Abstract
Cholestasis is a pathological common component of numerous liver diseases that results in hepatotoxicity, inflammation, and cirrhosis when untreated. While the predominant hypothesis in cholestatic liver injury remains hepatocyte apoptosis due to direct toxicity of hydrophobic bile acid exposure, recent work suggests the injury occurs through inflammatory necrosis. In order to resolve this controversy, we used novel plasma biomarkers to assess the mechanisms of cell death during early cholestatic liver injury. C57Bl/6 mice underwent bile duct ligation (BDL) for 6–72h, or sham operation. Another group of mice were given D-galactosamine and endotoxin as a positive control for apoptosis and inflammatory necrosis. Plasma levels of full length cytokeratin-18 (FL-K18), microRNA-122 (miR-122) and high mobility group box-1 protein (HMGB1) increased progressively after BDL with peak levels observed after 48h. These results indicate extensive cell necrosis after BDL, which is supported by the time course of plasma alanine aminotransferase activities and histology. In contrast, plasma caspase-3 activity, cleaved caspase-3 protein and caspase-cleaved cytokeratin-18 fragments (cK18) were not elevated at any time during BDL suggesting the absence of apoptosis. In contrast, all plasma biomarkers of necrosis and apoptosis were elevated 6h after Gal/End treatment. In addition, acetylated HMGB1, a marker for macrophage and monocyte activation, was increased as early as 12h but mainly at 48–72h. However, progressive neutrophil accumulation in the area of necrosis started at 6h after BDL. In conclusion, these data indicate that early cholestatic liver injury in mice is an inflammatory event, and occurs through necrosis with little evidence for apoptosis.
Keywords: Bile duct ligation, apoptosis, necrosis, biomarkers, cytokeratin-18, high mobility group box-1, microRNA-122, cholestasis
INTRODUCTION
Cholestasis is present in multiple pathologies including gall stone obstruction of the bile duct, biliary atresia, local tumor impingement and drug toxicity (Kim et al., 2000; Poupon et al., 2000; Yang et al., 2013b). Persistent cholestasis leads to injury of hepatocytes and bile duct epithelial cells, inflammation, and progression to fibrosis, cirrhosis and death. The predominant hypothesis concerning the mechanism of cholestatic liver injury is the accumulation of hydrophobic bile salts considered to be highly toxic (Perez and Briz, 2009; Maillette de Buy Wenniger and Beuers, 2010; Higuchi and Gores, 2003), with a heavy emphasis on glycochenodeoxycholate (GCDC) or its bile acid glycochenodeoxycholic acid (GCDCA) largely due to their accumulation in human serum during cholestatic conditions (Trottier et al., 2011). Papers from multiple laboratories over the past 20 years have shown an intricate mechanism by which GCDC at 30–50μM concentrations causes apoptosis in isolated rat hepatocytes (Spivey et al., 1993; Patel et al., 1995; Yerushalmi et al., 2001; Graf et al., 2002; Schoemaker et al., 2004; Rust et al., 2006). While GCDC-induced apoptosis is indisputable in rat hepatocytes, it is currently undetermined if this mechanism also applies to other animals or the human condition (Woolbright and Jaeschke, 2012).
Recent data in a bile duct ligation (BDL) model in the mouse demonstrated that the accumulating bile acids in serum and liver consist largely of hydrophilic bile acids including taurocholate, muricholic acid and tauromuricholic acid (Zhang et al., 2012). Hepatocytes exposed to these hydrophilic bile acids individually or in combination trigger chemokine formation and expression of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) but did not cause cell death (Allen et al., 2011; Zhang et al., 2012). In addition, animals deficient in CD18 or ICAM-1 are highly protected against BDL-induced liver injury suggesting that cholestatic liver injury in this mouse model is caused by neutrophils (Gujral et al., 2003a, 2004a). It has been suggested that the mode of cell death in the BDL model in rats (Schoemaker et al., 2003) or mice (Gujral et al., 2004b; Fickert et al., 2005; Nalapareddy et al., 2009; Mitchell et al., 2011) is mainly oncotic necrosis and not apoptosis. Nevertheless, this is controversial as others have concluded that apoptotic cell death is dominant (Higuchi and Gores, 2003). Part of the confusion is certainly caused by use of non-specific assays for apoptosis such as the TUNEL assay (Grasl-Kraupp et al., 1995; Jaeschke and Lemasters, 2003). However, one argument that has been used for the different interpretation is that a cell undergoing apoptosis is only identifiable for a limited time and thus many apoptotic cells may be missed when a longer time interval is used. In order to address this issue, we have used circulating biomarkers of apoptosis (caspase-cleavage fragment of cytokeratin-18 and caspase-3 enzyme activity) and necrosis (full-length cytokeratin-18, high mobility group box-1 protein and miR122) in the mouse BDL model that can accumulate and provide a signature pattern of resulting cell death mechanisms. Each of these markers has been successfully used in mice (Antoine et al., 2009; Williams et al., 2011; McGill et al., 2012) and humans (Starkey Lewis et al., 2011; Antoine et al., 2012, 2013; McGill et al., 2012; Dear et al., 2013) after acetaminophen overdose. In addition, we have compared our data in the BDL model with a traditional apoptosis/inflammation model, i.e. galactosamine/ endotoxin-induced liver injury (Leist et al., 1995; Jaeschke et al., 1998).
