Abstract
Objectives
To determine relationship of body mass index (BMI) with multiple cardiovascular risk factors.
Methods
Population-based surveys were performed and 1893 subjects aged 20–59 years evaluated. Data were collected using anthropometry and fasting glucose and lipid estimation. Statistical analyses were performed using curve fit and logistic regression.
Results
Body mass index was correlated significantly (Rho, R2) with weight (0.80, 0.64), waist (0.74, 0.55) and waist hip ratio (0.24, 0.06) (P < 0.05). Linear relationship was observed with systolic blood pressure (SBP) (0.39, 0.15), diastolic blood pressure (DBP) (0.29, 0.08), fasting glucose (0.13, 0.02), cholesterol (0.10, 0.01), high-density lipoprotein cholesterol (HDL-c) (−0.16, 0.03), and triglycerides (0.12, 0.01). Significant trends of risk factors with each increasing BMI unit (χ2 test, P < 0.001) were observed for hypertension (HTN) (214.4), diabetes (29.5), metabolic syndrome (108.9), and low HDL-c (40.5), and weaker trends with hypercholesterolemia (20.6), and hypertriglyceridemia (9.6). There was exponential relationship of BMI with age- and sex-adjusted odds ratios for HTN, diabetes, and metabolic syndrome.
Conclusion
Metabolic cardiovascular risk factors continuously worsen with increasing BMI.
Keywords: Cardiovascular diseases, Hypertension, Low income countries, Metabolic syndrome, Obesity, Risk factors
Introduction
Prospective Studies Collaboration has reported that there is a significant correlation of body mass index (BMI) with cardiovascular mortality.1 In a meta-analysis of about a million Caucasian subjects, who were prospectively followed for at least 2 years, it was reported that there was a U-shaped correlation of all-cause mortality with BMI; increased mortality in lower BMI arm was due to respiratory and infectious diseases while the higher BMI was associated with greater cardiovascular mortality. It was also reported that there is a continuous gradient of cardiovascular mortality starting with BMI of 21 kg/m2. Similar U-shaped curve has been reported in studies from USA, UK, and Korea.2–13 The US National Cancer Institute prospectively studied 1.46 million Caucasian subjects and reported a J-shaped mortality curve with lowest deaths at BMI of 22.5–24.9 and highest at > 30.0 kg/m2.6 Two prospective studies from India noted a reverse J-shaped curve with greatest all-cause mortality at BMI < 18 kg/m2.7,8 For cardiovascular mortality the relationship was not clear.7 A Korean study11 reported a linear increase in cardiovascular mortality as BMI increased from 18.5 kg/m2 to > 30 kg/m2 while the US cancer cohort study showed a J-shaped graph with the lowest mortality at BMI 20–22.4 kg/m2 and highest at 40–49 kg/m2.6
Relationship of metabolic cardiovascular risk factors with BMI has been studied in multiple populations in Europe, north America and Asia.2,14,15 These studies reported a variable trend in multiple metabolic risk factors with increasing BMI. Continuous linear relationship of hypertension (HTN) with increasing BMI has been reported in all the studies16 while variable results have been obtained with other cardiovascular risk factors such as diabetes and dyslipidaemia. Framingham Offspring study reported significant correlation of BMI with blood pressure (BP), glucose, cholesterol, and other lipids17 while a similar study in Chinese populations showed correlation with HTN and dyslipidaemia and not with diabetes.18
Indian National Family Health Surveys reported a rapid increase in BMI and prevalence of obesity in the country.19 Increasing urbanisation with associated dietary and physical activity transitions is fuelling the obesity epidemic in India.20 Increased BMI has been shown to be associated with increased cardiovascular risks in urban Indian populations.21 There is controversy regarding levels of BMI where cardiovascular risks increases in various low income countries.22 Studies have reported that BMI ≤ 25 kg/m2 is associated with increased cardiovascular risks while a few suggest that BMI ≤ 23 kg/m2 should be used as a cut-off for defining overweight.23 We performed cross-sectional studies in north India to identify prevalence of major cardiovascular risk factors.25,26 To correlate BMI and with multiple metabolic cardiovascular risk factors we analysed data using regression-based statistical techniques.
Methods
A series of cross-sectional epidemiological studies have been performed to determine cardiovascular risk factors in urban populations in Jaipur and Delhi. These studies were approved by the Institutional Ethics Committee and supported financially by different organisations. In Jaipur Heart Watch (JHW) series,26,27 we targeted men and women for complete socioeconomic, physical, and biochemical profiles in contrast to the others where biochemical measurements were obtained in random subjects. We conducted stratified cluster sampling on the Voters' lists in six locations representing an adult population of about 130,000 in Jaipur city in JHW-226 and two locations in JHW-3.27 The studies were representative of local population as reported earlier.27 In JHW-2, of the targeted population proportionate 960 men and 840 women, we evaluated 550 men (57.3%) and 573 women (68.2%) and in JHW-3, of the eligible 320 men and 280 women, we evaluated 226 (70.6%) and 232 (82.9%), respectively (overall response rate 62%). For the present analyses we included subjects 20–59 years of age (619 men, 661 women). In Delhi,25 data were obtained from a study by systematic random sampling among a population of about 30,000. The overall response rate was 80.5% as reported earlier.28 In brief, we collected information regarding demographic data, educational level, history of chronic illnesses such as coronary heart disease, HTN, diabetes, or high cholesterol levels, and smoking or tobacco intake. Income details were not inquired. Brief questions were asked to evaluate physical activity and diet but the results were considered inadequate and not included in the analyses. Physical examination was performed to assess height, weight, waist and hip circumference, and BP. Body mass index was calculated as weight (kg) divided by squared height (m). Waist hip ratio (WHR) was calculated. Fasting glucose was determined at a central laboratory using glucose peroxidase method and external quality control. Total cholesterol (TC) was measured using cholesterol oxidase-phenol 4-aminophenazone peroxidase method and high-density lipoprotein cholesterol (HDL-c) using an enzymatic method after precipitating non-HDL-c with a manganese-heparin substrate. Triglycerides were measured using the glycerol phosphate oxidase-peroxidase enzymatic method. Quality control measures were followed for estimation of TC, HDL-c and triglycerides (TG) while low-density lipoprotein cholesterol (LDL-c) was estimated using the Friedewald's formula.
