Abstract
Background
Previous studies demonstrated dysregulated expression of microRNAs (miRNAs) in the myocardium of patients with dilated cardiomyopathy (DCM). This study investigated levels of miRNAs in the circulation of DCM patients, and the value of miRNAs as biomarkers for DCM.
Methods and materials
In 45 DCM patients and 39 age- and sex-matched controls, circulating miR-423-5p, miR-126, miR-361-5p, miR-155, and miR-146a concentrations were measured and correlated to cardiac functional parameters, including left ventricular ejection fraction (LVEF) and N-terminal pro-brain natriuretic peptide (NT-proBNP).
Results
Plasma levels of miR-126 and miR-361-5P did not differ between the DCM and control groups (p = 0.331 and p = 0.784, respectively). Plasma levels of the immunity-associated miRNAs, miR-146a and miR-155, did not differ between the DCM and control groups (p = 0.437 and p = 0.702, respectively). Levels of circulating miR-423-5p were significantly greater in the DCM group (p = 0.003). Further, there was a positive correlation between plasma levels of miR-423-5p and NT-proBNP (r = 0.430, p = 0.003). MiR-423-5p distinguished DCM cases from controls with an area under the curve (AUC) receiver operating characteristic (ROC) curve of 0.674 (95% CI, 0.555–0.793).
Conclusions
Patients with DCM have elevated plasma miR-423-5p levels. The plasma concentration of miR-423-5p was positively correlated with the level of NT-proBNP. Circulating levels of miR-423-5p could be served as a diagnostic biomarker for heart failure caused by DCM. Plasma levels of immunity-associated miR-146a, -155, and -126 were not significantly different between DCM and control groups.
Keywords: Biomarkers, Dilated cardiomyopathy, Heart failure, MicroRNA
Abbreviations: AUC, area under the receiver–operator characteristic curve; NT-proBNP, N-terminal pro-brain natriuretic peptide; DCM, dilated cardiomyopathy; EF, ejection fraction; HF, heart failure; miRNA, microRNA; ROC, receiver–operator characteristic
1. Introduction
MicroRNAs (miRNAs) are endogenous small RNAs that are 21–25 nucleotides in length.1 Dysregulation of intracellular miRNA expression has been described in various diseases, including a number of cardiovascular conditions.2 Many studies have indicated that miRNAs are detectable and highly stable in plasma and other biological fluids. Furthermore, levels of circulating miRNAs have been shown to correlate with disease, thereby suggesting that miRNAs, might be valuable diagnostic biomarkers.2
Previous reports demonstrated a potential pathophysiological link between expression of miRNAs (miR-126, miR-155, miR-146a) and immune response for humans with dilated cardiomyopathy (DCM).3,4 Other studies showed up-regulated expression of miR-423-5p and miR-361-5p in DCM myocardium.5 The purpose of this study was to characterize levels of cardiac-associated miRNAs in the circulation of DCM patients and to determine their value as biomarkers for DCM.
2. Materials and methods
2.1. Participants
We recruited 45 DCM patients with New York Heart Association (NYHA) function classes I–IV, and a control group of 39 healthy age- and gender-matched volunteers, at the First Affiliated Hospital (Nanjing Medical University) after obtaining their written informed consent. Inclusion criteria for the DCM group were: a left ventricular ejection fraction (LVEF) less than 45%, as determined by two-dimensional and Doppler echocardiography (GE Vivid VII scanner with 1.7–3.4 MHz transducers); Exclusion criteria were: coronary heart disease (stenosis >50% of the luminal diameter in a major branch), as judged by coronary angiography; arterial hypertension (>160/100 mmHg); primary valvular heart disease; cardiomyopathy secondary to any systematic disease; clinical, sustained and rapid supraventricular arrhythmias; pericardial diseases; congenital heart diseases; cor pulmonale.6 Inclusion criteria for the control group were: absence of known coronary, valvular, or myocardial disease. Exclusion criteria for all participants were: pregnancy, dialysis, and known or treated malignancies. The Institutional Review Board of the First Affiliated Hospital approved the study protocol.
2.2. N-terminal pro-brain natriuretic peptide (NT-proBNP) measurements
NT-proBNP was measured using an electrochemiluminescence immunoassay (E170 Roche Diagnostics, Mannheim, Germany).
2.3. Plasma RNA purification
Blood samples were collected in tubes containing EDTA (Gong Dong EDTA K2), and plasma was isolated by centrifugation at 1500 ×g for 15 min at 4 °C. Total RNA was isolated from 400 μL of plasma using the mirVana PARIS kit (AM1556, Ambion) according to the manufacturer's instructions. Before RNA isolation, 25 fmol of synthetic Caenorhabditis elegans MicroRNA-39 (cel-miRNA-39; Qiagen, Germany) was added to the mixture as an internal reference.7 RNA isolated from plasma was subsequently re-suspended in 100 μL of RNase-free water, and then stored at −80 °C.