MATERIALS AND METHODS
Animals and experimental protocol
C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animals received humane care according to the criteria outlined in Guide for the Care and Use of Laboratory Animals. All experimental protocols were approved by the Animal Use Committees of the University of Kansas Medical Center. Thirty minutes before surgery, the mice were injected i.m. with a cocktail of anesthetics consisting of ketamine (225 mg/kg Ketaset; Fort Dodge Animal Health, Fort Dodge, IA), xylazine (11.4 mg/kg Rompun; Bayer, Shawnee Mission, KS), and acepromazine (2.3 mg/kg acepromazine maleate; VEDCO, St. Joseph, MO). A midline laparotomy was performed and the common bile duct was ligated twice with 4-0 silk sutures and then cut between the sutures. Sham-operated animals, which were subjected to the same operation except the ligation of the bile duct, served as controls. After BDL the abdomen of each animal was closed in two layers. Blood and liver samples were collected at the indicated time of death. Pieces of the liver were snap-frozen in liquid nitrogen or fixed in phosphate-buffered formalin for immunohistochemistry and histology.
Liver injury and plasma ALT levels
ALT activities were determined in the plasma by using the Pointe Scientific ALT Kit (Pointe Scientific Canton, MI) according to the manufacturer’s instructions. Sections of formalin-fixed paraffin-embedded liver samples were stained with hematoxylin and eosin (H&E) for evaluation of liver cell injury.
Hepatic neutrophil immunohistochemistry
Neutrophil accumulation in the livers was assessed by staining tissue sections using immunohistochemistry for Ly6B2 (AbD Serotec Raleigh, NC), a specific marker for neutrophils. Briefly, slides containing liver sections were deparaffinized, rehydrated, and then incubated with peroxidase suppressor (Sigma, St. Louis, MO) for 30 minutes. Vector Labs rat anti-mouse DAB detection system (Vector Labs Burlingame, CA) was used with the indicated primary antibody at 1:200 dilution.
Determination of circulating mechanism-based biomarkers of liver injury
Circulating biomarkers were determined as previously described for highly liver specific markers such as microRNA-122 (miR-122) by qRT-PCR (Starkey Lewis et al., 2011), unequivocal identification and absolute quantification of full length and caspase-cleaved cytokeratin-18 was determined by mass spectrometry as previously described (Antoine et al., 2009, 2010, 2012) and total HMGB1 (Antoine et al., 2009, 2010, 2012) by immunoassay and mass spectrometry. The absolute quantification of hyper-acetylated HMGB1 was determined by mass spectrometry as previously described as a biomarker of immune cell activation (Antoine et al., 2009). For miR-122 measurement, let-7d provided biological standardization and lin-4 served as an internal standard for the assay. The investigators measuring the plasma biomarkers were blinded to the treatment groups of the animals.
Western blotting
Flash frozen liver sections were homogenized in a chaps based buffer, and then centrifuged at 14000 rpm to remove cellular debris. The BCA assay (Pierce, Rockford, IL) was used to quantify protein levels in both liver and plasma. Briefly, equal quantities of protein were loaded into an Invitrogen Mini Blot electrophoresis system and transferred onto PVDF paper. The blot was probed with a caspase-3 antibody (Cell Signaling Danvers, MA) and visualized using an HRP conjugated secondary antibody (Santa Cruz Biotechnology Santa Cruz, CA).
Caspase activity assa
Caspase activity was determined by measuring the z-VAD-FMK-inhibitable cleavage of the fluorescent caspase-3 substrate Ac-DEVD-AMC (Sigma Aldrich St. Louis, MO) as described (Jaeschke et al., 1998).
Statistics
Statistical analysis was performed with Sigmaplot 8.0 (Systat Software, Inc., Chicago, IL). Data were assessed using one way ANOVA followed by Student-Newman-Keul’s post-hoc test for comparisons between means or Dunnett’s post-hoc test for comparisons to a control. For data not normally distributed, we used the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. P < 0.05 was considered significant.
RESULTS
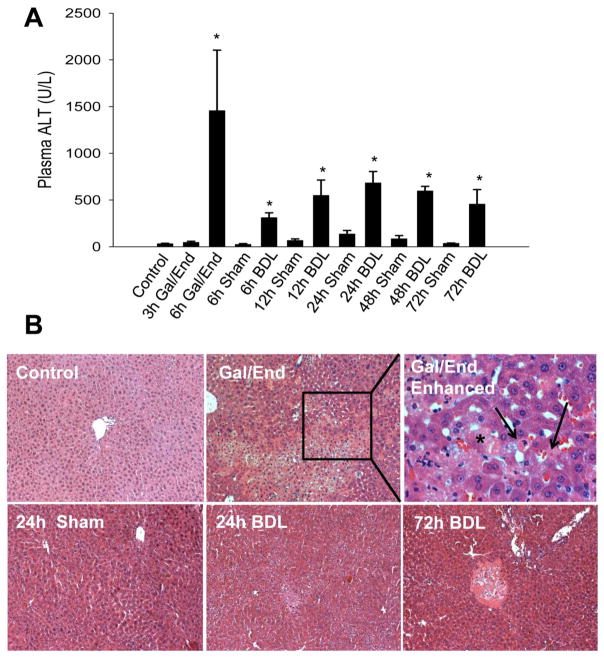
It is recognized that liver injury and bile infarcts peak between 48–72 h after BDL (Georgiev et al., 2008). Therefore, we investigated the time course of liver injury in C56Bl/6 mice between the onset of BDL and 72 h after BDL. Obstruction was confirmed by robust dilation of the gall bladder and the presence macroscopically of bile infarcts in the liver. ALT levels rose steadily through the first 24 hours before subsiding slightly at 48 h and 72 h after injury (Figure 1A). H&E staining confirmed the progressive increase of liver injury and showed the characteristic bile infarcts typically seen after BDL (Figure 1B). As an apoptosis control for these experiments, mice were treated for either 3 h or 6 h with 700 mg/kg D-galactosamine and 100 μg/kg endotoxin (Gal/End), a treatment regimen that is known to trigger TNF-mediated apoptosis (Leist et al., 1995) and inflammation-induced necrosis (Jaeschke et al., 1998). While there was no ALT release at 3 h, ALT levels were increased significantly 6 h post Gal/End treatment (Figure 1A). Histology indicated the presence of both necrotic and apoptotic cells (Figure 1B).