Diagnostic criteria
We used the diagnostic criteria as advised by American College of Cardiology clinical data standards.29 Smokers included subjects with present or past smoking. Isolated non-smoked tobacco use was also identified. Hypertension was diagnosed when the systolic BP (SBP) or diastolic BP (DBP) was ≤ 140/≤ 90 mmHg on a repeated single day measurements or the individual was a known hypertensive. Dyslipidaemia was defined by the presence of high TC (≤ 200 mg/dL), high LDL-c (≤ 130 mg/dL), low HDL-c (< 40 mg/dL), or high TG (≤ 150 mg/dL) according to National Cholesterol Education Program, Adult Treatment Panel III (NCEP, ATP III) guidelines.30 Diabetes was diagnosed when a subject provided history of previously diagnosed diabetes or the fasting blood glucose was ≤ 126 mg/dL. Metabolic syndrome was also defined according to the NCEP ATP III guidelines30 and presence of any three of the five criteria (high waist circumference [WC] > 100 cm men, > 90 cm women; BP ≤ 130 mmHg systolic and/or ≤ 90 mmHg diastolic; fasting hyperglycaemia ≤ 110 mg/dL; low HDL-c < 40 mg/dL men < 50 mg/dL women; and high TG ≤ 150 mg/dL) were considered diagnostic.
Statistical analysis
Continuous variables are reported as mean ± 1 standard deviation and ordinal variables in percent. Prevalence rates are reported in percent. Age- and sex-adjustment of various continuous variables (BMI, BP, glucose, and lipids) was performed within the statistical programme (SPSS version 15.0, SPSS Inc, Chicago, USA) using analysis of covariance (ANCOVA). Direct method was used for age adjustment of prevalence rates with standard Indian million population.31 Linear associations of BMI with continuous risk factor variables were calculated using Spearman's rho, linear regression, exponential regression and quadratic regression analysis within the statistical programme.32 Graphics to plot scatter distribution of BMI with numerical variables and box-plot graphs for BMI categories and numerical variables have been produced using SPSS programme. Significance has been evaluated using ANOVA for trend. Trends in prevalence rates have been calculated using Mantel Haenzel χ2. Age- and sex-adjusted odds ratios (OR) for risk factor prevalence at each BMI category were calculated using logistic regression analysis. P values < 0.05 are considered significant.
Results
We evaluated 1893 subjects (men 949, women 944) aged 20–59 years. Association of each kg/m2 unit increase in BMI with multiple anthropometric factors is shown in Table 1. Correlation analysis, with Spearman's Rho and R2, respectively, indicate a non-significant relationship with height and a significant association with weight (0.80, 0.64), waist (0.74, 0.55), hip (0.44, 0.21), and WHR (0.24, 0.06) (P < 0.05). There is a linear relationship with SBP (0.39, 0.15), DBP (0.29, 0.08), fasting glucose (0.13, 0.02), cholesterol (0.10, 0.01), HDL-c (–0.16, 0.03), and TG (0.12, 0.01) (Table 2). Scatter-plots and graphic analysis of association of BMI with risk factors shows a significant positive relationship with SBP, fasting glucose, TC, TG, and LDL-c, and negative correlation with HDL-c (Figure 1). Quadratic regression analysis shows similar associations of BMI with SBP, DBP, fasting glucose, TC, HDL-c, and TG (data not shown).
Table 1.
Mean anthropometric values at different body mass index levels among 1893 subjects (men 949, women 944) aged 20–59 years in north India during study years 1999–2004.
| BMI groups | Height | Weight | Waist | Hip | WHR |
|---|---|---|---|---|---|
| < 18 (n = 170) | 162.53 ± 9.57 | 43.17 ± 6.32 | 68.37 ± 7.41 | 81.72 ± 10.64 | 0.82 ± 0.08 |
| 18–18.9 (n = 69) | 163.49 ± 11.37 | 49.81 ± 7.05 | 73.89 ± 7.27 | 82.27 ± 13.91 | 0.86 ± 0.08 |
| 19–19.9 (n = 80) | 163.61 ± 9.71 | 52.49 ± 6.28 | 76.45 ± 7.98 | 85.35 ± 16.52 | 0.85 ± 0.08 |
| 20–20.9 (n = 87) | 164.75 ± 9.88 | 55.94 ± 6.71 | 77.71 ± 6.78 | 85.23 ± 15.61 | 0.87 ± 0.06 |
| 21–21.9 (n = 108) | 162.42 ± 10.01 | 57.09 ± 7.09 | 81.86 ± 8.29 | 88.48 ± 15.44 | 0.88 ± 0.08 |
| 22–22.9 (n = 126) | 163.71 ± 11.03 | 60.73 ± 8.21 | 82.77 ± 10.01 | 87.14 ± 18.64 | 0.89 ± 0.08 |
| 23–23.9 (n = 161) | 166.23 ± 9.91 | 65.10 ± 7.80 | 87.29 ± 8.37 | 92.14 ± 15.52 | 0.90 ± 0.08 |
| 24–24.9 (n = 152) | 166.21 ± 10.82 | 68.10 ± 8.88 | 90.47 ± 7.10 | 95.41 ± 14.27 | 0.92 ± 0.06 |
| 25–25.9 (n = 138) | 162.46 ± 11.37 | 67.68 ± 9.61 | 88.15 ± 11.48 | 95.51 ± 15.83 | 0.89 ± 0.12 |
| 26–26.9 (n = 146) | 163.35 ± 9.86 | 71.70 ± 11.44 | 93.01 ± 7.67 | 95.58 ± 19.36 | 0.91 ± 0.07 |
