Abstract
Background
Two-dimensional strain imaging allows rapid and accurate analysis of regional left ventricular (LV) principal strains in the longitudinal, radial, and circumferential directions. The aim of this study was to assess the ability of subtle differences in LV principal strains to characterize features of subclinical LV dysfunction in patients with systemic hypertension and apparently preserved LV systolic function.
Methods
2-dimensional echocardiographic (2DE) images of the LV were acquired in apical 4-chamber and parasternal short-axis at the basal, mid, and apical levels in 59 subjects, including 25 healthy controls (33 ± 4 yrs, 14 male) and 34 patients with systemic hypertension (36 ± 3 yrs, 24 male). Longitudinal (LS), circumferential (CS) and radial strains (RS) were quantified in an 18-segment model using a novel speckle tracking system (2D Cardiac Performance Analysis, TomTec Imaging System, Munich, Germany).
Results
In comparison with normal controls, peak LS was markedly attenuated in the subendocardial and subepicardial regions in patients with systemic hypertension. However, circumferential strain was reduced only in subepicardial region; radial strain was not significantly different in the two groups. The subendocardial-to-subepicardial gradient of circumferential deformation correlated with the radial strains in both controls and hypertensive patients (R = 0.87, p < 0.001).
Conclusions
Despite reduced longitudinal shortening, LV wall thickening in patients with systemic hypertension remains unaltered due to relatively preserved circumferential shortening. Characterizing the disparities in LV principal strains reveals the presence of subclinical LV dysfunction and provides unique insights into functional adaptations that maintain global LV ejection fraction in patients with systemic hypertension.
Keywords: Speckle tracking, Systemic hypertension, Left ventricular function
1. Background
Development of left ventricular hypertrophy (LVH) in patients with systemic hypertension is an established risk factor for the development of asymptomatic left ventricular (LV) dysfunction and congestive heart failure.1 Moreover, while conventional echocardiography can detect changes in LV diastolic dysfunction associated with LVH, global LV systolic function often remains preserved until late in the course of the disease, making subtle changes in LV contractile function difficult to interpret in the early stages.2,3
Subclinical changes in LV function can be identified by quantifying myocardial strain, a dimensionless measurement of deformation, expressed as a fractional or percentage change from an object's original dimension. Two-dimensional (2D) speckle tracking has recently emerged as a novel echocardiographic technique for rapid, offline, bedside analysis of regional LV strains in the longitudinal, radial, and circumferential directions.1,4–6 This technique analyzes myocardial motion by tracking natural acoustic reflections and interference patterns seen in two-dimensional echoardiographic images and has been validated with measurements obtained by sonomicrometry and magnetic resonance imaging.7 The aim of this study was to assess the ability of subtle differences in the LV strain patterns in the longitudinal, radial and circumferential directions to characterize features of subclinical LV dysfunction in patients with systemic hypertension and preserved LV ejection fraction (LVEF).
2. Methods
2.1. Study subjects
We included 34 young consecutive patients with systemic hypertension (36 ± 3 yrs, 24 male) and 25 age and sex matched healthy controls (33 ± 4 yrs, 14 male). Patients with established coronary artery disease, echocardiographic evidence of either regional or global wall motion abnormalities, valvular heart disease, diabetes mellitus and hypertrophic cardiomyopathy were excluded. Each study participant gave written, informed consent.
