Abstract
Objective
To study the associations between diet, exercise. and the serum lipid profile.
Materials and methods
Hospital based cross-sectional study. The study participants were selected through purposive sampling. The study participants comprised 316 men and women above 20 years of age from a disease-free cohort and included healthy subjects visiting the lifestyle clinic of CARE Hospitals, Hyderabad, India for health check-up.
Results
Among the participants of the study, 28.5% of the males and 42.2% of the females had hypercholesterolaemia. Body weight was significantly associated with total cholesterol and low-density lipoprotein (LDL) cholesterol. Of the subjects studied, males had a higher mean calorie and fat intake than the females. A positive association was observed between waist circumference and both total cholesterol and LDL cholesterol. Waist circumference was also positively correlated with systolic and diastolic blood pressure and triglycerides. There was a significant difference in the total cholesterol levels of subjects who exercised and those who were not involved in any physical activity. There was a significant difference between the high-density lipoprotein (HDL) cholesterol values of the subjects based on exercise levels. High-density lipoprotein cholesterol levels were significantly higher in males than in females and this is corroborated by the finding of increased exercise levels in males. Duration of exercise had a significant impact on the total cholesterol levels.
Conclusion
Our results confirm that diet and exercise routines significantly affect the serum lipid profile. Obesity and overweight constitute a risk factor for the development of hypercholesterolaemia and hypertriglyceridaemia.
Keywords: Cholesterol, Diet, Lipid profile, Obesity
Introduction
In the recent years India and other developing countries have witnessed a rapidly escalating epidemic of cardiovascular disease (CVD). It is predicted that, by 2020, coronary heart disease will be the leading cause of death in adult Indians.1
The increasing prevalence of overweight and obesity constitutes a major health crisis in India because of the associated increase in risk of coronary heart disease—approximately 12% of Indian males and 16% of Indian females are obese.2 Recent studies have indicated that the life expectancy of adults with severe obesity might be 15–20 years lower than normal individuals. A significant proportion of morbidity and mortality in obese adults are due to sudden cardiac arrest and congestive heart failure related to obesity.3
It has been reported that among others, smoking, dietary habits, and physical inactivity account for most of the risk of myocardial infarction worldwide in both sexes and at all ages in all regions.4 Physical activity and physical fitness have been identified as protective factors against the occurrence and progression of coronary heart disease and against premature mortality. Such associations among other factors have been related to improvement in the lipid profile.5
Lipid abnormalities are a widely accepted risk factor for ischaemic heart disease.6 Factors such as obesity, dietary changes and changes in exercise routines can influence adult lipid levels.7 There is a need to look at the diet of individuals in combination with their actual food intake in order to apply interventions that are effective at controlling their serum lipid profile, which is one of the major risk factors of CVD. The purpose of this study was to evaluate the role of diet and other lifestyle-related factors in the prevalence of hypercholesterolaemia in Indians.
Materials and methods
The study participants comprised of 316 males and females above 20 years of age, who were selected through purposive sampling. The mean age of the subjects was 42 years. The study was carried out in CARE Hospitals, Hyderabad city located in the Southern State of Andhra Pradesh in India. Healthy subjects visiting the lifestyle clinic of the hospital for health check-ups were chosen after obtaining their informed consent. Subjects with a history of CVD or diabetes mellitus as well as those with alcohol consumption greater than 80 g/day or long-term medication use were excluded from the study. The purpose of the study was explained to the potential subjects who visited the hospital for health check-up. The hospital's research and ethical committee approved of the study's procedures. The subjects had an individual interview along with blood collection and anthropometric assessment. Participation in the study was voluntary and all participants provided written informed consent. Information on food habits and dietary pattern was obtained by using a detailed interview schedule. Information on physical activity, i.e. time spent on exercise per day was collected. The type of exercise indulged in, i.e. aerobics, walking, jogging, swimming, etc. was also ascertained.