| 27–27.9 (n = 131) | 162.80 ± 9.99 | 73.10 ± 9.19 | 95.97 ± 8.25 | 100.57 ± 16.93 | 0.91 ± 0.07 |
| 28–28.9 (n = 101) | 159.65 ± 10.29 | 72.39 ± 9.60 | 95.10 ± 8.84 | 99.89 ± 19.33 | 0.90 ± 0.08 |
| 29–29.9 (n = 83) | 162.31 ± 10.14 | 77.62 ± 10.02 | 99.05 ± 9.56 | 102.25 ± 19.50 | 0.91 ± 0.12 |
| 30–30.9 (n = 99) | 163.26 ± 10.89 | 81.41 ± 10.90 | 104.79 ± 19.01 | 106.74 ± 21.38 | 0.95 ± 0.14 |
| 31–31.9 (n = 50) | 161.60 ± 10.26 | 82.57 ± 10.52 | 103.93 ± 9.66 | 101.11 ± 23.45 | 0.94 ± 0.09 |
| 32–32.9 (n = 53) | 160.86 ± 9.77 | 84.28 ± 10.62 | 103.15 ± 9.90 | 111.36 ± 18.59 | 0.90 ± 0.08 |
| 33–33.9 (n = 30) | 159.80 ± 10.37 | 85.04 ± 11.33 | 107.20 ± 12.54 | 102.09 ± 30.41 | 0.92 ± 0.09 |
| 34–34.9 (n = 26) | 157.54 ± 13.81 | 85.96 ± 15.73 | 101.75 ± 6.51 | 111.42 ± 20.28 | 0.87 ± 0.08 |
| 35–39.9 (n = 45) | 155.57 ± 8.13 | 88.44 ± 10.50 | 106.97 ± 7.12 | 118.0 ± 18.87 | 0.93 ± 0.31 |
| 40 + (n = 23) | 148.09 ± 16.83 | 96.70 ± 13.26 | 111.19 ± 14.84 | 125.75 ± 27.21 | 0.88 ± 0.13 |
| ANOVA (P) | 6.606 (0.000) | 179.554 (0.000) | 132.110 (0.000) | 28.275 (0.000) | 10.835 (0.000) |
| ANOVA trend (P) | 39.501 (0.000) | 3310.41 (0.000) | 2398.282 (0.000) | 504.523 (0.000) | 116.544 (0.000) |
| Spearman's Rho | –0.143 | 0.797 | 0.743 | 0.462 | 0.239 |
| R2 | 0.021 | 0.636 | 0.552 | 0.213 | 0.057 |
BMI: body mass index, WHR: waist hip ratio.
Table 2.
Mean values of biophysical and biochemical variables at different body mass index among 1893 subjects (men 949, women 944) aged 20–59 years in north India during study years 1999–2004.
| BMI groups | Systolic | Diastolic | Glucose | Total cholesterol | HDL cholesterol | Triglycerides |
|---|---|---|---|---|---|---|
| BP mmHg | BP mmHg | mg/dL | mg/dL | mg/dL | mg/dL | |
| < 18 (n = 170) | 109.81 ± 16.40 | 71.14 ± 10.92 | 80.60 ± 25.09 | 176.30 ± 32.47 | 42.80 ± 10.99 | 122.96 ± 57.13 |
| 18–18.9 (n = 69) | 111.79 ± 13.36 | 73.63 ± 8.98 | 83.79 ± 19.79 | 181.88 ± 40.16 | 40.98 ± 8.79 | 119.74 ± 44.47 |
| 19–19.9 (n = 80) | 112.95 ± 15.68 | 74.23 ± 12.96 | 84.98 ± 28.43 | 182.19 ± 41.11 | 43.08 ± 9.51 | 126.74 ± 53.24 |
| 20–20.9 (n = 87) | 113.26 ± 13.26 | 75.70 ± 9.98 | 84.68 ± 21.72 | 182.0 ± 43.42 | 41.40 ± 7.92 | 125.97 ± 52.80 |
| 21–21.9 (n = 108) | 113.93 ± 14.87 | 75.70 ± 11.75 | 98.30 ± 52.0 | 192.35 ± 43.21 | 40.95 ± 9.52 | 140.07 ± 82.61 |
| 22–22.9 (n = 126) | 115.07 ± 15.01 | 76.96 ± 12.0 | 92.51 ± 22.70 | 191.48 ± 41.34 | 42.38 ± 10.52 | 132.19 ± 56.79 |
| 23–23.9 (n = 161) | 118.20 ± 16.19 | 78.50 ± 11.01 | 89.23 ± 22.39 | 187.31 ± 39.80 | 38.44 ± 7.74 | 141.48 ± 63.61 |
| 24–24.9 (n = 152) | 118.67 ± 16.24 | 80.07 ± 11.05 | 92.89 ± 30.06 | 197.84 ± 45.48 | 38.21 ± 8.21 | 150.16 ± 75.35 |
| 25–25.9 (n = 138) | 120.08 ± 18.89 | 78.07 ± 15.19 | 96.60 ± 40.16 | 192.86 ± 44.72 | 40.74 ± 9.36 | 159.07 ± 89.02 |
| 26–26.9 (n = 146) | 124.49 ± 17.26 | 81.43 ± 10.93 | 90.80 ± 24.95 | 193.27 ± 40.24 | 37.80 ± 7.81 | 136.37 ± 65.27 |
| 27–27.9 (n = 131) | 127.49 ± 18.41 | 85.77 ± 14.05 | 95.45 ± 37.88 | 193.75 ± 37.49 | 38.27 ± 7.65 | 153.44 ± 96.01 |
| 28–28.9 (n = 101) | 123.91 ± 17.70 | 83.28 ± 10.82 | 100.37 ± 49.21 | 197.26 ± 40.05 | 39.01 ± 8.89 | 168.10 ± 87.10 |
| 29–29.9 (n = 83) | 129.01 ± 17.12 | 85.18 ± 12.15 | 97.45 ± 33.49 | 196.57 ± 44.54 | 39.61 ± 10.56 | 150.59 ± 67.63 |
| 30–30.9 (n = 99) | 135.92 ± 34.12 | 87.71 ± 13.68 | 98.61 ± 39.52 | 187.83 ± 32.63 | 38.42 ± 6.19 | 144.74 ± 84.54 |
| 31–31.9 (n = 50) | 122.96 ± 24.12 | 82.16 ± 16.26 | 101.84 ± 34.55 | 199.38 ± 39.68 | 37.86 ± 10.63 | 133.70 ± 51.65 |
| 32–32.9 (n = 53) | 127.74 ± 13.16 | 85.09 ± 7.90 | 101.13 ± 42.49 | 188.70 ± 36.45 | 40.13 ± 7.93 | 148.13 ± 73.61 |
| 33–33.9 (n = 30) | 131.60 ± 15.43 | 89.07 ± 10.32 | 85.60 ± 23.89 | 176.27 ± 29.42 | 36.23 ± 6.12 | 155.93 ± 15.41 |
| 34–34.9 (n = 26) | 138.38 ± 19.57 | 87.38 ± 9.75 | 99.27 ± 45.90 | 199.33 ± 34.23 | 37.65 ± 5.90 | 152.58 ± 63.79 |
| 35–39.9 (n = 45) | 139.36 ± 25.33 | 93.63 ± 12.10 | 93.84 ± 28.99 | 193.84 ± 42.57 | 37.71 ± 6.76 | 147.44 ± 65.81 |
| 40 + (n = 23) | 159.65 ± 58.22 | 124.52 ± 14.66 | 92.09 ± 19.49 | 195.87 ± 40.77 | 36.96 ± 5.07 | 148.74 ± 86.11 |
| ANOVA F value (P) | 20.870 (0.000) | 12.646 (0.000) | 3.389 (0.000) | 2.807 (0.000) | 4.536 (0.000) | 3.047 (0.000) |
| ANOVA for trend (P) | 338.122 (0.000) | 173.966 (0.000) | 32.595 (0.000) | 19.425 (0.000) | 48.485 (0.000) | 26.055 (0.000) |
| Spearman's Rho | 0.390 | 0.290 | 0.130 | 0.101 | −0.158 | 0.117 |
| R2 | 0.152 | 0.084 | 0.017 | 0.010 | 0.025 | 0.014 |
BMI: body mass index, BP: blood pressure, HDL: high-density lipoprotein.