2.4. Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Reverse transcription of miRNAs was performed using the TaqMan® MicroRNA Reverse Kit (Applied Biosystems, Foster, CA) according to the manufacturer's recommendations. The 15 μL RT reaction system contained 5 μL of RNA extract, 0.15 μL of 100 mM dNTPs (with dTTP), 1 μL of multiscribe reverse transcriptase (50 U/μL), 1.5 μL of 10 × RT buffer, 0.19 μL of RNase inhibitor (20 U/μL), 4.16 μL of RNase-free water, and 3 μL of 5 × miRNA-specific stem-loop RT primer (Applied Biosystems, Foster, CA). For real-time quantitative PCR (qRT-PCR), 1.33 μL of the cDNA product was used as a template in 20 μL reactions containing 1 μL of TaqMan® MicroRNA Assay, 7.67 μL of RNase-free water, and 10 μL of TaqMan® 2× Universal PCR Master Mix, No AmpErase® UNG (Applied Biosystems, Foster, CA). qRT-PCR was performed with 7900HT real-time PCR system (Applied Biosystems, Foster, CA). Triplicate measurements were obtained for each sample on a 384-well plate (Applied Biosystems, Foster, CA). This reaction contained a miRNA-specific forward primer, and a TaqMan® probe complementary to the 3′-end of the specific miNA sequence. Data were analyzed with SDS Relative Quantification Software version 2.2.2 (Applied Biosystems, Foster, CA), with the automatic Ct setting for assigning baseline and threshold for Ct determination. The relative expression level of each individual miRNA after normalization to cel-miRNA-39 was calculated using the 2−△△Ct method.8
2.5. Statistical analyses
Continuous data are presented as mean ± standard deviation (SD). Categorical data are presented as counts and proportions. Values were log-transformed when appropriate for statistical analysis. Between group comparisons were examined using unpaired Student's t-tests and χ2 tests for continuous and categorical variables, respectively. One-way ANOVA was used if more than two groups were compared. For correlation, Pearson's or Spearman's correlation coefficient was calculated for continuous and categorical data, respectively. Receiver Operating Characteristic (ROC) curve analysis was used to assess the diagnostic accuracy of miRNAs. The area under the ROC curve (AUC) was used as diagnostic index. Statistical significance was assumed at p < 0.05 and was two-sided. For all statistical analyses, the statistical software SPSS 17.0 (Statistical Package for the Social Sciences, Chicago, IL) for Windows was used.
3. Results
No significant differences in the clinical characteristics of the study populations were observed (Table 1). NT-proBNP measurements were only performed on DCM patients, most of whom were receiving diuretics, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers and β blockers. Plasma levels of miR-126 and miR-361-5P did not differ between the DCM and control groups (3.59 vs. 3.21, p = 0.331; 5.81 vs. 5.63; p = 0.784, respectively). Plasma levels of the immunity-associated miRNAs, miR-146a and miR-155, did not differ between the DCM and control groups (3.63 vs. 3.29, p = 0.437; 4.13 vs. 4.27, p = 0.702, respectively). miR-423-5p was the only microRNA that was significantly greater in DCM patients, compared with controls (5.851 vs. 4.619, p = 0.003) (Fig. 1). Plasma concentrations of miR-423-5p were positively correlated with NT-proBNP for the DCM group (Fig. 2 r = 0.430, p = 0.003). Plasma concentrations of miR-423-5p did not correlate with NYHA functional class or LVEF values. The diagnostic accuracy of miR-423-5p was evaluated using ROC curve analyses with an AUC of 0.674 (Fig. 3 95% confidence interval 0.555–0.793). By using a threshold miR-423-5PLnRQ of 4.017 as the lower limit for patients with DCM, there was a sensitivity of 87% and a specificity of 49% for patients with DCM patients.
Table 1.
Baseline clinical and echocardiographic characteristics of dilated cardiomyopathy (DCM) patients and healthy controls. Values are mean ± SD or number (%).
| Characteristic | Control | DCM | p-value |
|---|---|---|---|
| Age (years)a | 47.59 ± 11.85 | 47.76 ± 12.28 | 0.95 |
| Gender (male) | 25(64.1%) | 32 (71.1%) | 0.64 |
| Ejection fraction (%)a | 61.78 ± 4.15 | 33.80 ± 6.63 | <0.01 |
| Creatine (μmol/L)a | 73.67 ± 14.75 | 96.05 ± 30.68 | <0.01 |
| NT-proBNP (ng/L)b | – | 2733.50 (61–35,000) | |
| β-Blockers | – | 42 (93.3%) | |
| ACEI/ARB | – | 41 (91.1%) | |
| Diuretics | – | 41 (91.1%) | |
| NYHA grade | – | ||
| Class I | 2 (4.4%) | ||
| Class II | 21 (46.7%) | ||
| Class III | 14 (31.1%) | ||
| Class IV | 8 (17.8%) |
Abbreviations: DCM, dilated cardiomyopathy; NT-proBNP, N-terminal pro-brain natriuretic peptide; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; NYHA, New York Heart Association.