Anthropometry
Weight, height, waist circumference and fat fold thickness were recorded using standard procedures. Weight was measured using a Salter brand electronic digital scale (Model 920, Max capacity 150 kg). Height (to the nearest 0.1 cm) was measured using a wall fixed stadiometer (CMS Instruments, London). The triceps skin fold thickness was measured with a Slimguide skin fold callipers (Galaxy Informatics, India). Waist circumference was measured with an inelastic tape (Girth Measurer, Galaxy Informatics, India) used at the narrowest part of the torso at the end of expiration. Waist circumference of ≥90 cm was considered as the risk level for males and ≥80 cm for females. Body mass index (BMI) was computed and BMI of 23.0 was considered as the cut-off level for assessing the prevalence of overweight or obesity. The hip circumference was measured at the widest point of the buttocks by using an inelastic tape (girth measurer, Galaxy Informatics, India). The waist–hip ratio (WHR) was calculated.
Laboratory methods
Blood samples were taken in the morning after a fasting period of 10–14 hours. Fasting serum total cholesterol and triglyceride were assayed enzymatically. Cholesterol concentrations were determined in the Biochemistry Laboratory of CARE Hospitals, Hyderabad, India. Total cholesterol concentrations were measured enzymatically using a cholesterol kit (SYNCHRON CX Systems). Cholesterol Reagent was used to measure cholesterol concentration by a timed endpoint method.8 Low-density lipoprotein (LDL) cholesterol in serum was measured with a cholesterol LDL kit by a homogenous method based on an innovative detergent technology.9 High-density lipoprotein (HDL) cholesterol in serum was measured with a diagnostic test kit by enzymatic clearance assay.
The triglycerides in serum were measured with a triglycerides Group Policy (GPO) reagent kit. Triglycerides GPO reagent was used to measure the triglycerides concentration by a timed endpoint method.10 Blood pressure was recorded with a sphygmomanometer.
Dietary assessment
Information on family history, physical activity, food habits and dietary pattern was obtained by using a detailed interview schedule. The dietary intake was assessed by using the 24 hours recall method, which consisted of listing all foods and beverages consumed during the previous 24 hours using the standard cups developed by the National Institute of Nutrition, Hyderabad, India.11 The dietary intakes obtained in terms of standardised cups were converted into quantities of raw food ingredients and the energy and fat content was then computed using the Indian food composition tables.12
The schedule also consisted of questions to assess the dietary pattern. The subjects were asked about food choices, frequency of consuming sweets and desserts, monthly oil consumption, snack foods preferred and beverages and soft drinks consumed.
Physical activity assessment
Leisure-time physical activity was assessed with two questions. In the first question, the level of leisure-time physical activity was measured with five alternatives which included walking, jogging, aerobics, swimming, and cycling. The frequency and duration of leisure-time physical activity was determined in a question with four response alternatives ranging from 0 to ≥60 min/session.
Statistical analysis
The data was analysed using the SPSS for Windows version 15.0 (SPSS Inc. Chicago, IL, USA). Descriptive statistics were computed for anthropometric measurements and indices like BMI and WHR. The association between calorie intake and BMI with lipid profiles was assessed by χ2 test. Relationships between anthropometric measurements and lipid profile were assessed by correlation coefficients. Level of significance was considered as 0.05.
Results
Majority of the subjects were in the age group of 40–60 years. Among the participants of the study 28.5% of the males and 42.2% of the females had hypercholesterolaemia (Table 1). 71.5% of men and 57.8% of women had cholesterol levels below 199 mg/dL. Majority of the subjects reported brisk walking as the predominant leisure-time physical activity. Brisk walking was found to be the predominant form of exercise preferred by males (52.2%) and females (39.4%) (P < 0.05). The duration of brisk walking ranged from 30 to >60 min/day. The subjects were divided into 2 groups—those who exercised regularly and those who did not.
Table 1.
Lipid profile of the subjects studied.
| Parameter (mg/dL) | Prevalence (%) |
||
|---|---|---|---|
| Male | Female | P value | |
| Total cholesterol | |||
| <199 | 71.5 | 57.8 | 0.014 |
| ≥199 | 28.5 | 42.2 | |
| Triglycerides | |||
| <150 | 53.1 | 52.3 | 0.886 |
| ≥150 | 46.9 | 47.7 | |
| HDL cholesterol | |||
| ≥40 | 62.4 | 32.4 | 0.000 |
| <40 | 37.6 | 67.6 | |
| LDL cholesterol | |||
| <130 | 74.4 | 66.1 | 0.118 |
| ≥130 | 25.6 | 33.9 | |
HDL: high-density lipoprotein, LDL: low-density lipoprotein.
