Highlights
► We compare characteristics of our case with five previously reported cases. ► We discuss possible mechanisms of dissemination and metastasis to the distant site. ► We discuss treatment options, but poor outcome was noted in all six cases.
Keywords: Cervical carcinoma, Squamous cell, Metastasis, Eye, Orbit
Introduction
According to the American Cancer Society, there will be an estimated 12,710 new cases of cervical cancer with 4290 deaths in the US in 2011 (American Cancer Society). Even though the incidence and mortality of cervical cancer has decreased in the US, there are still many patients, particularly from minority groups, who continue to present with advanced stages of the disease. The lifetime probability of developing invasive cervical cancer is 1/145 women, with the most common routes of spread being local invasion of surrounding organs and through lymphatic channels (Monk and Tewari, 2007). Distant sites account for 27% of metastasis, however there are only 5 previously reported cases of orbital metastases (Monk and Tewari, 2007; Gosslee et al., 2009; Park et al., 2005; McCulley et al., 2002; Lee et al., 1997; Hertzanu et al., 1987).
Case report
A 59-year-old woman was evaluated for several months of foul-smelling vaginal discharge followed by symptoms of pelvic discomfort and difficulty voiding. She had not received a routine gynecologic examination in more than 10 years. A computed tomography (CT) scan of the pelvis and an exam under anesthesia (EUA) with tissue biopsies of the cervix and a paravaginal mass was performed. The CT scan revealed a spherical mass measuring 4.5 cm that completely replaced the uterine cervix without extension any further than the anterior vagina and immediately adjacent tissue [Fig. 1]. There was no evidence of hydroureter or para-aortic lymphadenopathy. A chest X-ray showed atelectasis, however no masses were present. Histology showed poorly differentiated squamous cell carcinoma. She was subsequently diagnosed with stage IIIA carcinoma of the cervix given periurethral and vaginal involvement. She was treated with concurrent external beam pelvic and groin radiation therapy and weekly Cisplatin, followed by brachytherapy. An EUA was performed during urethral interstitial implant placement and the cervical mass had resolved with radiation changes at the cervix. At her 3-month follow-up visit in clinic, she refused a pap test and pelvic exam due to fear of discomfort.
Fig. 1.
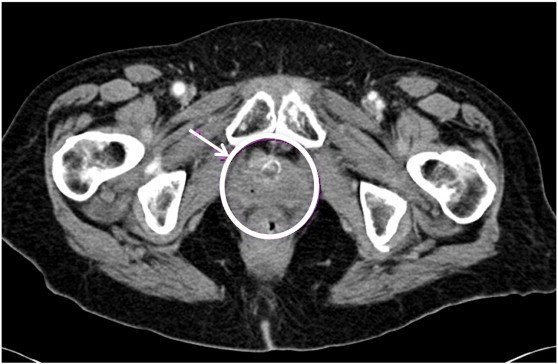
CT scan with pelvic lesion causing urethral obstruction due to mass effect.
Five months after initial presentation she was evaluated for a syncopal episode and fall. Work-up including head CT scan did not reveal any abnormalities. One month later, she presented with headaches, left eye proptosis, and epiphora with preserved vision. CT scan of the orbits revealed a fusiform enlargement of the left lateral rectus muscle with a fluid dense core and enhancing rim concerning for retrobulbar abscess versus neoplastic process. This abnormality was not reported on CT of the head including imaging of the orbits that was performed 1 month earlier. After excluding an infectious process, the patient was started on corticosteroids and was scheduled to return for outpatient ophthalmologic follow-up. Magnetic resonance imaging performed 2 weeks later showed an interval enlargement of the left retrobulbar orbital mass. She underwent surgical biopsy and pathology was positive for squamous cell carcinoma consistent with metastasis from cervical cancer [Fig. 2]. During this evaluation, the patient was offered HIV screening twice but she declined the test.
Fig. 2.
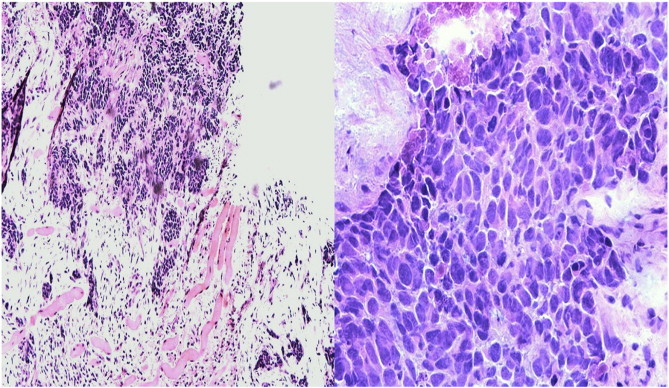
Metastatic moderately differentiated squamous cell carcinoma with tumor cells showing marked pleomorphism, hyperchromasia and necrosis (hematoxylin-eosin, original magnification × 100 and × 400).
