Abstract
Methods to systematically analyze in parallel the function of multiple protein or cell samples in vivo or ex vivo (i.e. functional proteomics) in a controlled gaseous environment have thus far been limited. Here we describe an apparatus and procedure that enables, for the first time, parallel assay of oxygen equilibria in multiple samples. Using this apparatus, numerous simultaneous oxygen equilibrium curves (OECs) can be obtained under truly identical conditions from blood cell samples or purified hemoglobins (Hbs). We suggest that the ability to obtain these parallel datasets under identical conditions can be of immense value, both to biomedical researchers and clinicians who wish to monitor blood health, and to physiologists studying non-human organisms and the effects of climate change on these organisms. Parallel monitoring techniques are essential in order to better understand the functions of critical cellular proteins. The procedure can be applied to human studies, wherein an OEC can be analyzed in light of an individual’s entire genome. Here, we analyzed intraerythrocytic Hb, a protein that operates at the organism’s environmental interface and then comes into close contact with virtually all of the organism’s cells. The apparatus is theoretically scalable, and establishes a functional proteomic screen that can be correlated with genomic information on the same individuals. This new method is expected to accelerate our general understanding of protein function, an increasingly challenging objective as advances in proteomic and genomic throughput outpace the ability to study proteins’ functional properties.
Keywords: Oxygen equilibrium curves, hemoglobin, blood, erythrocyte, functional proteomics
INTRODUCTION
Oxygen (O2) uptake from the environment and use by the organism are essential to almost all of the planet’s animal species, many of which rely on blood to bind and transport O2 throughout the body. The hemoglobin (Hb) O2-binding system is key to organisms’ abilities to operate, because of the necessity for O2 in energy metabolism as a final electron receptor in ATP synthesis.[1] Red blood cells (RBCs), which carry Hbs, contact the environment at the lungs or gills and circulate to subserve every tissue in the body.[2],[3] An exquisitely tuned O2- , CO2- and NO-sensor, Hb enables organisms to sense and adapt to their environments.[4] The functional flexibility in the O2-binding abilities of Hb are clearly modulated in both short- and long-term environmental changes.[5]
Reliable methods do currently exist for studying O2-binding of blood and Hb samples, and a vast body of literature supports fundamental discoveries concerning Hbs of humans and other species. These studies have been carried out largely or exclusively on single samples from individuals. Simultaneous samples from many individuals within a population are infrequent, owing in part to the inability to perform many O2- binding curves in true parallel. To date, no method can easily or practically handle replicate samples simultaneously, or multiple samples under identical conditions, a prerequisite for high-throughput approaches. These limitations, together with a lack of the instrument portability needed to study samples and the need for specialized apparatus, have also limited the ability to widely obtain O2 equilibrium curves (OECs) – an important clinical and biological property – in the clinic and the field.
The development of this parallel-monitoring apparatus presents numerous potential applications for O2-binding studies. In addition to revealing changes in blood O2-binding properties with changing environmental conditions, an equally important current challenge is to understand how blood O2-binding behaviors change with blood aging, particularly during blood-banking. For example, depletion of intraerythrocytic 2,3-DPG and ATP increases the O2 affinity of stored RBCs. A similar “storage deficit” occurs within hours in the intracellular levels of the nitric oxide (NO) derivative of Hb, S-nitrosohemoglobin (SNO-Hb). Studies are currently underway to identify interventions to eliminate this deficit. [6],[7],[8] More broadly, hospitals and blood banks must ensure that the stored blood they provide to transfused patients is safe and functional in terms of its O2-binding and related properties. To date, no technology for OECs is sufficiently widely available or efficient to guide medical decision-making or quality assurance in transfusion medicine. Similarly, single-sample throughput has limited the ability of scientists to determine the basis for inter-individual changes in OEC behavior during RBC storage.
Growing evidence suggests a relationship between morbidity and mortality in patients receiving blood as a function of storage time.[9],[10] There is substantial variation among the biological properties of RBC units stored conventionally.[11] A practical test capable of characterizing the OEC unique to a given unit of RBCs could in principle promote superior outcomes with RBC transfusion. Readily available OEC data from the recipient could further personalize this approach while aiding in inventory management of this precious medical resource.