Serum triglyceride levels were similar in both men and women (P = 0.886). Around 47% of the participants studied had serum triglycerides above the risk level of 150 mg/dL. Information regarding food habits revealed that majority of the participants of the study was non-vegetarian (61.5% females and 64.3% males). A significantly higher number of females had HDL cholesterol levels <40 mg/dL.
Table 2 represents the association of biochemical parameters and BMI with calorie intake. A higher percentage of subjects whose calorie intake was inadequate had BMI below the cut-off of 23 kg/m2 (76.8%) compared to those whose calorie intake was adequate (15%). There was a significant difference (P = 0.019) between subjects whose calorie intake was adequate and those whose calorie intake was inadequate with respect to BMI (Table 2). Significant association (P < 0.05) was observed between total cholesterol, HDL and LDL cholesterol with levels of calorie intake. There was no significant difference between subjects whose calorie intake was adequate and those whose calorie intake was inadequate with respect to triglyceride levels. Of the subjects whose calorie intake was adequate a higher percentage (63.7%) had total cholesterol levels ≥199 mg/dL than those whose calorie intake was inadequate (Table 2).
Table 2.
Association of biochemical parameters and with calorie intake.
| Calorie intake | Biochemical parameter | χ2 | P value | |
|---|---|---|---|---|
| Triglycerides (mg/dL) | ||||
| <150 | ≥150 | |||
| Inadequate | 58.5% | 41.5% | 1.4 | 0.230 |
| Adequate | 50.9% | 49.1% | ||
| Total cholesterol (mg/dL) | ||||
| <199 | ≥199 | |||
| Inadequate | 75.6% | 24.4% | 3.89 | 0.048* |
| Adequate | 36.3% | 63.7% | ||
| HDL cholesterol (mg/dL) | ||||
| ≥40 | <40 | |||
| Inadequate | 69.5% | 30.5% | 6.77 | 0.009** |
| Adequate | 47% | 53% | ||
| LDL cholesterol (mg/dL) | ||||
| <130 | ≥130 | |||
| Inadequate | 79.3% | 20.7% | 3.26 | 0.0007** |
| Adequate | 31.2% | 68.8% | ||
| BMI (kg/m2) | ||||
| <23.0 | ≥23.0 | |||
| Inadequate | 76.8% | 23.2 | 2.89 | 0.019* |
| Adequate | 15% | 85% | ||
P < 0.05
P < 0.01. BMI: body mass index, HDL: high-density lipoprotein, LDL: low-density lipoprotein.
The association of biochemical parameters and BMI with physical activity is presented in Table 3. A lower percentage (36.4%) of subjects who exercised regularly had triglyceride levels above the risk level of 150 compared to those who did not perform any exercise (48.7%) but the difference was not significant. There was a significant difference in the total cholesterol levels of subjects who exercised and those who were not involved in any physical activity (P = 0.047). Only 29% of the subjects who exercised regularly had total cholesterol levels over the risk level of 200 mg/dL (Table 3) compared to 62.3% of those who did not exercise. Although a lower percentage (72.1%) of subjects who exercised regularly had BMI above the Asian cut-off of 23, there was no significant difference between those who did not exercise (83.8%). There was a significant difference between the HDL cholesterol values of the subjects based on exercise levels (P = 0.012). 62.3% of those who exercised regularly had HDL levels ≥40 compared to only 38.1% of those who did not exercise regularly (Table 3). Exercise did not result in any significant effects on serum LDL cholesterol levels.
Table 3.
Association of biochemical parameters and body mass index with physical activity.
| Physical activity | Biochemical parameter | χ2 | P value | |
|---|---|---|---|---|
| Triglycerides (mg/dL) | ||||
| <150 | ≥150 | |||
| No exercise | 51.3% | 48.7% | 0.289 | 0.591 |
| Exercise | 63.6% | 36.4% | ||
| Total cholesterol (mg/dL) | ||||
| <199 | ≥199 | |||
| No exercise | 37.7% | 62.3% | 2.66 | 0.047* |
| Exercise | 71% | 29% | ||
| HDL cholesterol (mg/dL) | ||||
| ≥40 | <40 | |||
| No exercise | 38.1% | 61.9% | 3.48 | 0.012* |
| Exercise | 62.3% | 37.7% | ||
| LDL cholesterol (mg/dL) | ||||
| <130 | ≥130 | |||
| No exercise | 61.4% | 38.6% | 0.001 | 0.972 |
| Exercise | 71.6% | 28.4% | ||
| BMI (kg/m2) | ||||
| <23.0 | ≥23.0 | |||
| No exercise | 16.2% | 83.8% | 0.155 | 0.694* |
| Exercise | 27.9% | 72.1% | ||
P < 0.05. BMI: body mass index, HDL: high-density lipoprotein, LDL: low-density lipoprotein.
