Highlights
► First case of port-site metastasis after robotic staging surgery for uterine cancer. ► Changes to robotic surgical technique to reduce risk of port-site recurrence. ► Further areas of investigation worth examining in this aspect of robotic surgery.
Keywords: Robotic surgery, Gynecologic oncology, Port-site metastasis, Port-site recurrence, Robot-assisted
Introduction
The surgical management of gynecologic malignancy has traditionally been via laparotomy. Over the past few decades, the use of laparoscopy has been increasingly incorporated into the surgical management of gynecologic cancers. More recently, the use of robotic surgical systems has enabled the surgeon to perform more complicated procedures with improved dexterity and precision. The use of minimal access surgery is associated less morbidity, faster recovery, shorter hospitalization stay and increased patient satisfaction.
With the increased use of minimal access surgery in gynecologic cancer surgery, there has been a rising concern about the complication of recurrent disease at the laparoscopic port sites. Reymond et al. (1998) defined port-site metastasis as “early tumor recurrences that develop locally in the abdominal wall, within the scar tissue of one or more trocar sites or an incision wound after laparoscopy and these should not be associated with peritoneal carcinomatosis.” Since the first case of port-site metastasis was described by Dobronte et al. (1978) in 1978 after a diagnostic laparoscopy, there have been numerous reports of similar cases post-laparoscopic surgery although there is limited information available on the incidence of port site recurrences after robotic surgery.
The incidence of port-site metastasis after conventional laparoscopy is reported to be about 1–2% (Ramirez et al., 2003, 2004; Zivanovic et al., 2008). This is comparable to the incidence of metastases to drain sites and surgical incisions post-laparotomy. There are scant data on port site metastasis following robotic surgery for female genital tract cancers. Nodofor et al. (2011) reported a rate of 1.1% in a review of 181 patients undergoing robotic surgery for gynecologic malignancies. Other than this study, Sert (2010) described an unusual case of robotic port-site and pelvic recurrences after robotic radical hysterectomy for cervical cancer. Here we describe a case of port-site metastasis after robotic surgery (robot-assisted radical hysterectomy) for endometrial cancer.
Case report
A 72-year old, multiparous woman who had never been on hormone replacement therapy was referred for postmenopausal bleeding. Clinical examination found friable necrotic tissue at the endocervical canal which was arising from beyond the cervix. An outpatient endometrial biopsy confirmed a poorly-differentiated adenocarcinoma. The magnetic resonance imaging (MRI) scan showed a uterocervical mass invading more than 50% of the myometrium and involving the stroma of the upper half of the cervix. There were no significantly enlarged lymph nodes on the MRI scan. The patient underwent a robot-assisted modified radical hysterectomy with bilateral salpingo-oophorectomy, omentectomy and pelvic lymphadenectomy. The da Vinci® surgical system (Intuitive Surgical, Sunnyvale, CA) with 3 arms was used. In addition to the 12 mm supra-umbilical camera port, 2 instrument ports of 8 mm each were placed 10 cm lateral to the umbilicus on each side and one 5 mm assistant port was placed at the right upper quadrant. This was the fifty-eighth patient undergoing robot-assisted surgery for endometrial cancer. An intraoperative carbon dioxide (CO2) pneumoperitoneum of 12–14 mm Hg was maintained throughout the surgery. The primary specimen being the uterus, fallopian tubes and ovaries was removed en bloc through the vagina without using a specimen bag. The lymph nodes were removed via a Lina-McCartney tube device in the vagina. The estimated blood loss was 150mls. The histopathological report diagnosis was that of a serous adenocarcinoma of the endometrium grade 3 with more than 50% myometrial invasion into the outer half of the myometrium, cervical stromal involvement and extension in to the right parametrial tissue. Resection margins were clear and there was no evidence of malignancy in the pelvic lymph nodes, omentum or peritoneal washings. The patient was assigned a FIGO (2009) stage of IIIB (Creasman, 2009). The patient was offered the tumor group's recommendation of adjuvant chemotherapy followed by pelvic irradiation. The patient however, declined chemotherapy but received external beam whole pelvic radiation therapy and also brachytherapy. Five months after primary treatment, during routine clinical examination, 2 subcutaneous nodules, each about 4 cm in size, were found on the abdomen corresponding to the sites of the (8 mm) instrument ports. A computed tomography (CT) scan of the abdomen and pelvis showed solid-cystic lesions in the anterior abdominal wall and tiny peritoneal nodules (Fig. 1). There was no evidence of local recurrence at the vault or pelvis. A CT thorax was also done which was unremarkable. The patient had an ultrasound-guided fine needle aspiration of the subcutaneous nodule in the right abdominal wall and the cytology of this revealed a metastatic adenocarcinoma. She then underwent excision of both subcutaneous nodules and each tumor was found to be 5 × 6 cm in size, both involving the subcutaneous fat, fascia and muscle (Figs. 2, 3). The tumors were excised completely and the pathological examination found metastatic serous adenocarcinomas with similar histological features to those seen in the primary tumor specimen. Chemotherapy was subsequently initiated and the patient received six cycles of carboplatin and paclitaxel. A repeat CT scan after the third cycle of chemotherapy showed interval resolution in the previously noted peritoneal nodules as well as a reduction in size of the abdominal wall metastases. The patient is due to receive local radiotherapy on the metastatic sites.