2.2. Echocardiography
A complete 2DE was performed in all patients, using a commercially available ultrasound transducer and equipment (S4-2 probe, HD7, Philips). All acquisitions were performed by the same experienced operator with the patients in the left lateral position. Basic measurements included LV wall thickness by M-mode and LV diameter by 2D. LV volumes and LVEF were measured using the modified biplane Simpson method as recommended by the American Society of Echocardiography.8 To determine the timing of cardiac events, mitral inflow and LV outflow were recorded using pulsed Doppler echocardiography. 2DE images of the LV were acquired in apical 4-chamber (A4C), apical 3-chamber (A3C), apical 2-chamber (A2C) and parasternal short-axis at the basal, mid, and apical levels with same ultrasound machine. Three consecutive cardiac cycles loops were recorded at end expiration. The frame rate was kept between 70 Hz and 100 Hz. Longitudinal, circumferential and radial strains were quantified in an 18-segment model using a novel speckle tracking system (2D Cardiac Performance Analysis (2D CPA), TomTec Imaging System, Munich, Germany). LS was measured in all 3 views, A4C, A3C and A2C. Peak systolic strain was measured. 2D CPA is a speckle tracking based analysis tool that can analyze 2D data from various ultrasound machines and is an extension of velocity vector imaging software that has been previously validated with sonomicrometry4,9 and magnetic resonance imaging.1,10 2D CPA, similar to velocity vector imaging, determines myocardial motion from a user-defined tracing along the endocardial border. Endocardial and automated subepicardial borders are traced throughout one cardiac cycle by successive application of a series of tracking steps. From this motion, the myocardial velocity, longitudinal and radial strain are calculated for both endocardial and subepicardial regions along the trace. Longitudinal systolic strain from endocardial and subepicardial regions respectively was obtained from 6 segments and from lateral and septal wall segments in apical 4-chamber, three and two chamber views. Circumferential strain and radial strain were obtained from 6 segments in short-axis views of the LV. Offline analyses were independently performed by one observer who was not involved in image acquisition nor had knowledge of other echocardiographic measures of LV function. The intra-observer variabilities for endocardial longitudinal strain, epicardial longitudinal strain, endocardial circumferential strain and epicardial circumferential strain were found to be 10 ± 7%, 8 ± 7%, 11 ± 10%, 25 ± 22%, and 24 ± 20%, respectively. Also interobserver variabilities reported by us for the same measurement were −13.6 ± 6.3%, −12.6 ± 7.9%, 16 ± 15%, 26 ± 21%, and 28 ± 29%, respectively.11
2.3. Statistics
Continuous variables were expressed as mean ± SD. The differences between groups were analyzed by independent samples student t tests (MedCalc 11.2 software MariaKerke, Belgium). Annova and paired t test were used to assess the level of significance in the follow up group. Correlations between variables were tested by Pearson or Spearman correlation tests where appropriate. A p-value <0.05 was considered to be significant.
3. Results