The duration of exercise did not have a significant impact on triglyceride levels (Table 4). Total cholesterol levels were found to be lower in subjects who exercised regularly for >1 hour (P < 0.01). 76.5% of the subjects who exercised for more than an hour per day had total cholesterol levels below the risk level of 200 mg/dL compared to only 37.7% of subjects who did not exercise and 59.4% of those who exercised only 2–3 times/wk (Table 4).
Table 4.
Association of duration of exercise with biochemical parameters and body mass index.
| Exercise duration | Biochemical parameter | χ2 | P value | |
|---|---|---|---|---|
| Triglycerides (mg/dL) | ||||
| <150 | ≥150 | |||
| >1 hr/day | 58.8% | 41.2% | 2.84 | 0.672 |
| ½–1 hr/day | 48.6% | 36.4% | ||
| 2–3 times/wk | 62.9% | 37.1% | ||
| No exercise | 51.3% | 48.7% | ||
| Total cholesterol (mg/dL) | ||||
| <199 | ≥199 | |||
| >1 hr/day | 76.5% | 23.5% | 2.96 | 0.006* |
| ½–1 hr/day | 70.1% | 29.9% | ||
| 2–3 times/wk | 59.4% | 40.6% | ||
| No exercise | 37.7% | 62.3% | ||
| Body mass index (kg/m2) | ||||
| <23.0 | ≥23.0 | |||
| >1 hr/day | 17.6% | 82.4% | 0.896 | 0.925 |
| ½–1 hr/day | 18.7% | 81.3% | ||
| 2–3 times/wk | 17.1% | 82.9% | ||
| No exercise | 16.2% | 83.8% | ||
P < 0.01.
A significant percentage (64%) of the subjects who exercised for half an hour to one hour daily had HDL cholesterol ≥40 mg/dL compared to those who did not exercise regularly (Table 5). Our findings are similar to the results of Stein et al.13 who found that HDL levels rose significantly in groups training at higher intensity exercise when compared with a group training at lower intensity during 30-minute training sessions on a cycle ergometer performed 3 times per week. In our study duration of exercise had a significant effect on LDL cholesterol levels (P < 0.05). A higher percentage (70%) of those who exercised for half an hour or more daily had LDL cholesterol levels <130 mg/dL compared to those who did not exercise regularly (Table 5).
Table 5.
Association of duration of exercise with biochemical parameters.
| Exercise duration | Biochemical parameter | χ2 | P value | |
|---|---|---|---|---|
| HDL cholesterol (mg/dL) | ||||
| ≥40 | <40 | |||
| >1 hr/day | 64.7% | 35.3% | 4.67 | 0.052* |
| ½–1 hr/day | 64.5% | 35.5% | ||
| 2–3 times/wk | 45.7% | 54.3% | ||
| No exercise | 48.1% | 51.9% | ||
| LDL cholesterol (mg/dL) | ||||
| <130 | ≥130 | |||
| >1 hr/day | 70.6% | 29.4% | 0.693 | 0.064* |
| ½–1 hr/day | 70.1% | 29.9% | ||
| 2–3 times/wk | 63.2% | 36.8% | ||
| No exercise | 51.4% | 48.6% | ||
P < 0.05. HDL: high-density lipoprotein, LDL: low-density lipoprotein.
Discussion
In our study weight and BMI were positively and significantly correlated with calorie and fat intake (Figure 1). More women than men were found to be overweight or obese. The prevalence of overweight and obesity in terms of BMI, waist circumference and waist–hip ratio was significantly higher in women compared to men. Maximum percentage of the subjects studied (78.7% males and 82.9% females) had BMI above 23.0 which is the Asian cut-off. Analysis of the data also showed a significant correlation between waist circumference, hip circumference and total calorie and fat intake (Figure 1). Weight and BMI were positively and significantly correlated with calorie and fat intake (P < 0.01). There is an increasing trend of total calorie intake and total fat intake with the increasing BMI (Figure 1). There was no significant correlation between the waist–hip ratio and the total calorie or fat intake, which indicates that subjects with generalised obesity (high BMI) may not have central obesity (high WHR).14
Figure 1.