Figure 1.
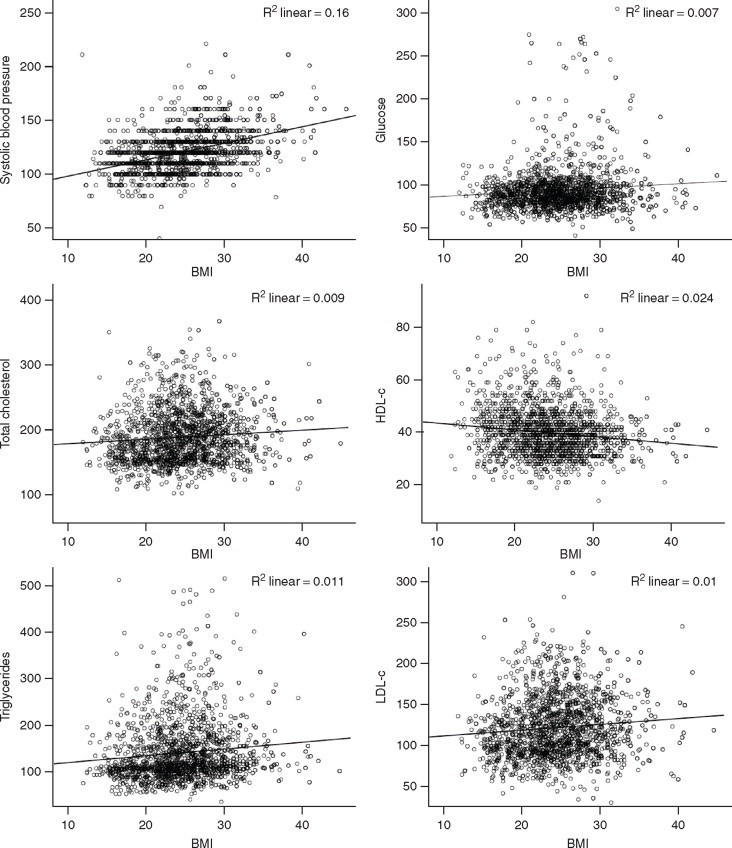
Scatter-plots of body mass index with systolic and diastolic blood pressure, fasting glucose, cholesterol lipoprotein lipids and triglycerides among 1893 subjects (men 949, women 944) aged 20–59 years in north India during study years 1999–2004. BMI: body mass index, HDL-c: high-density lipoprotein cholesterol, LDL-c: low-density lipoprotein cholesterol.
Prevalence of various risk factors at different BMI categories is shown in Table 3. Trend analysis reveals highly significant correlations of increasing BMI with prevalence of truncal obesity, HTN, diabetes, and metabolic syndrome (Ptrend < 0.0001), and weaker though significant correlations with high cholesterol, low HDL-c, and TG (Ptrend < 0.001). Relationship of prevalence of various cardiovascular risk factors with increasing BMI shows a strong exponential relationship with HTN (R2=0.87), hypercholesterolemia (0.29), diabetes (0.47), and the metabolic syndrome (0.63) (Figure 2). Graphic description of trends in ORs of association of each unit increase in BMI (baseline < 18 kg/m2) with prevalence of risk factors is depicted in Figure 3. Highly significant linear and exponential trends are observed for association with HTN, diabetes and the metabolic syndrome. The age- and sex-adjusted ORs and 95% confidence interval for association of HTN with BMI are: 1.49 (0.79–2.81) at 21–21.9 kg/m2, 2.58 (1.45–4.56) at 22–22.9 kg/m2, 4.49 (2.61–7.72) at 25–25.9 kg/m2 and 12.3 (7.58–19.96) at BMI ≤ 30 kg/m2. Similar associations are observed for diabetes 4.67 (1.77–12.36) at 21–21.9 kg/m2, 4.53 (1.74–11.77) at 22–22.9 kg/m2, 3.07 (1.15–8.21) at 25–25.9 kg/m2 and 6.21 (2.62–14.68) at BMI ≤ 30 kg/m2; as well as the metabolic syndrome 3.98 (1.67–9.52) at 21–21.9 kg/m2, 4.30 (1.84–10.02) at 22–22.9 kg/m2, 9.42 (4.25–20.86) at 25–25.9 kg/m2 and 10.48 (4.97–22.10) at BMI ≤ 30 kg/m2. Weaker, though significant, trends are observed for hypercholesterolemia, low HDL-c, and hypertriglyceridemia (Figure 3).
Table 3.