The study population consisted of 59 subjects, including 34 patients of systemic hypertension (36 ± 3 yrs, 24 male) and 25 age and sex matched healthy controls (33 ± 4 yrs, 14 male). Clinical and echocardiography data of patients with systemic hypertension and controls are shown in Table 1. No significant differences were found between the two groups in terms of age, sex or height. Patients with systemic hypertension weighed significantly more than the control group (p < 0.001). There was a significant higher systolic and diastolic blood pressure in patients with systemic hypertension than the control group (p < 0.001). Echocardiography parameters revealed significant septal wall thickness in hypertensive patients (p < 0.001). However there was no significant difference in LV mass in both the groups. There was no significant difference in the LV global ejection fraction between groups.
Table 1.
Clinical and echocardiographic characters between two groups.
| Characteristic | Controls |
Systemic hypertension |
p |
|---|---|---|---|
| (n = 25) | (n = 34) | ||
| Age (yrs) | 33 ± 4 | 36 ± 3 | NS |
| Men/women (n) | 14/11 | 24/10 | NS |
| Height (cm) | 168.2 ± 11 | 166.7 ± 10 | NS |
| Weight (kg) | 63.8 ± 8.6 | 75.2 ± 12.8 | <0.001 |
| Systolic blood pressure (mmHg) | 120 ± 7 | 164 ± 8 | <0.001 |
| Diastolic blood pressure (mmHg) | 78 ± 4 | 100 ± 8 | <0.001 |
| Ao (cm) | 2.3 ± 0.1 | 2.66 ± 0.3 | <0.001 |
| LA (cm) | 2.6 ± 0.1 | 3.24 ± 0.3 | <0.001 |
| LVDd (cm) | 5.0 ± 1.8 | 4.54 ± 0.6 | 0.008 |
| LVDs (cm) | 27 ± 1 | 2.66 ± 0.3 | NS |
| LV mass index g/m2 | 53 ± 8 | 62 ± 2 | NS |
| Septum wall thickness (cm) | 0.8 ± 0.2 | 1.5 ± 0.2 | <0.001 |
| Global ejection fraction % | 65 ± 5 | 67 ± 5 | NS |
| E peak velocity (cm/s) | 72 ± 14 | 84 ± 16 | <0.001 |
| A peak velocity (cm/s) | 61 ± 13 | 65 ± 15 | NS |
| e' (septal) cm/s | 11.7 ± 0.8 | 9.7 ± 2.8 | <0.001 |
| A' (septal), cm/s | 11.0 ± 0.3 | 10.8 ± 2.5 | NS |
| S' (Septal), cm/s | 13.4 ± 0.4 | 9.3 ± 2.3 | <0.001 |
| e'(lateral), cm/s | 12.4 ± 0.5 | 13.2 ± 2.8 | NS |
| A' (lateral), cm/s | 10.8 ± 1.9 | 11.6 ± 2.9 | NS |
| S' (lateral), cm/s | 14.1 ± 0.5 | 11.41 ± 2.6 | <0.001 |
In comparison with normal controls, peak LS was markedly attenuated in the subendocardial and subepicardial regions in patients with systemic hypertension as shown in Table 2 and Fig. 1. However, circumferential strain was reduced only in the subepicardial region. Radial strain in hypertensive patients was less than controls but it was not statistically significant (Fig. 2). The subendocardial-to-subepicardial gradient of circumferential deformation correlated with the radial strains in both controls and hypertensive patients (R = 0.87, p < 0.001). The comparison of LV mechanics between controls and systemic hypertension is shown as a graph in Fig. 3.
Table 2.
Comparison of left ventricular mechanics between two groups.
| Characteristic | Controls (n = 25) | Systemic hypertension (n = 34) | P |
|---|---|---|---|
| Longitudinal strain | |||
| Subendocardial (%) | −20.2 ± 4.7 | −13.4 ± 5.8 | <0.001 |
| Subepicardial (%) | −17.5 ± 4.4 | −11.3 ± 5.6 | <0.001 |
| Circumferential strain | |||
| Subendocardial (%) | −23.5 ± 8.8 | −19.0 ± 9.6 | 0.06 |
| Subepicardial (%) | −6.6 ± 2.1 | −5.1 ± 3.4 | 0.03 |
| Radial strain (%) | 20.3 ± 11.3 | 17.2 ± 10.3 | 0.23 |
Fig. 1.
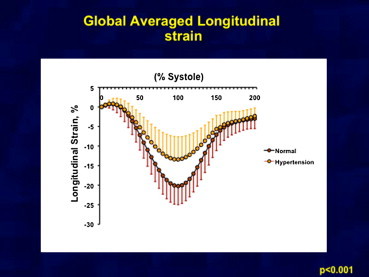
Showing average of subendocardial longitudinal strain in two groups. There is significant difference in longitudinal strain in both groups.
Fig. 2.
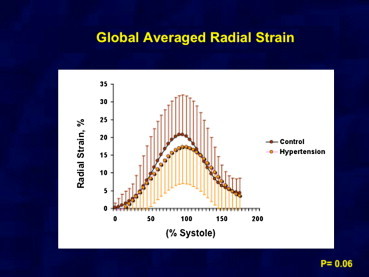
Showing average of radial strain in two groups. No significant difference in radial strain seen in two groups.
Fig. 3.
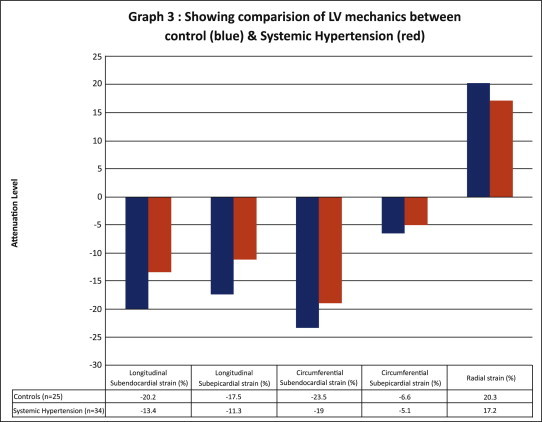
Graph showing comparison of LV mechanics between control (blue) and systemic hypertension (red).