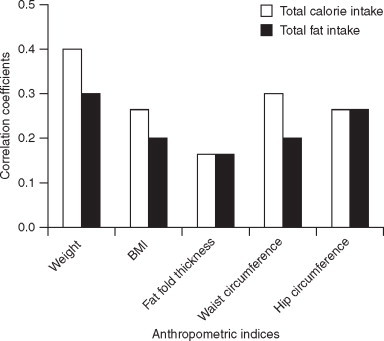
Correlation matrix of anthropometric indices with dietary intake. BMI: body mass index.
In our study more women than men had hypercholesterolaemia (Table 1). Regarding serum cholesterol levels, it has been shown that both exercise and weight loss have a greater influence on lowering LDL cholesterol and raising HDL cholesterol levels in men than in women and in older or post-menopausal women.15 25.6% of men and 33.9% of women had high LDL cholesterol levels (≥130 mg/dL) but the difference was not significant (Table 1).
In our study increased calorie intake was positively correlated with total cholesterol levels (Table 2). It has been reported that dietary factors, particularly habitual dietary fat consumption and the amount and type of fat in a meal are major determinants of postprandial lipaemic response.16 Chen et al.17 reported that the magnitude of postprandial lipaemia within an individual is directly proportionate to the fat content of the meal. Among the subjects studied, a higher percentage (68.8%) of those whose calorie intake was adequate had LDL cholesterol levels ≥130 (P < 0.01). These results are in agreement with those of Polychronopoulos et al.18 whose data revealed that greater adherence to a Mediterranean diet was associated with 23% lower likelihood of having hypercholesterolaemia after controlling for age, sex, BMI, smoking habits, and physical activity status. A higher percentage of subjects (69.5%) whose calorie intake was inadequate had HDL cholesterol levels ≥ 40 than those whose calorie intake was adequate (Table 2). It has been reported that excess weight gain tends to lower HDL cholesterol and raise LDL cholesterol.
A higher percentage of males were found to be performing exercise for half an hour or >1 hour daily than females (Figure 2). 58.7% of the women studied reported that they did not find time for exercise (Figure 2). There was a significant difference in the total cholesterol levels of subjects who exercised and those who were not involved in any physical activity (Table 3). Only 29% of the subjects who exercised regularly had total cholesterol levels over the risk level of 200 mg/dL (Table 3) compared to 62.3% of those who did not exercise. Our findings are similar to those of Lopez et al.19 who reported a moderate effect of exercise on decreasing serum total cholesterol and a more marked effect on decreasing serum triglycerides in young individuals after a 7-week period of exercise.
Figure 2.
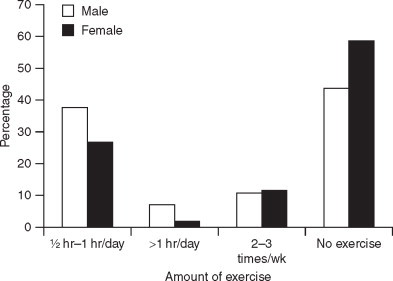
Exercise frequency of subjects (n = 316).