Mean + SD.
Median (interquartile range).
Fig. 1.

Circulating miRNAs in research cohorts. Expression of miRNAs in plasma was obtained from patients with DCM (n = 45) and control subjects (n = 39), as determined by TaqMan qRT-PCR. Values were normalized to cel-miR-39. Abbreviations: DCM: dilated cardiomyopathy. *Significant differences between DCM and control groups (p < 0.01). Plasma levels of miR-146a, -155, -126 and -361-5p were not significantly different between DCM and control groups.
Fig. 2.
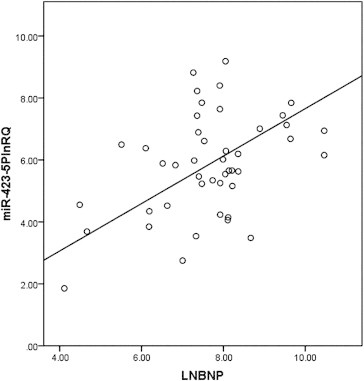
Positive correlation between NT-proBNP and miR-423-5P in patients with dilated cardiomyopathy (DCM). A significant relationship was observed (r = 0.430; p = 0.003; n = 45).
Fig. 3.
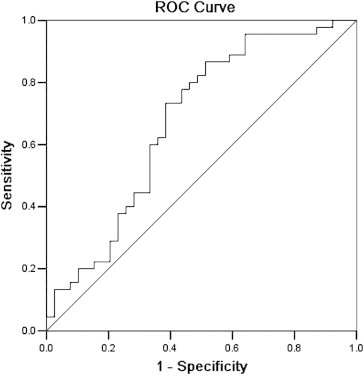
Diagnostic accuracy of miRNA-423-5p. A receiver–operator characteristic (ROC) curve was established to determine the diagnostic power for distinguishing DCM cases from healthy people. The area under the curve (AUC) was 0.674 (95% confidence interval 0.555–0.793). Use of a miR-423-5pLnRQ threshold of 4.017 as the lower limit for patients in the DCM group, resulted in a sensitivity of 87% and a specificity of 49% for the identification of DCM patients.
4. Discussion
Previous reports demonstrated a potential pathophysiologic link between expression of miRNAs (miR-126, miR-155, miR-146a) and immune response in human DCM.3,4 It was also demonstrated that circulating levels of some miRNAs were indices of patients with HF,9,10 although there was limited evaluation of levels in patients with DCM. We did not confirm dysregulation of miR-126 and miR-361-5P plasma levels in DCM patients, in contrast to reports of patients with ischemic heart disease by Fukushima and Chen.11,12 This difference may be explained, in part, by decreased expression of miR-126 in diabetes mellitus and coronary heart disease.13,14 In addition, elevated expression of miR-361-5p is associated with metastatic colorectal cancer,15 whereas its involvement in heart failure is undetermined.
Thomas Thum's study demonstrated up-regulated expression of miR-423-5p in DCM myocardium.5 In addition, Tijsen et al. found that miR-423-5p was able to distinguish heart failure from healthy controls and patients with dyspnea attributable to other causes (with an area under the curve of 0.91).9 Similarly, Yaron Goren reported that plasma levels of miR-423-5p were higher in HF patients than in controls.10 A possible mechanism for this is that expression of miR-423-5P in cell is up-regulated by ANP.16 We also found that miR-423-5p mean plasma levels were significantly higher in DCM patients than in healthy subjects, but the degree of increase and diagnostic value are conspicuously lower than for the previous studies. This may be because the previous studies included ischemic heart disease. This is supported by Tutarel's data, that patients with reduced systemic right ventricle ejection fraction miR-423-5p levels were not elevated.17 Consequently, microRNA levels associated with different categories of heart failure may vary. Therefore, further studies are needed to evaluate the diagnostic value of miR-423-5P levels in plasma to identify patients with heart failure.
Our study showed that miR-423-5p levels are also positively related to NT-proBNP levels in patients with DCM. Similarly, Tijsen demonstrated a correlation between the plasma levels of miR-423-5p and NT-proBNP.9 Consequently, circulating levels of miR-423-5p may reflect the severity of DCM.