4. Discussion
In hypertensive patients with abnormal diastolic LV filling, LV systolic function is commonly considered normal if the global ejection fraction (GEF) and fractional shortening (FS) are normal.12,13 However, the GEF and FS only reflect the global cardiac contractile function and do not take regional systolic abnormalities into consideration. The above results show that 2D speckle tracking is able to detect sub-clinical myocardial dysfunction in hypertensive patients with LVH despite normal global systolic parameters.
2D speckle tracking echocardiography is a relatively new technique that can be used in conjunction with two or three-dimensional echocardiography to detect the multidirectional components of LV deformation. The tracking system is based on gray-scale B-mode images and is obtained by automatic measurement of the distance between two pixels of an LV segment during the cardiac cycle, independent of the angle of insonation. The integration of 2D speckle tracking with real-time cardiac ultrasound imaging overcomes some of the limitations of previous work in the field and has the potential to provide a unified framework to more accurately quantify the regional and global function of the LV. 2D speckle tracking holds promise to reduce inter- and intra-observer variability in assessing regional LV function and improve patient care while reducing health care costs by early identification of subclinical disease.14
The longitudinal fiber shortening was less in the LVH group with normal GEF and FS than the corresponding segments in the control group, a finding that was consistent with the results of Poulsen et al.15 The subendocardial longitudinal-oriented myocardial fibers have shown to be particularly vulnerable to ischemia leading to a dominant decrease in shortening in the longitudinal axis.16,17 Manaka et al found that myocardial systolic impairment in hypertensive LVH may originate at the endocardial side and significantly move to the epicardium compared with control, and the impairment may progress with increased LVH, but this study was limited to the longitudinal direction.18 Our findings in patients with hypertension and decreased tissue tracking values in the longitudinal fibers might be explained by the presence of regional subendocardial myocardial ischemia and increased perivascular and interstitial fibrosis, which was previously demonstrated in patients with hypertension.19 It is also possible that the level of end-systolic wall stress is different between the groups, affecting the subendocardial fiber shortening. Furthermore, the hypertrophic myocardium itself may impede contraction. These pathophysiologic changes are likely to lead to decreased longitudinal systolic contraction and might also result in a heterogeneous segmental diastolic impairment affecting the global diastolic function. There was no significant difference in circumferential strain values in both the groups. Radial strain was reduced, although it was not significant reduction as compared to controls. Kosmala et al20 and Imbalazano et al21 have reported reduced radial strain in hypertensive patients along with reduced longitudinal study. 2D strain has a limitation of through plane motion, although least in longitudinal direction.
Hypertension and LVH are important risk factors for developing chronic congestive heart failure and, although patients with congestive heart failure and a normal LVEF have a lower mortality risk than those with reduced EF, the mortality risk is still significantly increased over control patients.22 The assessment of LV systolic longitudinal contraction by 2D speckle tracking gives new insight in myocardial function in hypertension that might improve pathophysiologic understanding and identify patients at high risk who would benefit from regression of LV hypertrophy following a more aggressive antihypertensive treatment program. The combination of depressed longitudinal systolic contraction and abnormal diastolic LV filling may play a key role in the development of acute and chronic heart failure in patients with hypertension.
4.1. Study limitations
The present study is a single-center observational study. It is limited by the relative small sample size. Also torsion and rotational characteristics have not been discussed.
5. Conclusion
Despite reduced longitudinal shortening, LV wall thickening in patients with systemic hypertension remains unaltered because of relatively preserved circumferential shortening. Characterizing the disparities in LV principal strains unmasks presence of subclinical LV dysfunction and provides unique insights regarding functional adaptations that maintain global LV ejection fraction in patients with systemic hypertension.
Conflicts of interest
All authors have none to declare.