Prevalence of cardiovascular risk factors at different body mass index among 1893 subjects (men 949, women 944) aged 20–59 years in north India during study years 1999–2004.
| BMI groups | WHR > 0.8/0.9 | Hypertension | Diabetes mellitus | Metabolic syndrome | Cholesterol ≥ 200 mg/dL | LDL cholesterol ≥ 130 mg/dL | HDL cholesterol < 40 mg/dL | Triglycerides ≥ 150 mg/dL |
|---|---|---|---|---|---|---|---|---|
| < 18 (n = 170) | 38 (22.5) | 13 (7.7) | − | 8 (4.7) | 30 (17.6) | 37 (21.9) | 68 (40.2) | 33 (19.4) |
| 18–18.9 (n = 69) | 30 (44.1) | 10 (14.3) | 2 (2.9) | 3 (4.4) | 18 (26.1) | 18 (26.1) | 33 (47.8) | 12 (17.4) |
| 19–19.9 (n = 80) | 35 (43.6) | 13 (16.5) | 2 (2.5) | 7 (8.8) | 23 (28.8) | 22 (27.5) | 34 (42.5) | 21 (26.2) |
| 20–20.9 (n = 87) | 40 (46.5) | 9 (10.5) | 1 (1.1) | 3 (3.4) | 23 (26.4) | 23 (26.4) | 31 (35.6) | 21 (24.1) |
| 21–21.9 (n = 108) | 65 (60.2) | 17 (16.0) | 9 (8.3) | 18 (16.7) | 41 (38.0) | 41 (38.3) | 57 (53.3) | 34 (31.5) |
| 22–22.9 (n = 126) | 86 (69.4) | 31 (25.2) | 6 (4.8) | 22 (17.6) | 47 (37.3) | 46 (36.8) | 53 (42.4) | 36 (28.6) |
| 23–23.9 (n = 161) | 113 (71.1) | 42 (26.8) | 8 (5.0) | 35 (21.2) | 55 (34.2) | 58 (36.0) | 101 (62.7) | 47 (29.2) |
| 24–24.9 (n = 152) | 125 (83.3) | 44 (29.5) | 10 (6.6) | 41 (27.0) | 64 (42.1) | 69 (45.7) | 86 (57.0) | 49 (32.2) |
| 25–25.9 (n = 138) | 103 (75.7) | 38 (27.7) | 11 (8.0) | 44 (31.9) | 53 (38.4) | 46 (33.3) | 71 (51.4) | 52 (37.7) |
| 26–26.9 (n = 146) | 119 (83.3) | 51 (35.7) | 10 (6.8) | 40 (27.8) | 57 (37.7) | 62 (42.8) | 96 (66.2) | 40 (27.4) |
| 27–27.9 (n = 131) | 115 (89.1) | 67 (51.5) | 9 (6.9) | 42 (32.1) | 49 (37.4) | 54 (41.2) | 82 (62.6) | 41 (31.3) |
| 28–28.9 (n = 101) | 76 (77.6) | 39 (39.4) | 8 (7.9) | 40 (41.4) | 45 (44.6) | 41 (41.0) | 64 (64.0) | 45 (44.6) |
| 29–29.9 (n = 83) | 71 (86.6) | 36 (43.9) | 8 (9.6) | 28 (34.1) | 33 (39.8) | 36 (43.4) | 50 (60.2) | 33 (39.8) |
| 30–30.9 (n = 99) | 92 (95.8) | 52 (53.6) | 12 (12.1) | 33 (33.7) | 31 (31.3) | 34 (35.1) | 63 (64.9) | 28 (28.3) |
| 31–31.9 (n = 50) | 47 (94.0) | 19 (38.0) | 6 (12.0) | 17 (34.0) | 26 (52.0) | 25 (50.0) | 32 (64.0) | 11 (22.0) |
| 32–32.9 (n = 53) | 39 (75.0) | 23 (43.4) | 6 (11.3) | 16 (30.2) | 18 (34.0) | 18 (34.0) | 27 (50.9) | 19 (35.8) |
| 33–33.9 (n = 30) | 25 (83.3) | 21 (70.0) | 1 (3.3) | 9 (30.0) | 7 (23.3) | 7 (24.1) | 20 (66.7) | 8 (26.7) |
| 34–34.9 (n = 26) | 20 (76.9) | 15 (57.7) | 4 (15.4) | 12 (46.2) | 11 (42.3) | 9 (34.6) | 20 (76.9) | 9 (34.6) |
| 35–39.9 (n = 45) | 41 (91.1) | 35 (77.8) | 6 (13.3) | 18 (40.0) | 20 (44.4) | 23 (51.1) | 28 (62.2) | 14 (31.1) |
| 40 + (n = 23) | 18 (78.3) | 16 (69.6) | 2 (8.7) | 6 (26.1) | 10 (43.5) | 9 (39.1) | 13 (56.5) | 5 (21.7) |
| Chi-square for trend (P) | 258.463 (0.000) | 214.417 (0.000) | 29.501 (0.000) | 108.913 (0.000) | 20.652 (0.000) | 18.599 (0.000) | 40.502 (0.000) | 9.625 (0.000) |
BMI: body mass index, LDL: low-density lipoprotein, HDL: high-density lipoprotein, WHR: waist hip ratio, Hypertension – known hypertension or blood pressure ≥ 140/≥ 90 mmHg; Metabolic syndrome – NCEP ATP III guidelines 30, Diabetes – known diabetes or fasting glucose ≥ 126 mg/dL.
Figure 2.
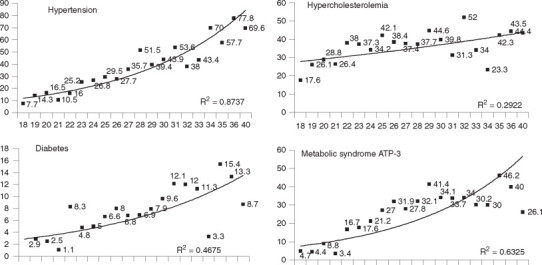
Relationship of prevalence (%) of various cardiovascular risk factors with increasing body mass index (x-axis) in 1893 subjects (men 949, women 944) aged 20–59 years in north India during study years 1999–2004. There is a significant relationship of increasing BMI with hypertension (known hypertension or blood pressure ≤ 140/≤ 90 mmHg; R2 = 0.87), diabetes (known diabetes or fasting glucose ≤ 126 mg/dL; R2 = 0.47), and the metabolic syndrome (NCEP ATP III guidelines; R2 = 0.63), and a weaker relationship with hypercholesterolemia > 200 mg/dL (R2 = 0.29). No evidence of a J- or U-shaped association is observed.