Figure 1.
Liver injury in C56Bl/6 mice after treatment with galactosamine/endotoxin (Gal/End), bile duct ligation (BDL) or sham-operation. A) Plasma alanine aminotransferase (ALT) activities after Gal/End, BDL or sham-operation (6–72h). Data represent mean ± SE of n=4–6 animals per group. *P<0.05 (compared to untreated control or sham. B) Representative H&E-stained liver sections at 100x or a 400x enhanced Gal/End picture. Apoptotic cells are marked with black arrows; a necrotic cell is indicated by an asterisk.
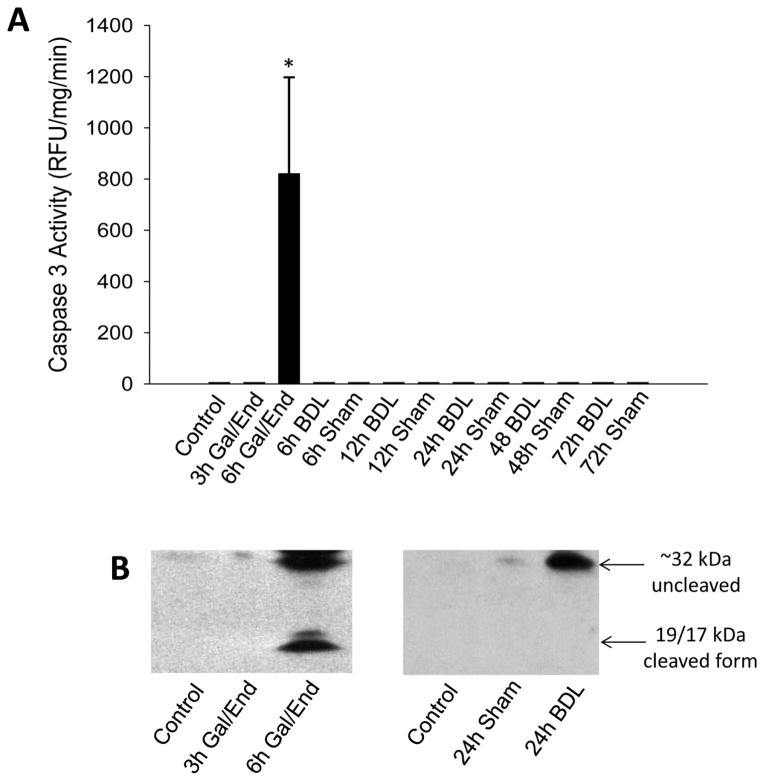
Because it is known that apoptotic cell death is a fairly rapid process and the resulting apoptotic bodies can be removed, it has been argued that the extent of apoptosis may be underestimated when tissue sections are evaluated at a single time point (Giucciardi and Gores, 2005). In order to overcome this problem, plasma biomarkers were evaluated. We have previously shown that during apoptosis, caspase-3 activities can be measured in serum after Gal/End in rats and mice (Gujral et al., 2003b; McGill et al., 2012) but not after APAP overdose in mice or humans (McGill et al., 2012). No evidence of caspase-3 activity in plasma was found at any time after BDL (Figure 2A). In contrast, substantial plasma caspase-3 activities were measured 6 h after Gal/End (Figure 2A). The plasma caspase-3 activity measurements were supported by western blots showing that both procaspase-3 and an active cleavage product were detectable in the 6 h Gal/End samples (Figure 2B). However, procaspase-3 proteins were only detected in BDL samples with substantial injury (e.g. 24 h) but not in sham-operated animals (Figure 2B).
Figure 2.
Assessment of apoptosis in C56Bl/6 mice after treatment with galactosamine/endotoxin (Gal/End), bile duct ligation (BDL) or sham-operation. A) Caspase-3 activity was measured in plasma after BDL or Gal/End treatment. Data represent mean ± SE of n=3 animals per group. *P<0.05 (compared to untreated control). B) Western blot of procaspase-3 (32 kD) and its active 19 and 17 kD fragments in plasma of an untreated control, 3 or 6 h after Gal/End or 24 h after BDL as a representative time point.