High-density lipoprotein cholesterol levels were significantly higher in males than in females (Table 1) and this is corroborated by the finding of increased exercise levels in males (Figure 2). There was a significant difference between the HDL cholesterol values of the subjects based on exercise levels. About 62.3% of those who exercised regularly had HDL levels ≥ 40 compared to only 38.1% of those who did not exercise regularly (Table 3). The association between plasma concentration of HDL cholesterol and the incidence and severity of coronary heart disease has been well-recognised.20 Programmes of increased physical activity, particularly those based upon running or jogging, have attracted attention as being among the few potentially effective and physiologically desirable means of increasing plasma HDL cholesterol concentrations. Several longitudinal studies have been conducted in initially sedentary, but healthy, individuals to measure the effect of increased physical activity on plasma lipoprotein concentrations. Our findings confirm the results of other studies which report that increased exercise significantly elevates plasma HDL cholesterol concentrations.21 Total cholesterol levels were found to be lower in subjects who exercised regularly for >1 hour (P < 0.01). 76.5% of the subjects who exercised for more than an hour per day had total cholesterol levels below the risk level of 200 mg/dL compared to only 37.7% of subjects who did not exercise and 59.4% of those who exercised only 2–3 times/wk (Table 4). Current beliefs suggest that regular participation in physical activity produces favourable lipid changes. There is specific evidence supporting the benefits of both higher intensity as well as longer duration exercise programmes for producing specific alterations in serum lipid concentrations especially serum HDL cholesterol.22
In our study duration of exercise had a significant effect on LDL cholesterol levels (Table 5). A higher percentage (70%) of those who exercised for half an hour or more daily had LDL cholesterol levels <130 mg/dL than those who did not exercise regularly.
Those who are overweight tend to have high total cholesterol and high LDL cholesterol partly on the basis of diet, which is usually high in saturated fats and cholesterol and partly on the basis of inactivity. In our study body weight was significantly associated with total cholesterol and LDL cholesterol (Figure 3). There was a negative association between weight and HDL cholesterol but this was not significant. Many studies have reported that obesity, as defined on the basis of BMI, is consistently related to increased blood pressure and unfavourable lipid profiles.23 Waist circumference, however, may be a stronger predictor than BMI for the identification of metabolic and CVD-associated risk factors.24 In our study, BMI was positively and significantly associated with systolic blood pressure (Figure 3). A positive association was observed between waist circumference and both total cholesterol and LDL cholesterol. Waist circumference was also positively correlated with systolic and diastolic blood pressure and triglycerides (Figure 3). It has been reported that a large waist circumference is significantly inversely associated with HDL cholesterol levels and significantly positively associated with LDL cholesterol levels and blood pressure.25
Figure 3.
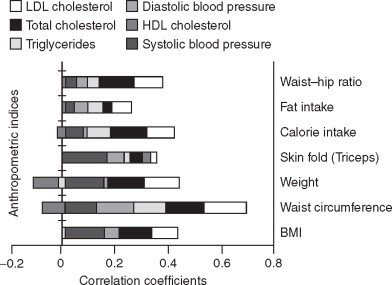
Correlation coefficients between hypertension and lipid profiles with anthropometric indices. HDL: high-density lipoprotein, LDL: low-density lipoprotein.
Conclusion
In conclusion, our findings provide support for the potentially beneficial effects of both diet and exercise on the serum lipid profile. The most important lifestyle factors which affect the serum lipid profile are diet composition, body weight and physical activity. The modification of blood lipid levels will be beneficial especially to those who are at higher risk of coronary heart disease. Screening for these abnormalities is essential and must be followed by active and effective interventions. Interventions may be more effective if they are targeted at specific socio-demographic sub-groups. Dietary advise to younger people should address undesirable aspects of food patterns. Combining campaigns to improve diet with efforts to increase physical activity may be needed to effectively reduce CVD risk.
Acknowledgements
This study was supported by a grant provided by the Management Committee of Kasturba Gandhi College for Women, Secunderabad, India. The study was carried out in CARE Hospital, Banjara Hills, Hyderabad, India. The authors are grateful to all the subjects who participated in the study.
References
- 1.Yajnik CS. The insulin resistance epidemic in India: fetal origins, later lifestyle or both? Nutr Rev. 2001;59:1–9. doi: 10.1111/j.1753-4887.2001.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 2.NFHS-3. Third National Family Health Survey. International Institute for Population Sciences 2006; Mumbai, India.