References
- 1.Rovner A., de las Fuentes L., Waggoner A.D., Memon N., Chohan R., Dávila-Román V.G. Characterization of left ventricular diastolic function in hypertension by use of Doppler tissue imaging and color M-mode techniques. J Am Soc Echocardiogr. 2006;19:872–879. doi: 10.1016/j.echo.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Lorell B.H., Carabello B.A. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 3.Edvardsen T., Rosen B.D., Pan L. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging – the Multi-Ethnic Study of Atheroscleosis (MESA) Am Heart J. 2006;151:109–114. doi: 10.1016/j.ahj.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Reisner S.A., Lysyansky P., Agmon Y., Mutlak D., Lessick J., Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630–633. doi: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Helle-Valle T., Crosby J., Edvardsen T. New non-invasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112:3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- 6.Nakai H., Takeuchi M., Nishikage T., Kokumai M., Otani s, Lang R.M. Effect of aging on twist- displacement loop by 2- dimensional speckle tracking imaging. J Am Soc Echocardiogr. 2006;19:880–885. doi: 10.1016/j.echo.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Geyer H., Caracciolo G., Abe H. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Schiller N.B., Shah P.M., Crawford M. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:e358–e367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 9.Thomas G. Response to “non-Doppler two-dimensional strain imaging by echocardiography-from technical considerations to clinical applications”. J Am Soc Echocardiogr. 2007;20:1020. doi: 10.1016/j.echo.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Helle-Valle T., Crosby J. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112:3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- 11.Caracciolo G., Eleid M., Abe H., Sengupta P.P. Non-uniform recovery of left ventricular transmural mechanics in ST-segment elevation myocardial infarction. Cardiovasc Ultrasound. 2012;8:31. doi: 10.1186/1476-7120-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Cao T., Duan Y., Yuan L., Wang Z. Velocity vector imaging in assessing myocardial systolic function of hypertensive patients with left ventricular hypertrophy. Can J Cardiol. 2007;23:957–961. doi: 10.1016/s0828-282x(07)70857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edvardsen T., Skulstad H., Uhrheim S., Ihlen H. Regional myocardial function during acute myocardial ischemia assessed by strain Doppler echocardiography. J Am Coll Cardiol. 2001;37:726–730. doi: 10.1016/s0735-1097(00)01160-8. [DOI] [PubMed] [Google Scholar]
- 14.Saha S.K., Kiotsekoglou A., Toole R.S. Value of two-dimensional speckle tracking and real time three-dimensional echocardiography for the identification of subclinical left ventricular dysfunction in patients referred for routine echocardiography. Echocardiography. 2012;29:588–597. doi: 10.1111/j.1540-8175.2011.01631.x. [DOI] [PubMed] [Google Scholar]
- 15.Poulsen S.H., Andersen N.H., Ivarsen P.I., Mogensen C.E., Egeblad H. Doppler tissue imaging reveals systolic dysfunction in patients with hypertension and apparent “isolated” diastolic dysfunction. J Am Soc Echocardiogr. 2003;16:724–731. doi: 10.1016/S0894-7317(03)00403-6. [DOI] [PubMed] [Google Scholar]
- 16.Jones C., Raposo L., Gibson D. Functional importance of the long axis dynamics of the human left ventricle. Br Heart J. 1990;63:215–220. doi: 10.1136/hrt.63.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcos-Alberca P., Garcia-Fernandez M., Ledesma M.J. Intramyocardial analysis of regional systolic and diastolic function in ischemic heart disease with Doppler tissue imaging: role of the different myocardial layers. J Am Soc Echocardiogr. 2002;15:99–108. doi: 10.1067/mje.2002.120634. [DOI] [PubMed] [Google Scholar]
- 18.Manaka M., Tanaka N., Takei Y., Kurohane S., Takazawa K., Yamashina A. Assessment of regional myocardial systolic function in hypertensive left ventricular hypertrophy using harmonic myocardial strain imaging. J Cardiol. 2005;45:53–60. [PubMed] [Google Scholar]
- 19.Querejeta R., Varo N., López B. Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation. 2000;101:1729–1735. doi: 10.1161/01.cir.101.14.1729. [DOI] [PubMed] [Google Scholar]
- 20.Kosmala W., Plaksej R., Strotmann J.M. Progression of left ventricular functional abnormalities in hypertensive patients with heart failure: an ultrasonic two-dimensional speckle tracking study. J Am Soc Echocardiogr. 2008;21:1309–1317. doi: 10.1016/j.echo.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Imbalzano E., Zito C., Carerj S. Left ventricular function in hypertension: new insight by speckle tracking. Echocardiography. 2011;28:649–657. doi: 10.1111/j.1540-8175.2011.01410.x. [DOI] [PubMed] [Google Scholar]
- 22.Aurigemma G.P., Gottdiener J.S., Shemanski L., Gardin J., Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]