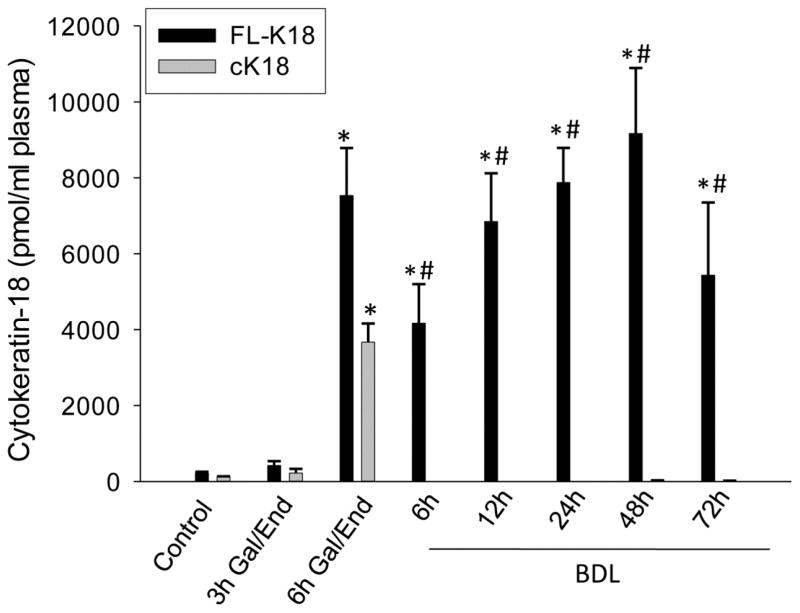
Cytokeratin 18 is a filamentous protein that can be released by damaged cells into the extracellular space reflecting cell death. The full-length protein (FL-K18) represents an unmodified cellular component that is passively released by necrotic cells whereas the release of a caspase-specific cleavage product of cytokeratin 18 (cK18) provides evidence for intracellular caspase activation and potentially apoptotic cell death (Biven et al., 2003). This method offers a wider analytical window enhancing the ability to simultaneously detect apoptosis and necrosis of epithelial cells. When FL-K18 and cK18 levels were measured in plasma of BDL and sham-operated mice, a progressive increase of FL-K18 levels was observed up to 48 h with a slight decline at 72 h after BDL (Figure 3). However, cK18 levels never exceeded sham-operated levels of <150 pmol/ml serum at any time after BDL (Figure 3). In contrast, both molecular forms of keratin-18 substantially increased during Gal/End-induced apoptosis and inflammation-induced necrosis at 6 h (Figure 3).
Figure 3.
Full length (FL-K18) and caspase-cleaved (cK18) cytokeratin-18 levels in plasma after galactosamine/endotoxin (Gal/End) treatment, bile duct ligation (BDL) or sham-operation. The values of sham-operation (<150 pmol/ml) were subtracted from the BDL values. Data represent mean ± SE of n=4–6 animals per group. *P<0.05 (compared to untreated control); #P<0.05 (compared to cK18 values)
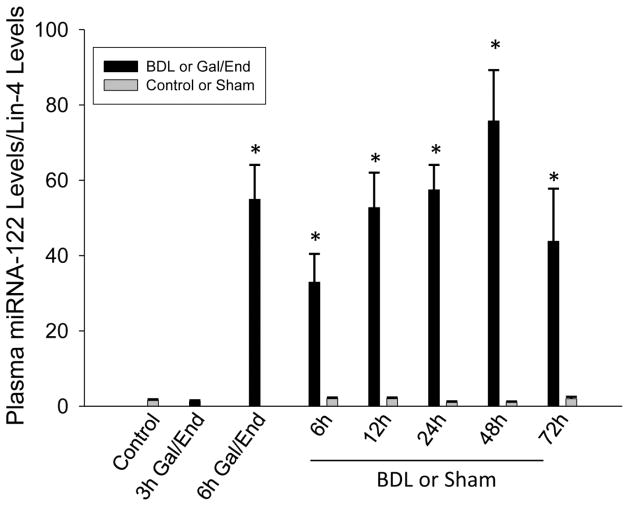
Serum miR-122 levels have been associated with APAP-induced necrosis in experimental animals and humans (Wang et al., 2009; Starkey Lewis et al., 2011). Similar to FL-K18, miR-122 levels increased progressively up to 48 h and moderately declined afterwards (Figure 4). Only traces of miR-122 were detectable in sham-operated animals. In addition, serum miR-122 levels increased substantially at 6 h after Gal/End (Figure 4).
Figure 4.
Plasma micro-RNA 122 (miR-122) levels were measured after galactosamine/endotoxin (Gal/End) treatment, bile duct ligation (BDL) or sham-operation. The values of miR-122 are expressed as ratio to the reference let-7d and the internal standard, Lin-4. Data represent mean ± SE of n=4–6 animals per group. *P<0.05 (compared to untreated control); #P<0.05 (compared to sham-operated control).
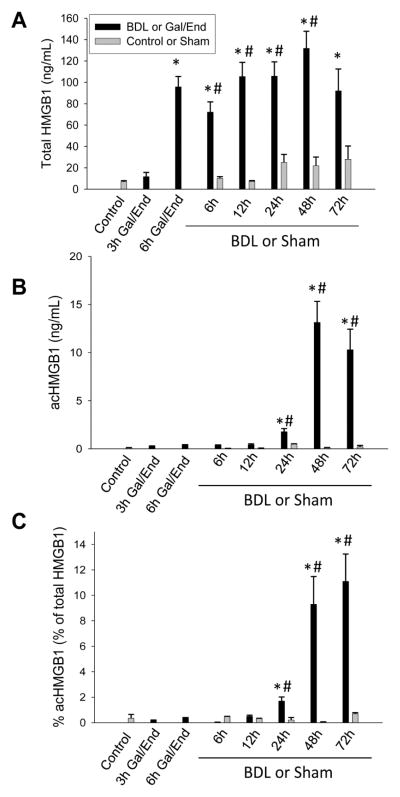
High mobility group box 1 (HMGB1) protein is a nuclear protein that can be passively released during necrotic cell death (Scaffidi et al., 2002) or actively secreted by activated inflammatory cells such as macrophages (Erlandsson Harris and Andersson, 2004). Recent work indicated that the acetylated form of HMGB1 is actively secreted mainly by inflammatory cells in response to inflammatory stimuli (Bonaldi et al., 2003). Total HMGB1 levels increased dramatically starting 6 h post BDL and further increased up to 48 h followed by a moderate decline at 72 h (Figure 5A). The increase in HMGB1 levels 6 h after Gal/End was similar to the BDL animals after surgery. In contrast to miR-122 and FL-K18, control and sham-operated animals showed low baseline levels of HMGB1 with some elevation 1–3 days after BDL (Figure 5A). Analysis of the acetylated form of HMGB1 (acHMGB1) demonstrated close to undetectable levels after Gal/End and at 6 h after BDL surgery (Figure 5B). AcHMGB1 levels were significantly increased at 12 h after BDL and peaked between 48 and 72 h (Figure 5B). However, as indicated by the percentage of acHMGB1 compared to the total HMGB1, the acetylated form was only a relevant component at later time points after BDL reaching levels of 10% of the total HMGB1 compliment (Figure 5C).