MiR-146a and miR-155 are immunity-associated miRNAs that may contribute to the development of heart failure.18 Prior studies showed that miR-146a and miR-155 levels are increased in the myocardium of heart failure patients.4,19 Corsten showed that inflammation-miRNA-155 plays a central role in the induction of cardiac inflammation and failure.20 Our study showed no dysregulation in the plasma levels of miR-146a and miR-155 in patients with DCM. We suspect that these two miRNAs are sufficiently localized that any variation in myocardial levels is not detectable in plasma.
There are several limitations to this study. Although our cohort of participants is relatively large when compared with previous miRNAs studies, one of the major limitations of this study is sample size. Secondly, we have not completed long-term follow-ups of patients. Consequently, the potential for miR-423-5p to be served as prognostic indicator for DCM needs to be studied.
In conclusion, our results suggest that patients with dilated cardiomyopathy have elevated plasma levels of miR-423-5p. In addition, plasma concentration of miR-423-5p may reflect the severity of DCM. The potential for miR-423-5P to discriminate between heart failure caused by DCM patients and healthy people warrants further investigation in larger populations. Plasma levels of immunity-associated miR-146a, -155, and -126 were not significantly different between DCM and healthy groups.
Conflicts of interest
All authors have none to declare.
Acknowledgments
This study supported by “Twelve Five-Year” National Science and Technology Support Program (2011BAI11B08) and National Natural Science Foundation Project (Grant Number:81170201/H0208).
References
- 1.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S.K., Bang C., Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet. 2010;3:484–488. doi: 10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- 3.Satoh M., Minami Y., Takahashi Y. A cellular microRNA, let-7i, is a novel biomarker for clinical outcome in patients with dilated cardiomyopathy. J Card Fail. 2011;17:923–929. doi: 10.1016/j.cardfail.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Horie T., Ono K., Nishi H. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc Res. 2010;87:656–664. doi: 10.1093/cvr/cvq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thum T., Galuppo P., Wolf C. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 6.Richardson P., McKenna W., Bristow M. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 7.Kroh E.M., Parkin R.K., Mitchell P.S. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 2010;50:298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 9.Tijsen A.J., Creemers E.E., Moerland P.D. MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010;106:1035–1039. doi: 10.1161/CIRCRESAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 10.Goren Y., Kushnir M., Zafrir B. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail. 2012;14:147–154. doi: 10.1093/eurjhf/hfr155. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima Y., Nakanishi M., Nonogi H. Assessment of plasma miRNAs in congestive heart failure. Circ J. 2011;75:336–340. doi: 10.1253/circj.cj-10-0457. [DOI] [PubMed] [Google Scholar]
- 12.Chen C., Yang S.L., Wang F. Plasma microRNA-361-5p as a biomarker of chronic heart failure. Heart. 2010:A189. [Google Scholar]
- 13.Zampetaki A., Kiechl S., Drozdov I. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 14.Fichtlscherer S., De Rosa S., Fox H. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 15.Schou J.V., Andersen K.K., Jensen B.V. Prediction of survival in patients with metastatic colorectal cancer treated with third-line cetuximab and irinotecan through changes in microRNA expression in whole blood during treatment. J Clin Oncol. 2011;29(15 suppl) abstr 3532. [Google Scholar]
- 16.Kotlo K.U., Hesabi B., Danziger R.S. Implication of microRNAs in atrial natriuretic peptide and nitric oxide signaling in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2011;301:C929–C937. doi: 10.1152/ajpcell.00088.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tutarel O., Dangwal S., Bretthauer J. Circulating miR-423_5p fails as a biomarker for systemic ventricular function in adults after atrial repair for transposition of the great arteries. Int J Cardiol. 2011 Dec 20 doi: 10.1016/j.ijcard.2011.11.082. Epub (ahead of print) [DOI] [PubMed] [Google Scholar]
- 18.Quinn S.R., O'Neill L.A. A trio of microRNAs that control Toll-like receptor signalling. Int Immunol. 2011;23:421–425. doi: 10.1093/intimm/dxr034. [DOI] [PubMed] [Google Scholar]
- 19.da Costa Martins P.A., Bourajjaj M., Gladka M. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation. 2008;118:1567–1576. doi: 10.1161/CIRCULATIONAHA.108.769984. [DOI] [PubMed] [Google Scholar]
- 20.Corsten M.F., Schroen B., Van De Vrie M. Absence of microRNA-155 protects against adverse cardiac inflammation and hypertrophy during pressure overload and prevents heart failure. Eur J Heart Fail Supplements. 2011;10:S5–S6. [Google Scholar]


