lessnick SL, Lunsford LB, Lewis BC, Delattre O, Zucman J, Thomas G, Denny CT. Proc Natl Acad Sci U S A. 1993a;90:5752–6. doi: 10.1073/pnas.90.12.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May WA, Lessnick SL, Braun BS, Klemsz M, Lewis BC, Lunsford LB, Hromas R, Denny CT. Mol Cell Biol. 1993b;13:7393–8. doi: 10.1128/mcb.13.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AJ, Young JC, Pendergast AM, Pondel M, Landau NR, Littman DR, Witte ON. Mol Cell Biol. 1991;11:1785–92. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, Van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale A, Brown PO, Botstein D. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Rabbitts TH. Nature. 1994;372:143–9. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- Sorensen PH, Lessnick SL, Lopez-Terrada D, Liu XF, Triche TJ, Denny CT. Nat Genet. 1994;6:146–51. doi: 10.1038/ng0294-146. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Thompson AD, Braun BS, Arvand A, Stewart D, Chen E, May WA, Korenberg J, Denny CT. Oncogene. 1996;13:2649–2658. [PubMed] [Google Scholar]
- Thompson AD, Teitell MA, Arvand A, Denny CT. Oncogene. 1999;18:5506–13. doi: 10.1038/sj.onc.1202928. [DOI] [PubMed] [Google Scholar]
- Vogt PK, Aoki M, Bottoli I, Chang HW, Fu S, Hecht A, Iacovoni JS, Jiang BH, Kruse U. Cell Growth Differ. 1999;10:777–84. [PubMed] [Google Scholar]
- Vukmirovic OG, Tilghman SM. Nature. 2000;405:820–2. doi: 10.1038/35015690. [DOI] [PubMed] [Google Scholar]
- Welford SM, Gregg J, Chen E, Garrison D, Sorensen PH, Denny CT, Nelson SF. Nucleic Acids Res. 1998;26:3059–65. doi: 10.1093/nar/26.12.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead I, Kirk H, Kay R. Molecular & Cellular Biology. 1995;15:704–10. doi: 10.1128/mcb.15.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucman J, Melot T, Desmaze C, Ghysdael J, Plougastel B, Peter M, Zucker JM, Triche TJ, Sheer D, Turc-Carel C, et al. Embo J. 1993;12:4481–7. doi: 10.1002/j.1460-2075.1993.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]