She subsequently began radiation therapy to her left orbit. Despite treatment, the orbital mass nearly doubled in size over the next month [Fig. 3]. She was hospitalized again due to poor pain control and intractable nausea and vomiting. Follow-up positron emission tomography CT scans of the body also revealed interval development of widely metastatic disease involving lung, liver, adrenals, bone, and scalp. Despite palliative radiation therapy her clinical status continued to deteriorate and she was eventually sent home on hospice care. She died 10 months after the initial diagnosis of cervical cancer and 4 months after the diagnosis of orbital metastasis.
Fig. 3.
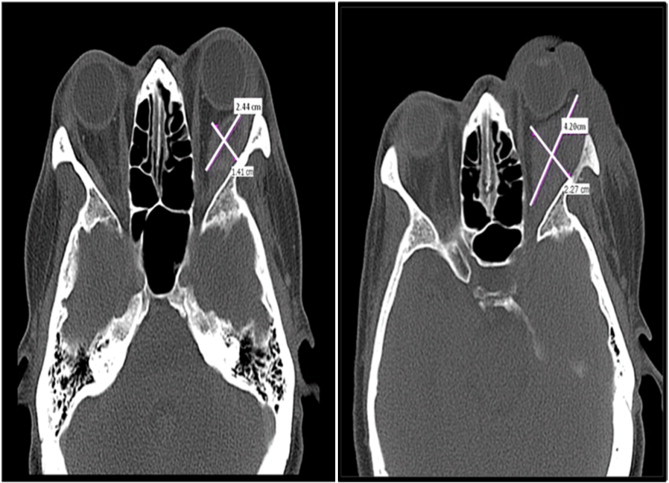
Coronal view of CT scans showing interval enlargement in retrobulbar mass noted 1 month after initial presentation, despite palliative radiation therapy.
Comment
There are only five cases of cervical cancer metastatic to the orbit that have been reported in the literature (Gosslee et al., 2009; Park et al., 2005; McCulley et al., 2002; Lee et al., 1997; Hertzanu et al., 1987). Symptoms at the time of diagnosis included orbital pain, proptosis, lid swelling, and diplopia with variable effects on visual acuity (Gosslee et al., 2009; Park et al., 2005; McCulley et al., 2002; Lee et al., 1997; Hertzanu et al., 1987). Stage at initial diagnosis of cervical carcinoma ranged from FIGO IB1 to IV, though upon presentation with orbital metastasis all but one case had concurrent distant metastases (Gosslee et al., 2009; Park et al., 2005; McCulley et al., 2002; Lee et al., 1997; Hertzanu et al., 1987). Time from primary diagnosis to orbital metastasis was highly variable, ranging from 4 months to 10 years (Gosslee et al., 2009; Park et al., 2005; McCulley et al., 2002; Lee et al., 1997; Hertzanu et al., 1987). Histology was also variable, including 3 squamous cell carcinomas, one adenocarcinoma, and one undifferentiated carcinoma whose cervical origin was confirmed by HPV PCR of the tissue biopsy (Gosslee et al., 2009; Park et al., 2005; McCulley et al., 2002; Lee et al., 1997; Hertzanu et al., 1987). Treatment of orbital metastases is usually palliative and consists of radiation therapy. Surgical excision and chemotherapy may also be considered, but their benefit has not been proven. All patients had poor outcomes despite attempts of treatment with chemotherapy, radiation and/or surgery, with death ranging from 3–10 months after diagnosis of orbital metastasis (Gosslee et al., 2009; Park et al., 2005; McCulley et al., 2002; Lee et al., 1997; Hertzanu et al., 1987).
As mentioned previously, cervical cancer metastasis occurs through local invasion of surrounding tissue and through lymphatic dissemination. Hematogenous dissemination, although uncommon, is most likely to involve the liver, bones or lungs (Monk and Tewari, 2007). Presumably, the cases discussed above represent a rare instance of hematogenous dissemination to the orbit. Previous discussion of cervical cancer metastatic to the central nervous system (CNS) suggests migration via the venous plexus of Batson and the inner vertebral plexus (Tangjitgamol et al., 2004). The orbit can be considered an extension of the same route, as the venous drainage of the eye communicates with the dural venous sinuses of the CNS via the cavernous sinus and ophthalmic veins. We hypothesize that hematogenous metastases to the eye and orbit are more rare than brain metastases because the ophthalmic veins are significantly smaller caliber vessels than the CNS venous sinuses and are also much further away from the venous drainage of the spine.