- 3.Gidding SS, Nehgme R, Heise C, Muscar C, Linton A, Hassink S. Severe obesity associated with cardiovascular deconditioning, high prevalence of cardiovascular risk factors, diabetes mellitus/hyperinsulinemia and respiratory compromise. J Pediatr. 2004;144:766–769. doi: 10.1016/j.jpeds.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 5.Haskell WL. Exercise-induced changes in plasma lipids and lipoproteins. Prev Med. 1984;13:23–36. doi: 10.1016/0091-7435(84)90038-0. [DOI] [PubMed] [Google Scholar]
- 6.Pais P, Pogue J, Gerstein H. Risk factors for acute myocardial infarction in Indians: a case-control study. Lancet. 1996;348:358–363. doi: 10.1016/s0140-6736(96)02507-x. [DOI] [PubMed] [Google Scholar]
- 7.El-Hazmi MA, Warsy AS. Prevalence of plasma lipid abnormalities in Saudi children. Ann Saudi Med. 2001;21:21–25. doi: 10.5144/0256-4947.2001.21. [DOI] [PubMed] [Google Scholar]
- 8.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 9.Nakamura M, Taniguti Y, Yamamoto M, Hino K, Manabe M. Homogenous assay of serum LDL-cholesterol on an auto analyzer. Clin Chem. 1997;43:S260–S261. [Google Scholar]
- 10.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 11.Thimmayamma BVS. A Hand Book of Schedules and Guidelines in Socioeconomic and Diet Surveys, 1987. National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India.
- 12.Gopalan CB, Ramasastri V, Balasubramanian SC. Nutritive Value of Indian Foods, 1989. (As revised and updated by Narasinga Rao BS, Deosthala YG, Pant KC.) National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India.
- 13.Stein RA, Michielli DW, Glantz MD. Effects of different exercise training intensities on lipoprotein cholesterol fractions in healthy middle-aged men. Am Heart J. 1990;119:277–283. doi: 10.1016/s0002-8703(05)80017-1. [DOI] [PubMed] [Google Scholar]
- 14.Chadha SL, Gopinath N, Katyal I, Shekhawat S. Dietary profile of adults in an urban and a rural community. Indian J Med Res. 1995;101:258–267. [PubMed] [Google Scholar]
- 15.Glick M, Michel AC, Dorn J, Horwitz M, Rosenthal T, Trevisan M. Dietary cardiovascular risk factors and serum cholesterol in on old order Mennonite community. Am J Public Health. 1988;88:1202–1205. doi: 10.2105/ajph.88.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry SEE. Postprandial lipaemia-the influence of diet and its link to coronary heart disease. Nutrition Bulletin. 2005;30:314–322. [Google Scholar]
- 17.Chen YD, Skowronski R, Coulston AM, Pietarinen J, Hollenbeck CB, Reaven GM. Effect of acute variations in dietary fat and carbohydrate intake on retinyl ester content of intestinally derived lipoproteins. J Clin Endocrinol Metab. 1992;74:28–32. doi: 10.1210/jcem.74.1.1727825. [DOI] [PubMed] [Google Scholar]
- 18.Polychronopoulos E, Panagiotakos DB, Polystipioti A. Diet, lifestyle factors and hypercholesterolemia in elderly men and women from Cyprus. Lipids Health Dis. 2005;4:17. doi: 10.1186/1476-511X-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez A, Vial R, Balart L, Arroyave G. Effect of exercise and physical fitness on serum lipids and lipoproteins. Atherosclerosis. 1974;20:1–9. doi: 10.1016/0021-9150(74)90073-2. [DOI] [PubMed] [Google Scholar]
- 20.Raz I, Rosenbilt H, Kark JD. Effect of moderate exercise on serum lipids in young men with low high density lipoprotein cholesterol. Arteriosclerosis. 1988;8:245–251. doi: 10.1161/01.atv.8.3.245. [DOI] [PubMed] [Google Scholar]
- 21.Marrugat J, Elosua R, Covas MI, Molina L, Rubies-Prat J. Amount and intensity of physical activity, physical fitness, and serum lipids in men. The MARATHOM Investigators. Am J Epidemiol. 1996;143:562–569. doi: 10.1093/oxfordjournals.aje.a008786. [DOI] [PubMed] [Google Scholar]
- 22.Nieman DC, Haig JL, Fairchild KS, De Guia ED, Dizon GP, Register UD. Reducing-diet and exercise training effects on serum lipids and lipoproteins in mildly obese women. Am J Clin Nutr. 1990;52:640–645. doi: 10.1093/ajcn/52.4.640. [DOI] [PubMed] [Google Scholar]
- 23.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willet WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–921. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 24.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76:743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 25.Vijayalakshmi P, Anitha N. Assessing the causative factors and nutritional profile of selected obese subjects. Ind J Nutr Dietet. 2003;40:436–446. [Google Scholar]