Figure 5.
Total (A) and acetylated (B) forms of plasma high mobility group box 1 (HMGB1) protein were measured after galactosamine/endotoxin (Gal/End) treatment, bile duct ligation (BDL) or sham-operation. C. Percentage of acHMGB1 compared to the total HMGB1 was calculated. Data represent mean ± SE of n=4–6 animals per group. *P<0.05 (compared to untreated control); #P<0.05 (compared to sham-operated control).
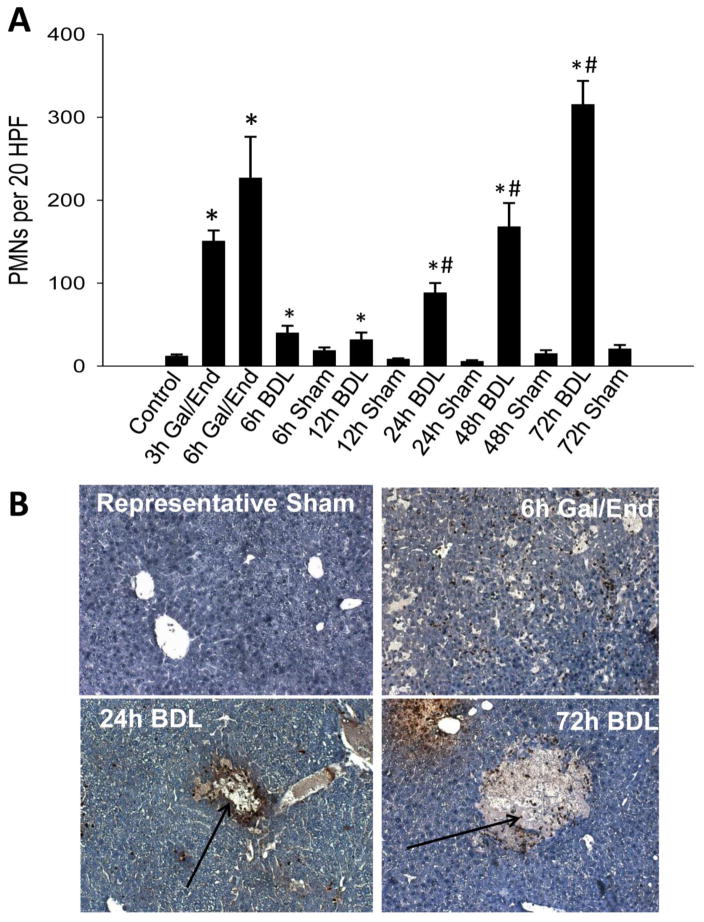
Previous studies suggested that neutrophils are critical for the injury after BDL in mice (Gujral et al., 2003a). To assess how this rise in HMGB1 levels correlated with neutrophil recruitment, neutrophil levels were quantified via immunohistochemistry in liver sections. Neutrophil counts were increased significantly in all BDL samples. The time course shows a moderate infiltration of neutrophils during the first 12 h and then a more progressive increase up to 72 h (Figure 6A). In contrast, high neutrophil levels were already observed 3 h after Gal/End with only a moderate further increase at 6 h (Figure 6A). Whereas neutrophils were located predominantly in sinusoids at 3 h (data not shown) and both in sinusoids and extravasated into the parenchyma at 6 h after Gal/End (Figure 6B), most neutrophils after 24–72 h after BDL are located within the area of necrosis (Figure 6B).
Figure 6.
Neutrophil accumulation in livers after galactosamine/endotoxin (Gal/End) treatment, bile duct ligation (BDL) or sham-operation. Neutrophils were stained using an anti-neutrophil allotypic antibody against Ly6B2. A) The numbers of neutrophils were counted in 20 randomly selected high power fields (HPF) per section. Data represent mean ± SE of n=4–6 animals per group. *P<0.05 (compared to untreated control); #P<0.05 (compared to sham-operated control). B) Representative images (x100) of stained liver sections are shown. After BDL, neutrophils were largely associated with areas of infarct (arrows).
DISCUSSION
The objective of the current investigation was to use plasma biomarkers to assess mode of cell death and inflammation over time in a murine model of obstructive cholestasis (BDL). In addition to the mechanistic insight, the results also provide reference data for the interpretation of translational studies in humans where plasma but no tissue is available in most cases (Antoine et al., 2012; McGill et al., 2012).