Fig. 1.
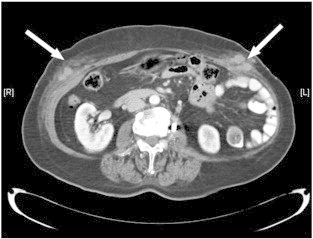
CT scan of the abdomen showing the port-site metastases (arrows).
Fig. 2.
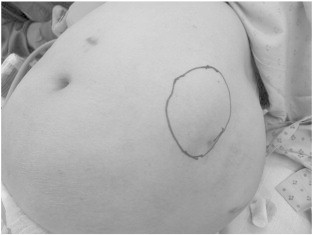
Pre-operative marking (circle) of the port-site metastasis on the left abdominal wall.
Fig. 3.
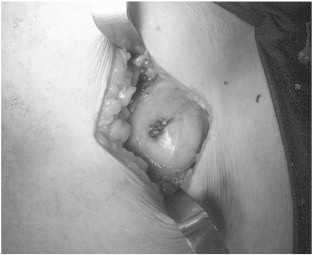
Intra-operative view of port-site metastatic tumor.
Discussion
In the current literature, there is a paucity of data regarding the incidence of port-site metastasis after robotic surgery for gynecological malignancy. So far, only one study by Nodofor et al. (2011) has studied port-site metastasis after robotic procedures for suspected gynecological malignancies and reported the incidence to be low at 1.1% (2 cases in 181 surgeries), which is comparable to conventional laparoscopic surgery. In that study, the first case of port-site metastasis described a robot-assisted hysterectomy and bilateral salpingo-oophorectomy for a left adnexal mass and final pathology revealed a metastatic gallbladder adenocarcinoma to the left ovary. The second case of port site metastasis was an endometrial adenocarcinoma metastatic to the cervix and the adnexae. The patient underwent a robot-assisted total hysterectomy and salpingo-oophorectomy after chemotherapy and pelvic irradiation. In each of the above cases, port-site metastasis occurred 3 weeks and 11 months respectively after the robotic surgeries.
Our case report is the first case of port-site metastasis following robot-assisted standard endometrial cancer staging surgery. Our patient underwent a modified radical hysterectomy, bilateral salpingo-oophorectomy, pelvic lymphadenectomy and omentectomy. In our patient, it is likely that the port sites were not the only sites of recurrence with peritoneal nodules noted on CT, although these nodules were not histologically investigated.
A number of mechanisms have been proposed for the development of port-site metastasis including hematogenous spread, direct wound contamination due to tumor implantation, the effects of CO2 pneumoperitoneum, aerosolization of tumor cells (“chimney effect”), local immune reaction and most importantly, surgical technique. Patient selection is also a crucial factor as more advanced disease is associated with an increased likelihood of port-site metastasis (Pearlstone et al., 1999).
Several preventive measures have been proposed to reduce the occurrence of port-site metastasis including minimizing tissue trauma and the transfer of instruments, rinsing the trocars in povidone-iodine and the instrument tips when changing instruments, resecting the tumor with disease-free margins, the use of specimen bags for retrieval, removing all intra-abdominal fluid before trocar removal, irrigation of trocar sites with povidone-iodine and closure of peritoneal trocar sites of 10 mm or more. For all our robotic cases since this case, we now routinely rinse all the trocar sites. There is also minimal instrument change with one transfer usually during the closure of the vault when a needle driver is substituted for the “hot shears” monopolar scissors held in the “left hand”. In this case, the tumor resection margins were clear as well. Perhaps for future cases, we will implement further preventive techniques like utilizing a lower CO2 pneumoperitoneum pressure and a protective bag for specimen retrieval.