Figure 3.
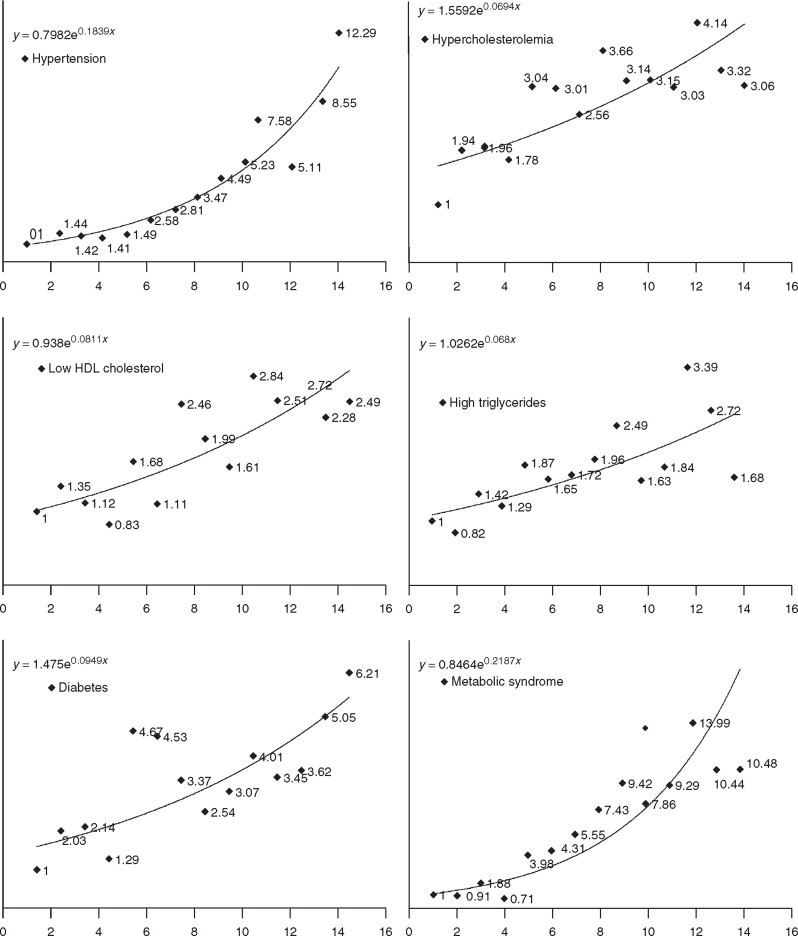
Trends in age adjusted odds ratios for BMI and various risk factors in 1893 subjects (men 949, women 944) aged 20–59 years in north India during study years 1999–2004. A steep relationship of increasing BMI with hypertension (known hypertension or blood pressure ≤ 140/≤ 90 mmHg), metabolic syndrome (NCEP ATP III guidelines), and diabetes (known diabetes or fasting glucose ≤ 126 mg/dL), and a weaker relationship with hypercholesterolaemia (total cholesterol ≤ 200 mg/dL), low HDL cholesterol < 40 mg/dL, hypertriglyceridemia ≤ 150 mg/dL, and diabetes is noted. BMI: body mass index, HDL: high-density lipoprotein.
Discussion
This study in urban Asian Indians shows that there is a significant linear association of multiple cardiovascular risk factors with BMI. This is confirmed by linear regression, quadratic regression, and non-linear analyses. As compared to BMI < 18 kg/m2 there is a stepwise increment of multiple risk factors, specifically HTN, diabetes, and metabolic syndrome with increasing BMI. Logistic regression analysis shows that at BMI of 20.0–20.9 kg/m2 the ORs for HTN, diabetes, and the metabolic syndrome are 1.5–2, at BMI of 22–22.9 kg/m2, 2.5–4 and at BMI > 30 kg/m2 the ORs are in range of 10–14 implying a continuous linear relationship of various metabolic risk factors with increasing obesity.
National studies in India have reported a significant increase in overweight and obesity in the country. The serial National Family Health Surveys (NFHS) reported a significant increase in overweight in successive NFHS-2 (1998–99)–NFHS-3 (2005–2006).19 The mean prevalence of overweight (BMI ≤ 25 kg/m2) increased from 10.6% to 12.6% (P < 0.001). However, there has been a significant increase in overweight/obesity in urban subjects in the last 20 years. The JHW studies in middle-class urban locations reported that prevalence of age adjusted overweight/obesity increased from 20.4% in the first study (1992–1994) to 46.8% in the fifth study (2008–2010) (Mantel Haenszel χ2 for trend P < 0.001).33 The mean BMI also increased significantly (age- and sex-adjusted quadratic regression b = 0.99 ± 0.10 per study, P < 0.001). Global Burden of Metabolic Risk Factors Study reported increasing trend in mean BMI all over the world.34 From 1980–2008, the mean BMI increased by 0.4 kg/m2 per decade in men and by 0.5 kg/m2 in women. Increasing mean BMI was also reported for India and many low income countries of Asia, Africa, and Oceania.35
Relationship of increasing BMI with cardiovascular mortality in different regions of the world shows discordant trends. While a J-shaped relationship has been reported among Caucasians,1 the relationship is U-shaped in Chinese and East Asians,9,10 and an almost flat relationship among Asian Indians.7,8 A recent study that evaluated association between BMI and risk of death in more than a million Asians reported that BMI > 35 kg/m2 as well as < 15 kg/m2 was associated with greater all-cause as well as cardiovascular mortality.35 The trends were different in East Asians where a J-shaped curved was observed while among the Indians and Bangladeshis the trends in cardiovascular mortality were not clear. This study suggests that there could be ethnic differences in association of BMI with cardiovascular risk but not many studies have evaluated ethnic or racial differences in relationship of obesity with burden of cardiovascular risks.