Apoptosis and Necrosis During Early Cholestatic Liver Injury
A predominant hypothesis in cholestatic liver injury remains the idea that hydrophobic bile salts such as GCDC induce apoptosis in hepatocytes (Guicciardi and Gores, 2005; Maillette de Buy Wenniger and Beuers, 2010). While this mechanism applies to cell death of cultured rat hepatocytes exposed to GCDC in vitro (Spivey et al., 1993; Patel et al., 1995; Yerushalmi et al., 2001; Graf et al., 2002; Schoemaker et al., 2004; Rust et al., 2006), caspase levels and apoptosis after BDL in rats in vivo are very limited mainly due to the induction of anti-apoptotic proteins (Schoemaker et al., 2003). In mice, conclusions of apoptotic cell death after BDL were mainly based on TUNEL-positive cells and the protection in Fas receptor-deficient lpr mice (Miyoshi et al., 1999). However, no relevant caspase activation was detected after BDL and caspase inhibitors, which were highly effective in the Gal/End or Fas antibody-induced apoptosis models, did not protect against BDL-induced liver injury (Gujral et al., 2004b). The current biomarker data using full length cytokeratin-18 (FL-K18) and the caspase cleavage product (cK18), clearly indicate a lack of caspase activation from the onset of BDL to the peak of tissue damage at 2–3 days. In contrast, the dramatic increase of FL-K18 levels in plasma indicates extensive cell necrosis. Importantly, the parallel assessment of these parameters before and during liver injury after treatment with Gal/End, a model of TNF-induced parenchymal cell apoptosis and inflammation-induced necrosis (Leist et al., 1995; Jaeschke et al., 1998; Lawson et al., 1998), confirmed that substantial amounts of FL-K18 and of the caspase-cleaved fragments are released into the plasma during active necrotic and apoptotic cell death. Thus, based on the biomarker data it can be concluded that the liver injury during the first 3 days after BDL consists almost exclusively of necrosis and not apoptosis. This conclusion was further confirmed by the presence of uncleaved pro-caspase-3 in plasma after BDL and not of the active fragment. Again, the positive control for apoptosis (Gal/End) showed the presence of both pro-caspase-3 and the active fragments in plasma.
Although these plasma biomarker data are consistent and correlate with a number of tissue evaluations concluding the absence of apoptosis in the murine BDL model (Gujral et al., 2004b; Fickert et al., 2005; Nalapareddy et al., 2009; Mitchell et al., 2011), there are in vivo studies that claim a dominant role of apoptotic cell death (Miyoshi et al., 1999; Higuchi et al., 2001). Most of these studies rely on the TUNEL assay as an indicator of apoptosis. However, the TUNEL assay detects DNA strand breaks, which occur during apoptosis but also during necrotic cell death (Grasl-Kraupp et al., 1995; Gujral et al., 2001) suggesting that this assay is not specific for apoptosis. In addition, the argument that the Fas receptor-deficient lpr mouse is protected is not proof for apoptotic cell death (Miyoshi et al., 1999). Lpr mice show reduced inflammatory injury during BDL (Gujral et al., 2004b) and have increased Nrf2 activation, which makes them less susceptible to oxidant stress-induced injury (Williams et al., 2013). Thus, off-target effects appear to be responsible for the protection against BDL-induced injury in these mice. Likewise, the protection after anti-sense treatment knocking down the pro-apoptotic protein Bid (Higuchi et al., 2001) could not be reproduced in Bid-deficient mice (Nalapareddy et al., 2009) and therefore, off target effects need to be considered.
Circulating microRNAs have been used as biomarkers for various disease processes. MiR-122 is particularly enriched in the liver (Chang et al., 2004) and was shown to be a sensitive indicator of liver injury after APAP overdose in mice (Wang et al., 2009) and humans (Starkey Lewis et al., 2011; Antoine et al., 2013; Dear et al., 2013). APAP-induced cell injury involves predominantly necrosis in both species (Gujral et al., 2002; McGill et al., 2012; Antoine et al., 2012). Our data showed a progressive increase of miR-122 levels in plasma after BDL but not after sham-operation. The time course of miR-122 release after BDL was very similar to the necrosis marker FL-K18. In addition, miR-122 levels increased 6 h after Gal/End, which involves both apoptotic and necrotic cell death at this time (Leist et al., 1995; Jaeschke et al., 1998). Thus, the release of miR-122 further supports the conclusion of extensive necrotic cell death after BDL.
A third marker of necrosis used in our study was the nuclear protein and damage associated molecular pattern (DAMP), HMGB1, which, according to current theory, is not released during apoptotic cell death (Scaffidi et al., 2002). Again, HMGB1 levels are increased during necrotic cell death after APAP overdose in mice (Scaffidi et al., 2002; Antoine et al., 2009; Williams et al., 2011) and humans (Antoine et al., 2012, 2013). Total HMGB1 levels dramatically increased during BDL but plasma levels changed only marginally after sham-operation. The time course, progressive increase up to 48 h after BDL and then a moderate decline, was similar to FL-K18 and miR-122 levels. Together all 3 plasma biomarkers consistently indicate necrotic cell death after BDL and plasma caspase activities and cK18 levels demonstrate the lack of apoptosis. These plasma biomarkers accurately reflect what can be observed by histology with the added advantage of representing a more global picture for the entire liver.
Neutrophils and Inflammation as a Cause of Cholestatic Liver Injury
Our previous studies provided strong evidence for neutrophilic inflammation being the key mechanism of BDL-induced liver injury in the mouse (Gujral et al., 2003a, 2004a; Kim et al., 2006; Allen et al., 2011). Neutrophils cause target cell necrosis by generating reactive oxygen (Jaeschke, 2006; 2011). BDL triggers progressive recruitment of neutrophils into the liver causing extensive oxidant stress and cell death (Gujral et al., 2003a, 2004a). HMGB1 is a known damage associated molecular pattern (DAMP), which can bind to toll like receptor 4 (TLR4) and the receptor for advanced glycation end products (RAGE) and induce generation of pro-inflammatory mediators, which can activate and recruit leukocytes to sites of inflammation (Yang et al., 2013a). However, the fact that TLR4-deficient mice did not show reduced hepatic neutrophil accumulation and injury after BDL did not support a role of HMGB1 or endotoxin in neutrophil recruitment (Allen et al., 2011). In addition, the release of HMGB1 from necrotic cells and the formation of pro-inflammatory cytokines and chemokines by macrophages in the circulation are inconsistent with the focal bile infarcts caused by neutrophils. It is more likely that chemotactic factors are derived from bile as the extensive pressure building up during bile duct obstruction leads to local ruptures of cholangioles and leakage of bile into the parenchyma (Fickert et al., 2002). Preliminary data from our laboratory have suggested that chemotactic osteopontin fragments in bile can initiate the neutrophilic inflammatory response after BDL (Yang et al., 2011).