In addition to applications for human blood, this parallel and potentially high-throughput method can be used in research stations to analyze all types of non-human blood for comparative studies and to discover physiological responses to changing environments. As our planet moves into an era of increasing temperature and rapid environmental change, we must develop successful methods for understanding mechanisms behind the effects of these changes on organismic physiology.[12] Global climate change has become a critical issue in discussions of the future of our planet, for both humans and other species, and may seriously impact numerous organisms and ecosystems, with varying effects within a single species. For example, fish RBCs show relatively frequent polymorphisms in Hb types, demonstrating individual-to-individual variations within a single species.[13] It has not been possible to date, however, to extensively study O2-binding properties of the RBCs from individual fish, or to simultaneously analyze replicate samples from multiple species living under varying environmental conditions. Nor has it been possible to perform OECs “in the field,” free of potential transport-associated artifacts including the passage of time.
Here we describe and present results from a microplate-based O2 tonometric approach that allows, for the first time, parallel monitoring of samples. Our findings support the facility and replicability of this approach. The device and approach can promote accelerated functional studies of proteins and cells so as to keep pace with the rapid growth of proteomic and genomic knowledge.
METHODS
Sample preparation
Animal and human blood samples were obtained under IACUC- and IRB-approved protocols. Standard antecubital phlebotomy and heparin-coated syringes were used for human blood draws, and samples were typically used within four hours of acquisition. Blood was centrifuged for 3 minutes at 2500 RCF at room temperature (25°C), plasma and white blood cells were removed, and then RBCs were washed with phosphate-buffered saline (PBS, pH 7.4). The chelating agent diethylenetriaminepentaacetic acid (DTPA, 0.1 mM) was typically added to inhibit metal-dependent methemoglobin formation (Hb oxidation). RBCs were suspended at a hematocrit (Hct) of 20–25% (volume/volume) for studies, which was ideal for examining the “A” and “B” visible (500–650 nm) spectral bands. No significant difference in the resulting binding curves was seen when varying spectral regions (Soret vs. A/B visible) were used to calculate the degree of Hb O2 saturation.
RBC hemolysates were prepared by hypotonic lysis in four volumes of water containing DTPA, 0.1 mM. Chromatographically purified HbA0 (HbA) was a kind gift from Curacyte/Apex Biosciences (Durham, NC). Chemical modifications were performed in order to modify intraerythrocytic Hbs and create well-characterized changes in O2 - binding characteristics. These modifications allowed us to create our own examples of Hb with known differences in O2 equilibria.
Chemicals
All chemicals were from Sigma Chemicals (St. Louis, MO) unless otherwise noted. In some cases hemolysates or HbA0 were dialyzed in various buffers (as described in each accompanying figure legend). Specifically, thin-film dialysis of the Hb vs. Hepes or Bis-Tris buffers of varying PO4 concentrations with DTPA present (0.1 mM) was used to change the buffered PO4 concentrations, in order to study the effect of PO4 concentration on O2 affinity of HbA0.
Analyte preparation
A standard, microplate-based spectrophotometer (SpectraMax 190 by Molecular Devices, or BMG Labtech’s Fluostar Omega) was used to obtain the spectra needed to construct OECs from blood and Hb samples. Temperature was kept constant by the instrument’s thermostatically controlled sample chamber and experiments were done at temperatures ranging from 18–37°C.
The multi-cuvette tonometer cell was designed and built by Bonaventura and Perez. Figure 1 shows a typical 8-well tonometer used for some of the experiments. The tonometer’s dimensions are typically ~127 × 85.5 × 17 mm, and a standard microplate reader readily accommodates the device via the reader’s drawer. The cell’s length and width are nearly identical to those of a typical microplate. The height is slightly greater but does not impede these readers. Presently, the novel tonometer can analyze 8–24 samples simultaneously, depending on the layout of the inner microcuvette-holding insert plate. The number of simultaneous O2 equilibria can theoretically be increased further by raising the number of and shrinking the size of the inserted microcuvettes. A simple modification of the drawer’s “door” intended to seal the loaded microplate reader will allow tubing (for gas flow) to remain connected to the cell throughout an experiment.
Figure 1.