In the Framingham Offspring Study,17 the BMI was significantly and linearly associated with SBP, fasting glucose levels, plasma TC, very LDL-c, and LDL-c, and was inversely and linearly associated with HDL-c (P < 0.001) in non-smoking men and women. US National Health Surveys—National Health Examination Survey (1960–1962), National Health and Nutrition Examination Survey (NHANES)-I (1971–1975), NHANES-II (1976–1980), NHANES-III (1988–1994); and NHANES-IV (1999–2000)36 also reported a linear relationship of multiple cardiovascular risk factors with BMI. Cross-sectional observational data was analysed among African Americans in Jackson Heart Study (JHS) and Caucasians in Framingham Heart Study (FHS) in 4030 (mean age 54 years, 64% women) and 5245 (mean age 51 years, 54% women) participants, respectively.37 Prevalence of all risk factors except high TG and low HDL was substantially higher in JHS (P < 0.001) and BMI was associated with increasing prevalence of most cardiovascular disease (CVD) risk factors within each race. For diabetes mellitus, HTN, and low HDL, steeper relationships to BMI were observed in FHS than in JHS (P values < 0.001–0.016). There were larger proportional increases in risk factor prevalence with increasing BMI in Caucasians than in African Americans. The authors concluded that the higher prevalence rates of cardio metabolic risk factors at nearly all levels of BMI in African Americans, suggest that additional factors contribute to the burden of CVD risk in African Americans. Direct comparison of these studies with our observations is not appropriate, but increasing OR of multiple risk factors with increasing BMI in our study and its linear relationship suggesting additional or more varied factors adding to the burden of CVD risk in an Indian population cannot be ignored.
This study has several limitations and multiple strengths. The study is confined to urban populations in north India and may not be representative of the general population. However, since diabetes and cardiovascular epidemic in India is essentially an urban phenomenon these data are important.38 Moreover, the extremes of BMI distribution to evaluate the risk factor associations is available only in urban locations and therefore this is proper sampling. A small sample size in comparison to large prospective epidemiological studies in USA, Europe, and Asia is a limitation. However, this is not a prospective study but an analytical study to evaluate significance of risk factor associations of BMI with continuous variables such as BP, glucose and lipids. For such analyses, the present sample size is considered adequate.39 Thirdly, we have not evaluated association of cardiovascular risk factors with other measures of obesity such as WC and WHR which are considered by some as more important determinants of cardiovascular risk.40 In the present study there is a significant correlation of BMI with these measures. Since, the measurement error in evaluating hip and waist size is large41 we used BMI which is less prone to error. Methodologies of measurement and importance of WC is also variable in different studies and populations.42,43 Strengths of the study include large sample size, representative population and in-depth assessment of multiple risk factors.
In conclusion, there is a major burden of premature CVD in the underdeveloped world.44 It is important to optimise the risk stratification among populations to guide appropriate intervention. Although, observational cohort studies play a crucial role in defining the important risk factors and guide evidence base for interventions, observational studies as the present one provides knowledge regarding broad risk factors, such as high BMI and obesity, that could be targeted for early intervention so that the anticipated epidemic of CVD could be mitigated or even thwarted.45 Our study in this Asian Indian population reveals cluster of multiple risk factors with increasing BMI irrespective of any arbitrary cutoff levels. This study highlights the fact that cardiovascular risks increase within the so-called range of normal BMI and there is a linear increase in multiple risk factors, such as HTN, diabetes, and metabolic syndrome, with each unit increase in BMI of > 19 kg/m2. It has been previously noted that multiple cardiovascular risk factors such as smoking, SBP, dyslipidaemia, and hyperglycaemia also have a linear relationship with cardiovascular risk.46 The present study shows that BMI also has a similar relationship. Large prospective studies in Europe, north America, and East Asia have reported a continuous relationship of cardiovascular outcomes with increasing BMI.1,35 Whether Asian Indians also have a similar relationship for cardiovascular outcomes is a matter of future studies. However, considering the relationship of BMI with HTN, dyslipidaemia and dysglycaemia, in the present study there is a need of healthcare policy makers and providers to initiate individual- and population-level measures to control body weight in Asian Indian populations and to prevent a rise in BMI among the non-obese populations of this region.
References
- 1.Whitlock G, Lewington S, Sherliker P. Body mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslam DW, James WPT. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petreli JM, Rodriguez C, Heath C. Body mass index and mortality in prospective cohorts of US adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Schatzkin A, Harris TB. Overweight, obesity and mortality in a large prospective cohorts of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 5.Romero-Corral A, Montoru VM, Somers VK. Association of body weight with total mortality and with coronary artery disease: a systemic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 6.De Gonzalez AB, Hatge P, Cerhan JR. Body mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pednekar MS, Hakama M, Heberts JR, Gupta PC. Association of body mass index with all-cause and cause-specific mortality: findings from a prospective cohort study in Mumbai (Bombay), India. Int J Epidemiol. 2008;37:525–534. doi: 10.1093/ije/dyn001. [DOI] [PubMed] [Google Scholar]
- 8.Sauvaget C, Ramadas K, Thomas G, Vinoda J, Thara S, Sankaraanarayanan R. Body mass index, weight change and mortality risk in a prospective study in India. Int J Epidemiol. 2008;37:990–1004. doi: 10.1093/ije/dyn059. [DOI] [PubMed] [Google Scholar]
- 9.Mhurchu CN, Rodgers A, Pan WH, Gu DF, Woodward M. Asia Pacific Cohort Studies Collaboration. Body mass index and cardiovascular disease in the Asia Pacific region: an overview of 33 cohorts involving 310,000 participants. Int J Epidemiol. 2004;33:751–758. doi: 10.1093/ije/dyh163. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Yang G, Zhou M. Body mass index and mortality from ischaemic heart disease in a lean population: 10 year prospective study of 220,000 adult men. Int J Epidemiol. 2006;35:14150. doi: 10.1093/ije/dyi215. [DOI] [PubMed] [Google Scholar]