The acetylated form of HMGB1 is known to be actively secreted by monocytes/macrophages (Bonaldi et al., 2003; Erlandsson Harris and Andersson, 2004). Our data indicate that only traces of acHMGB1 are being released during the first 24 h after BDL. However, at 48 and 72 h approximately 10% of the circulating HMGB1 proteins are acetylated. This suggests increasing monocyte/macrophage activation at 2–3 days after BDL. Moreover, the delayed release of HMGB1 and detection in the circulation compared to TNFα has been shown in murine models of sepsis (Wang et al., 1999). Indeed, inflammatory mononuclear phagocytes are detectable in the liver 3 days after BDL in mice (Duwaerts et al., 2013). Our current acHMGB1 time course data are highly consistent with the presence of mononuclear phagocytes and the delayed release of HMGB1 from activated immune cells. These cells are in a more pro-inflammatory state than the resident macrophages (Kupffer cells) at this time (Duwaerts et al., 2013). This would suggest that these infiltrating monocytes are more likely the source of acHMGB1 than the resident Kupffer cells. Nevertheless, a systemically released mediator like HMGB1 cannot direct neutrophils to focal areas of necrosis and, therefore, may not be a relevant therapeutic target. Thus, the passively released, hypoacetylated form of HMGB1 is a sensitive marker of cell death and the hyperacetylated form indicates monocyte/macrophage cell activation during obstructive cholestasis. Translational studies may be warranted to assess if plasma HMGB1 levels, as demonstrated for APAP overdose (Antoine et al., 2012), may predict negative clinical outcome (acute liver failure and death).
Summary and Conclusions
Using the plasma biomarkers full length cytokeratin-18 (FL-K18), miR-122 and HMGB1, we demonstrated progressive necrotic cell death after BDL in mice. Each of these necrosis biomarkers was more responsive than plasma ALT. On the other hand, using the apoptosis biomarkers plasma caspase-3 enzyme activities, active caspase-3 fragments and the caspase-cleaved fragment of cytokeratin-18 (cK18), no evidence for apoptotic cell death was detected within the 3 day period after BDL. In contrast, in a positive control for apoptosis and necrosis, the Gal/End model, these parameters were readily detectable. The findings with the plasma biomarkers after BDL or Gal/End are in full agreement with histological assessments in the liver. Thus, the panel of plasma biomarkers indicates that liver cell death after BDL is caused exclusively by necrosis. In addition, these biomarkers may be useful to assess the mechanisms of cell death after obstructive cholestasis in humans which may ultimately lead to new therapies and stratified medicine.
Highlights.
The mechanism of cell death during cholestasis remains a controversial topic.
Plasma biomarkers offer new insight into cell death after bile duct ligation.
Cytokeratin-18, microRNA-122 and HMGB1 levels implicate necrosis.
Acetylated HMGB1 levels rise late after BDL confirming inflammation.
BDL-induced liver injury involves mainly inflammation, necrosis but no apoptosis.
Acknowledgments
This work was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916, and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM12345 and, from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 from the National Institute of Environmental Health Sciences. DJA, REJ and BKP would like to acknowledge the financial support from the Medical Research Council (G0700654). DJA would also like to acknowledge additional financial support from a Wellcome Trust Research Fellowship.
Abbreviations
- ALT
alanine aminotransferase
- BDL
bile duct ligation
- cK18
caspase-cleaved cytokeratin-18 fragment
- DAMP
damage associated molecular pattern
- FL-K18
full length cytokeratin-18
- H&E
hematoxylin and eosin
- HMGB1
high mobility group box 1
- Gal/End
galactosamine/endotoxin
- GCDC
glycochenodeoxycholate
- ICAM-1
intercellular adhesion molecule-1
- miR-122
micro RNA-122
- TNF-α
tumor necrosis factor-alpha
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, Park BK. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112:521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16:479–490. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bivén K, Erdal H, Hägg M, Ueno T, Zhou R, Lynch M, Rowley B, Wood J, Zhang C, Toi M, Shoshan MC, Linder S. A novel assay for discovery and characterization of pro-apoptotic drugs and for monitoring apoptosis in patient sera. Apoptosis. 2003;8:263–268. doi: 10.1023/a:1023672805949. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R, Zaret KS, Taylor JM. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- Dear JW, Antoine DJ, Starkey-Lewis P, Goldring CE, Park BK. Letter to the Editor: Early detection of paracetamol toxicity using circulating liver microRNA and markers of cell necrosis. Br J Clin Pharmacol. 2013 doi: 10.1111/bcp.12214. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duwaerts CC, Gehring S, Cheng CW, van Rooijen N, Gregory SH. Contrasting responses of Kupffer cells and inflammatory mononuclear phagocytes to biliary obstruction in a mouse model of cholestatic liver injury. Liver Int. 2013;33:255–265. doi: 10.1111/liv.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34:1503–1512. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- Fickert P, Trauner M, Fuchsbichler A, Zollner G, Wagner M, Marschall HU, Zatloukal K, Denk H. Oncosis represents the main type of cell death in mouse models of cholestasis. J Hepatol. 2005;42:378–385. doi: 10.1016/j.jhep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H, Trauner M. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- Georgiev P, Jochum W, Heinrich S, Jang JH, Nocito A, Dahm F, Clavien PA. Characterization of time-related changes after experimental bile duct ligation. Br J Surg. 2008;95:646–656. doi: 10.1002/bjs.6050. [DOI] [PubMed] [Google Scholar]