Images of an 8-well sample tonometry cell (a), a cell inset (b), and an individual sample within a microcuvette (c). Also visible (right, 1a) are inlet and outlet gas ports and the microplate reader’s underlying drawer. Samples are positioned in the inset plate (white) designed to align as would a standard microplate in a reader. In (b) the inset overlies a microplate template to illustrate the alignment with a layout pre-programmed in a typical reader. Each sample microcuvette assembly (c) comprises an annular, plastic support (black) and two layers of thin polymer film sandwiching a layer of blood cells (or Hb) suspended in buffer, and secured with stacked O-rings.
Microcuvette and cell assembly
Samples were prepared in microcuvettes constructed for the tonometry cell using a technique developed at the Duke University Marine Lab. In summary, a suspension of hemolysate, RBCs, or other O2-binding protein is sandwiched between two sheets of Teflon, Saran Wrap™, or another appropriate O2-permeable membrane with a maximal thickness of 1 micrometer. Saran Wrap was used for the experiments reported here. To prepare samples, the O2-permeable membrane is stretched over a black plastic ring and secured in place with an O-ring. 10 uL of sample is pipetted as a drop onto this first membrane layer. A second membrane layer is pre-stretched and secured on a larger plastic ring. This second layer is carefully lowered onto the droplet of Hb sample, flattening it to produce a thin sample layer. The cuvette is secured and completed by the stacked application of a second O-ring. Each assembled microcuvette is then placed into a clear plastic insert secured in the multi-cuvette tonometer (Figure 1b–c). The positions of cuvette seats within the tonometer correspond with the centers of templated microplate wells, enabling the microplate’s wells to be optimally positioned. After all cuvettes are placed in the tonometer, a rubber gasket and the transparent top plate of optical glass are secured with a retaining plate that isolates the inside of the tonometer from external gases other than those deliberately added via the gas inlet line. See Lapennas et al.[14] for more details on thin-film cuvette sample preparation.
Gas exposure and OECs
A gas-mixing apparatus (MCQ Gas Mixer, Italy) was used to mix, hydrate and flow pre-purified N2, O2, and (for some experiments) CO2 into the tonometer. Complete deoxygenation was achieved via 500 standard cubic centimeters/minute (sccm) N2 flow through airtight fittings in the tonometer. The sample was then scanned, and serial spectra were examined to ensure complete deoxygenation. This step was followed by increases in O2 concentration (pO2). Specifically, pO2 is increased stepwise until the pO2 is sufficiently high to fully saturate all of Hb’s O2-binding sites. Generally, incremental changes in pO2 were made in the present study by adjusting the pO2 (%O2) setting of the gas mixer with a 3-minute equilibration interval before scanning at each subsequent pO2.
An alternative method for changing the pO2 within the tonometer, involving gas injections through a silicone septum within the cell’s lateral injection port, was occasionally used (as in Supplemental Figure 1). This method eliminates the need for a gas delivery/mixing system or periodic resealing of the fittings, and presents an even more mobile and universally feasible technique for producing the pO2 changes. However, the air injection technique requires precise measurements of the internal volume of the tonometer, and barometric pressure and humidity, during the experiment, making this technique challenging for practical high-throughput use.
Time to thermodynamic equilibrium upon changes in pO2
Prior to actual sampling, experiments were conducted on stepwise pO2 changes to determine the time required to establish equilibrium for Hb at each O2 partial pressure (pO2). For these experiments, each change in pO2 was followed immediately by a 20-minute tracking period (with measurements made every 20 seconds) of the OD values of wavelengths 540, 556 and 576 nm. Typical %O2s (volume/volume) included 0%, 0.2%, 0.6%, 1%, 3%, and 15%, in either ascending or descending order.
In cases where the system is under allosteric control, (i.e. where the functions between the protein and ligands are complex, as in homotropic and heterotropic allosteric systems), true equilibrium must be obtained at each stage of the measurements.[15] In our experiments, Hb was fully deoxygenated before sampling, and deoxygenation was confirmed both by comparison of the experimental spectra with reference spectra and by the lack of any further change upon further exposure to strictly anoxic gas.