- 11.Jee SH, Sull JW, Park J. Body mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 12.Gu H, He J, Duan X. Body weight and mortality among men and women in China. JAMA. 2006;295:776–783. doi: 10.1001/jama.295.7.776. [DOI] [PubMed] [Google Scholar]
- 13.Zhou M, Offer A, Yang G. Body mass index, blood pressure, and mortality from stroke: a nationally representative prospective study of 212,000 Chinese men. Stroke. 2008;39:753–759. doi: 10.1161/STROKEAHA.107.495374. [DOI] [PubMed] [Google Scholar]
- 14.Kopelman PG. Obesity as a medical problem. Nature. 2004;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 15.Klein S, Burke LE, Bray GA. Clinical implications of obesity with specific focus on cardiovascular disease. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 16.Timpson NJ, Harbord R, Davey-Smith G. Does greater adiposity increase blood pressure and hypertension risk? Hypertension. 2009;54:84–90. doi: 10.1161/HYPERTENSIONAHA.109.130005. [DOI] [PubMed] [Google Scholar]
- 17.Lamon-Fava S, Wilson PWF, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women: The Framingham Offspring Study. Arterioscl Thromb Vasc Biol. 1996;16:1509–1515. doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- 18.Wildman RP, Gu D, Reynolds K, Duan X, Wu X, He J. Are waist circumference and body mass index independently associated with cardiovascular disease in Chinese adults? Am J Clin Nutr. 2005;82:1195–1202. doi: 10.1093/ajcn/82.6.1195. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Chen HJ, Shaikh S, Mathur P. Is obesity becoming a public health problem in India: examining the shift from under- to over-nutrition problems over time. Obesity Rev. 2009;10:456–474. doi: 10.1111/j.1467-789X.2009.00568.x. [DOI] [PubMed] [Google Scholar]
- 20.WHO Consultation Obesity: preventing and managing the global epidemic. WHO Tech Rep Ser. 2000;894:1–37. [PubMed] [Google Scholar]
- 21.Gupta R, Gupta VP, Bhagat N, Rastogi P, Sarna M, Deedwania PC. Obesity is major determinant of coronary risk factors in India: Jaipur Heart Watch studies. Indian Heart J. 2008;60:26–33. [PubMed] [Google Scholar]
- 22.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 23.Misra A. Revision of limits of body mass index to define overweight and obesity are needed for the Asian ethnic groups. Int J Obesity and Relat Metab Disord. 2003;27:1294–1296. doi: 10.1038/sj.ijo.0802412. [DOI] [PubMed] [Google Scholar]
- 25.Misra A, Sharma R, Pandey RM, Khanna N. Adverse profiles of dietary nutrients, anthropometry and lipids in urban slum dwellers of northern India. Eur J Clin Nutr. 2001;55:727–734. doi: 10.1038/sj.ejcn.1601214. [DOI] [PubMed] [Google Scholar]
- 26.Gupta R, Gupta VP, Sarna M. Prevalence of coronary heart disease and risk factors in an urban Indian population: Jaipur Heart Watch-2. Indian Heart J. 2002;54:59–66. [PubMed] [Google Scholar]
- 27.Gupta R, Sarna M, Thanvi J, Rastogi P, Kaul V, Gupta VP. High prevalence of multiple coronary risk factors in Punjabi Bhatia community: Jaipur Heart Watch-3. Indian Heart J. 2004;57:646–652. [PubMed] [Google Scholar]
- 28.Misra A, Vikram NK, Gupta R, Pandey RM, Wasir JS, Gupta VP. Waist circumference cut-off points and action levels for Asian Indians for identification of abdominal obesity. Int J Obesity. 2006;30:106–111. doi: 10.1038/sj.ijo.0803111. [DOI] [PubMed] [Google Scholar]
- 29.Cannon CP, Battler A, Brindis RG. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 30.National Cholesterol Education Program Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 31.Sundar Rao PSS, Richard J. 3rd Ed. Vol. 19. Prentice-Hall; New Delhi, India: 1996. pp. 135–152. (An Introduction to Biostatistics). [Google Scholar]
- 32.Selvin S. Statistical Analysis of Epidemiological Data. Oxford University Press; New York: 1992. [Google Scholar]
- 33.Gupta R, Guptha S, Gupta VP, Agrawal A, Gaur K, Deedwania PC. Twenty year trends in cardiovascular risk factors in India and influence of educational status. Eur J Cardiovasc Prev Rehabil. 2011 Sep 26 doi: 10.1177/1741826711424567. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 34.Finucane MM, Stevens GA, Cowan MJ, On behalf of the Global Burden of Metabolic Risk Factors for Chronic Diseases Collaborating Group (Body Mass Index) National, regional and global trends in body mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.0 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng W, McLerran DF, Rolland B. Association between body mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–729. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregg EW, Cheng YJ, Caldwell BL, Flegal KM, Venkatnarayan KM, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in US Adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 37.Taylor HA, Coadt SA, Levy D. Relationships of BMI to cardiovascular risk factors differ by ethnicity. Obesity. 2010;18:1638–1645. doi: 10.1038/oby.2009.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta R, Joshi PP, Mohan V, Reddy KS, Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 39.Blackburn H, Reddy KS, McKeigue P. Cardiovascular Survey Methods. World Health Organization; Geneva: 2002. [Google Scholar]
- 40.Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist: hip ratio as predictors of cardiovascular risk: a review of the literature. Eur J Clin Nutr. 2010;64:16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 41.Whitlock G, Lewington S, Mhurchu CN. Coronary heart disease and body mass index: a systematic review of the evidence from large prospective cohort studies. Semin Vasc Med. 2002;2:369–381. doi: 10.1055/s-2002-36766. [DOI] [PubMed] [Google Scholar]
- 42.Ross R, Berentzen T, Bradshaw AJ. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obesity Rev. 2008;9:312–325. doi: 10.1111/j.1467-789X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal SK, Misra A, Aggarwal P. Waist circumference measurement by site, posture, respiratory phase, and meal time: implications for methodology. Obesity Silver Spring. 2009;17:1056–1061. doi: 10.1038/oby.2008.635. [DOI] [PubMed] [Google Scholar]
- 44.Gersh B, Mayosi B, Sliwa K, Yusuf S. The epidemic of cardiovascular diseases in the developing world: global implications. Eur Heart J. 2010;31:642–648. doi: 10.1093/eurheartj/ehq030. [DOI] [PubMed] [Google Scholar]
- 45.Gupta R, Deedwania PC. Interventions for cardiovascular disease prevention. Cardiol Clinics. 2011;29:15–34. doi: 10.1016/j.ccl.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Law MR, Wald NJ. Risk factor thresholds: their existence under scrutiny. BMJ. 2002;324:1570–1572. doi: 10.1136/bmj.324.7353.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uncited Reference
- 24.Misra A, Khurana L. Obesity related non-communicable disease: South Asians vs White Caucasians. Int J Obesity. 2011;35:167–187. doi: 10.1038/ijo.2010.135. [DOI] [PubMed] [Google Scholar]