- Graf D, Kurz AK, Reinehr R, Fischer R, Kircheis G, Häussinger D. Prevention of bile acid-induced apoptosis by betaine in rat liver. Hepatology. 2002;36:829–839. doi: 10.1053/jhep.2002.35536. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003a;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Farhood A, Jaeschke H. Oncotic necrosis and caspase-dependent apoptosis during galactosamine-induced liver injury in rats. Toxicol Appl Pharmacol. 2003b;190:37–46. doi: 10.1016/s0041-008x(03)00154-6. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004a;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology. 2004b;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Gores GJ. Bile acid regulation of hepatic physiology: IV. Bile acids and death receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G734–G738. doi: 10.1152/ajpgi.00491.2002. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Miyoshi H, Bronk SF, Zhang H, Dean N, Gores GJ. Bid antisense attenuates bile acid-induced apoptosis and cholestatic liver injury. J Pharmacol Exp Ther. 2001;299:866–873. [PubMed] [Google Scholar]
- Jaeschke H. Mechanisms of Liver Injury. II Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–3486. [PubMed] [Google Scholar]
- Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- Kim WR, Ludwig J, Lindor KD. Variant forms of cholestatic diseases involving small bile ducts in adults. Am J Gastroenterol. 2000;95:1130–1138. doi: 10.1111/j.1572-0241.2000.01999.x. [DOI] [PubMed] [Google Scholar]
- Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Parenchymal cell apoptosis as a signal for sinusoidal sequestration and transendothelial migration of neutrophils in murine models of endotoxin and Fas-antibody-induced liver injury. Hepatology. 1998;28:761–767. doi: 10.1002/hep.510280324. [DOI] [PubMed] [Google Scholar]
- Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55 kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J Immunol. 1995;154:1307–1316. [PubMed] [Google Scholar]
- Maillette de Buy Wenniger L, Beuers U. Bile salts and cholestasis. Dig Liver Dis. 2010;42:409–418. doi: 10.1016/j.dld.2010.03.015. [DOI] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Mahrouf-Yorgov M, Mayeuf A, Robin MA, Mansouri A, Fromenty B, Gilgenkrantz H. Overexpression of Bcl-2 in hepatocytes protects against injury but does not attenuate fibrosis in a mouse model of chronic cholestatic liver disease. Lab Invest. 2011;91:273–282. doi: 10.1038/labinvest.2010.163. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- Nalapareddy P, Schüngel S, Hong JY, Manns MP, Jaeschke H, Vogel A. The BH3-only protein bid does not mediate death-receptor-induced liver injury in obstructive cholestasis. Am J Pathol. 2009;175:1077–1085. doi: 10.2353/ajpath.2009.090304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T, Bronk SF, Gores GJ. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest. 1994;94:2183–2192. doi: 10.1172/JCI117579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupon R, Chazouillères O, Poupon RE. Chronic cholestatic diseases. J Hepatol. 2000;32:129–140. doi: 10.1016/s0168-8278(00)80421-3. [DOI] [PubMed] [Google Scholar]
- Rust C, Wild N, Bernt C, Vennegeerts T, Wimmer R, Beuers U. Bile acid-induced apoptosis in hepatocytes is caspase-6-dependent. J Biol Chem. 2009;284:2908–2916. doi: 10.1074/jbc.M804585200. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schoemaker MH, Conde de la Rosa L, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology. 2004;39:1563–1573. doi: 10.1002/hep.20246. [DOI] [PubMed] [Google Scholar]
- Schoemaker MH, Gommans WM, Conde de la Rosa L, Homan M, Klok P, Trautwein C, van Goor H, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol. 2003;39:153–161. doi: 10.1016/s0168-8278(03)00214-9. [DOI] [PubMed] [Google Scholar]
- Spivey JR, Bronk SF, Gores GJ. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J Clin Invest. 1993;92:17–24. doi: 10.1172/JCI116546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DG, Antoine DJ, French NS, Dhaun N, Webb DJ, Costello EM, Neoptolemos JP, Moggs J, Goldring CE, Park BK. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54:1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- Trottier J, Białek A, Caron P, Straka RJ, Milkiewicz P, Barbier O. Profiling circulating and urinary bile acids in patients with biliary obstruction before and after biliary stenting. PLoS One. 2011;6:e22094. doi: 10.1371/journal.pone.0022094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Antoine DJ, Shaw PJ, Benson C, Farhood A, Williams DP, Kanneganti TD, Park BK, Jaeschke H. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol Appl Pharmacol. 2011;252:289–297. doi: 10.1016/j.taap.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, McGill MR, Farhood A, Jaeschke H. Fas receptor-deficient lpr mice are protected against acetaminophen hepatotoxicity due to higher glutathione synthesis and enhanced detoxification of oxidant stress. Food Chem Toxicol. 2013;58:228–235. doi: 10.1016/j.fct.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol. 2012;18:4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013a;93:865–873. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Köck K, Sedykh A, Tropsha A, Brouwer KL. An updated review on drug-induced cholestasis: Mechanisms and investigation of physicochemical properties and pharmacokinetic parameters. J Pharm Sci. 2013b May 7; doi: 10.1002/jps.23584. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Ramachandran A, Yan HM, Fickert P, Trauner M, Jaeschke H. Osteopontin promotes neutrophil extravasation and initiates liver injury through a MMP3-mediated pathway in obstructive cholestasis in mice (abstract) Hepatology. 2011;54(Suppl 1):772A. [Google Scholar]
- Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001;33:616–626. doi: 10.1053/jhep.2001.22702. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]