Due to the paucity of case reports on cervical cancer metastatic to the orbit we are unable to identify any trend of histology, stage, treatment or survival. If we consider the orbit an extension of the CNS, there is more data available to comment on these features. Histologically, CNS metastases are seen more frequently with neuroendocrine and poorly differentiated cervical carcinoma (Weed et al., 2003; Chura et al., 2007). The histology in the presented case is poorly differentiated squamous cell carcinoma, consistent with these observations. One of the more recent case series of brain metastases from cervical cancer reported 9 patients with stage IIB or lower and 3 patients with stage IIIA or higher (Chura et al., 2007). The significance of this data is debatable since there is a higher incidence of lower stages of cervical cancer due to early detection. Time from initial diagnosis to CNS metastasis ranged from 1 month to 8 years and treatment options included steroids alone, radiation with steroids, surgery followed by radiation or radiation followed by chemotherapy (Chura et al., 2007). The study concluded that radiation therapy improved neurological symptoms, but additional chemotherapy increased survival significantly (Chura et al., 2007). The distribution of histology, time to diagnosis of metastasis, treatment and survival of these cases is similar to the 6 reviewed cases of orbital metastasis, and therefore it may be worth applying the data collected on improved survival to future cases of orbital metastasis.
Even though CT scans of the abdomen and chest are not part of the clinical staging evaluation of cervical cancer, this imaging is imperative to exclude extra-pelvic disease. During our patient's initial evaluation, there was a “probably enlarged” mediastinal lymph node noted on CT chest that may have represented undiagnosed metastatic disease. Given that there is a significant rate of recurrence, it is important to continue short interval follow-up of cervical cancer patients in an effort to promote early detection of recurrence or persistent disease. Patients that present with specific new-onset symptoms should be evaluated early and distant metastases should always be on the differential. Treatment options for metastatic disease may vary. Treatment of orbital metastases in particular is usually palliative and consists of radiation therapy with or without steroids and chemotherapy. Surgical excision may also be considered, however the finding of orbital metastasis in and of itself is an ominous sign.
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Accepted and presented as poster presentation at the 77th Annual Meeting of the Central Association Obstetrician and Gynecologists. Las Vegas, Nevada. October 27–30, 2010.
References
- American Cancer Society. Last revised 10/26/2011. Accessed Dec 6, 2011. http://www.cancer.org/Cancer//CervicalCancer/DetailedGuide/cervical-cancer-key-statistics
- Chura J.C., Shukla K., Argenta P.A. Brain metastasis from cervical carcinoma. Int. J. Gynecol. Cancer. 2007;17(1):141–146. doi: 10.1111/j.1525-1438.2007.00808.x. [DOI] [PubMed] [Google Scholar]
- Gosslee J.M., Misra R.P., Langford M.P., Vekovius B., Byrd W.A., Flynn S.B. Orbital metastasis of keratinizing squamous cell cervical carcinoma with giant cells: a case report. Int. Ophthalmol. 2009;29:39–44. doi: 10.1007/s10792-007-9162-6. [DOI] [PubMed] [Google Scholar]
- Hertzanu Y., Vellet A.D., Fain B.A., Ferreira M.M., Ninin D.T. Eye metastases in carcinoma of the cervix: a case report. S. Afr. Med. J. 1987;71:53–54. [PubMed] [Google Scholar]
- Lee H.M., Choo C.T., Poh W.T. Orbital metastasis from carcinoma of cervix. Br. J. Ophthalmol. 1997;81:329–331. doi: 10.1136/bjo.81.4.329b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley T.J., Yip C.C., Bullock J.D., Warwar R.E., Hood D.L. Cervical carcinoma metastatic to the orbit. Ophthal. Plast. Reconstr. Surg. 2002;18:385–387. doi: 10.1097/00002341-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Monk B.J., Tewari K.S. Invasive cervical cancer. In: DiSaia P.J., Creasman W.T., editors. Clinical Gynecologic Oncology. 7th ed. Mosby; Philadelphia, PA: 2007. pp. 63–64. [Google Scholar]
- Park T.W., Theuerkauf I., Nuschin M. Orbital metastases of unknown origin: HPV typing identifies the primary tumor. Acta Obstet. Gynecol. Scand. 2005;84:702–704. doi: 10.1111/j.0001-6349.2005.0383b.x. [DOI] [PubMed] [Google Scholar]
- Tangjitgamol S., Levenback C.F., Beller U., Kavanagh J.J. Role of surgical resection for lung, liver, and central nervous system metastases in patients with gynecological cancer: a literature review. Int. J. Gynecol. Cancer. 2004;14(3):399–422. doi: 10.1111/j.1048-891x.2004.14326.x. [DOI] [PubMed] [Google Scholar]
- Weed J.C., Graff A.T., Shoup B., Tawfik O. Small-cell undifferentiated (neuroendocrine) carcinoma of the uterine cervix. J. Am. Coll. Surg. 2003;197:44–51. doi: 10.1016/S1072-7515(03)00120-0. [DOI] [PubMed] [Google Scholar]