We started out with a practice of routinely infiltrating 1% marcaine into the subcutaneous tissue as a prelude to port insertion. This was done to improve patient analgesia. We have stopped doing this after this case of port site metastasis. We felt that this practice could create a potential space for tumor to seed, especially under the pressure of a pneumoperitoneum. We have empirically adopted this approach based on a biologically plausible mechanism and it would be worthwhile investigating this in future studies. A subcutaneous emphysema could also be a potential space in which tumor could seed and clinical factors that result in the formation of a subcutaneous emphysema could also potentially increase the risk of port site metastasis or metastasis to the subcutaneous tissue. Finally, the use of powered cutting is an aspect that could be explored in contributing to aerosolizing the tumor and predisposing to port-site metastasis. Changes in practice were based on data gleaned from the laparoscopic literature as port-site metastasis for robot-assisted endometrial cancer staging has not been previously reported. We modified our practice based on empirical derivations of possible mechanisms for port site metastases such as intra-abdominal pressure and the possibility of the injection of local anesthetic in the pre-peritoneal space before port insertion creating a potential space for tumor and bacteria to seed.
Robot-assisted surgery is a relatively new treatment modality in gynecology with the US FDA approving it for gynecological surgery in 2005. As more gynecological procedures, both benign and for cancer, are performed with robot-assistance there will be an exponential growth in clinical experience and the corresponding body of evidence with regards to outcomes and complications. At this juncture in robotic gynecological oncology surgery, it is difficult to ascertain if the risk for port-site metastasis is any different than traditional laparoscopic surgery as we are reporting the very first documented case of port-site metastasis associated with robotic surgery for endometrial cancer staging. Although it would be reasonable to assume that robot-assisted surgery should be no different than traditional laparoscopy, this is an empiric observation, and an outcome that should be continually monitored as experience with robotic surgery in endometrial cancer continues to increase and as this modality continues to become ubiquitous. We hope that our case presentation contributes to this growing clinical experience, stimulates further investigation and ultimately improves patient outcomes.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int. J. Gynaecol. Obstet. 2009;105(2):109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Dobronte Z., Wittmann T., Karacsony G. Rapid development of malignant metastases in the abdominal wall after laparoscopy. Endoscopy. 1978;10:127–130. doi: 10.1055/s-0028-1098280. [DOI] [PubMed] [Google Scholar]
- Nodofor B.T., Soliman P.T., Schmeler K.M., Nick A.M., Frumovitz M., Ramirez P.T. Rate of port-site metastasis is uncommon in patients undergoing robotic surgery for gynecological malignancies. Int. J. Gynecol. Cancer. 2011;21(5):936–940. doi: 10.1097/IGC.0b013e3182174609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstone D.B., Mansfield P.F., Curley S.A., Kumparatana M., Cook P., Feig B.W. Laparoscopy in 533 patients with abdominal malignancy. Surgery. 1999;125:67–72. [PubMed] [Google Scholar]
- Ramirez P.T., Wolf J.K., Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol. Oncol. 2003;91:179–189. doi: 10.1016/s0090-8258(03)00507-9. [DOI] [PubMed] [Google Scholar]
- Ramirez P.T., Frumovitz M., Wolf J.K., Levenback C. Laparoscopic port-site metastases in patients with gynaecological malignancies. Int. J. Gynecol. Cancer. 2004;14(6):1070–1077. doi: 10.1111/j.1048-891X.2004.14604.x. [DOI] [PubMed] [Google Scholar]
- Reymond M.A., Schneider C., Kastl S., Hohenberger W., Kockerling F. The pathogenesis of port-site recurrences. J. Gastrointest. Surg. 1998;2:406–414. doi: 10.1016/s1091-255x(98)80030-9. [DOI] [PubMed] [Google Scholar]
- Sert B. Robotic port-site and pelvic recurrences after robot-assisted laparoscopic radical hysterectomy for a stage IB1 adenocarcinoma of the cervix with negative lymph nodes. Int. J. Med. Robot. 2010;6(2):132–135. doi: 10.1002/rcs.295. [DOI] [PubMed] [Google Scholar]
- Zivanovic O., Sonoda Y., Diaz J.P., Levine D.A., Brown C.L., Chi D.S., Barakat R.R., Abu-Rustum N.R. The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol. Oncol. 2008;111(3):431–437. doi: 10.1016/j.ygyno.2008.08.024. [DOI] [PubMed] [Google Scholar]