Analysis of HbO2 saturation and plotting OECs
O2 equilibria were measured spectrophotometrically, and compared to standard spectra obtained for deoxygenated, oxygenated and methemoglobin (met) Hb. MetHb is the oxidized or ferric form of Hb, which does not bind O2 but is spectrally distinct and can alter both the calculated amount of O2 bound to the Hb and the measured O2 affinity. Absorbance spectra of the fully oxygenated samples and progressive equilibrium positions from fully liganded to unliganded Hb were calculated as a percentage change at each of the pO2s established in the tonometer. Intermediate degrees of Hb O2 ligation were determined by calculating the HbO2 fractional saturation (HbO2 saturation) as previously described. Specifically, we calculated the averaged fractional change in multiple spectral features (typically at wavelengths 542, 555, 577, and 690 nm) that distinguish deoxygenated from oxygenated Hb. [4] OECs were then plotted as logarithm (log) of the PO2 vs. HbO2 saturation.
Non-human blood samples
Blood samples were obtained from various organisms in order to demonstrate the wide range of specimens that can be studied in parallel in the apparatus described here.[16] Fish Hb and RBCs were prepared according to methods described by Bonaventura et al.[17].
RESULTS
Time to equilibrium
We measured the time to equilibration of the absorbance changes in Hb after changes in pO2 (Supp. Figs. 1 and 2), and concluded that three minutes between stepwise changes in pO2 and subsequent testing was sufficient for full equilibrium between the gas and solution phases. Specifically, after a pO2 change, 97% of the total absorbance change was observed within one minute and the change was complete by three minutes (Supp. Fig. 2). Therefore, we allowed 3 minutes between changes in pO2 and the subsequent scan. We typically began experiments with fully deoxygenated Hb, and increased the pO2 stepwise until the sample reached 100% HbO2 saturation. As expected, similar times to equilibrium were observed irrespective of whether the Hb studied was initially in the deoxygenated or oxygenated state.
Validation with replicate standards studied in parallel
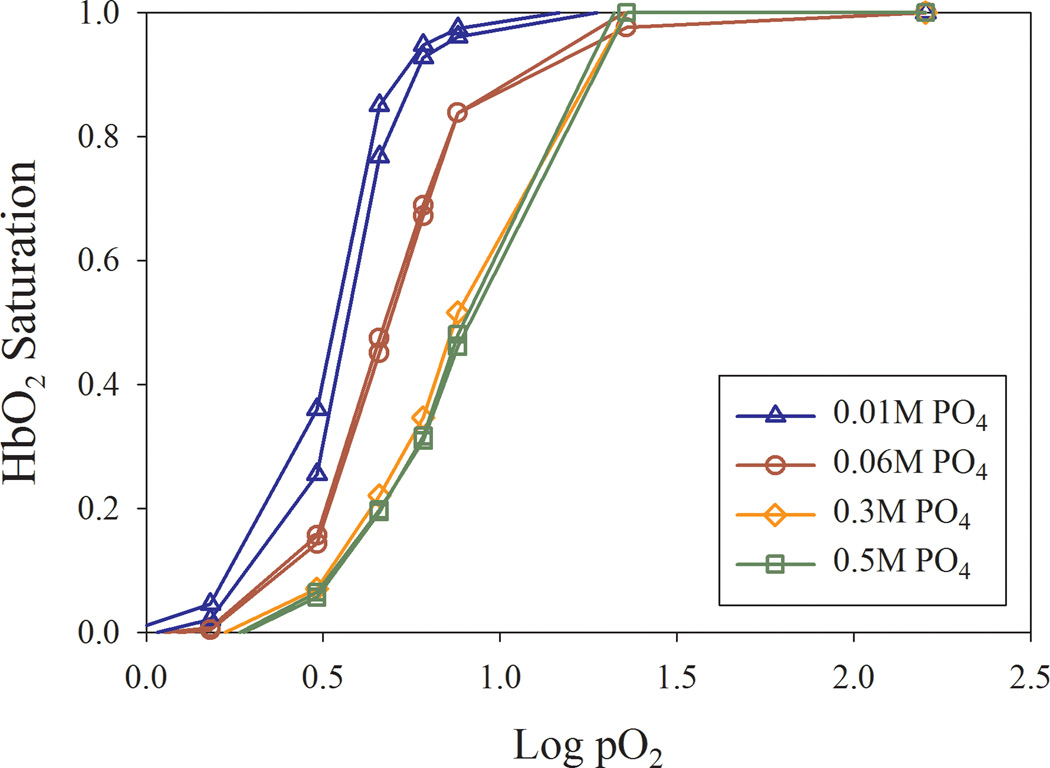
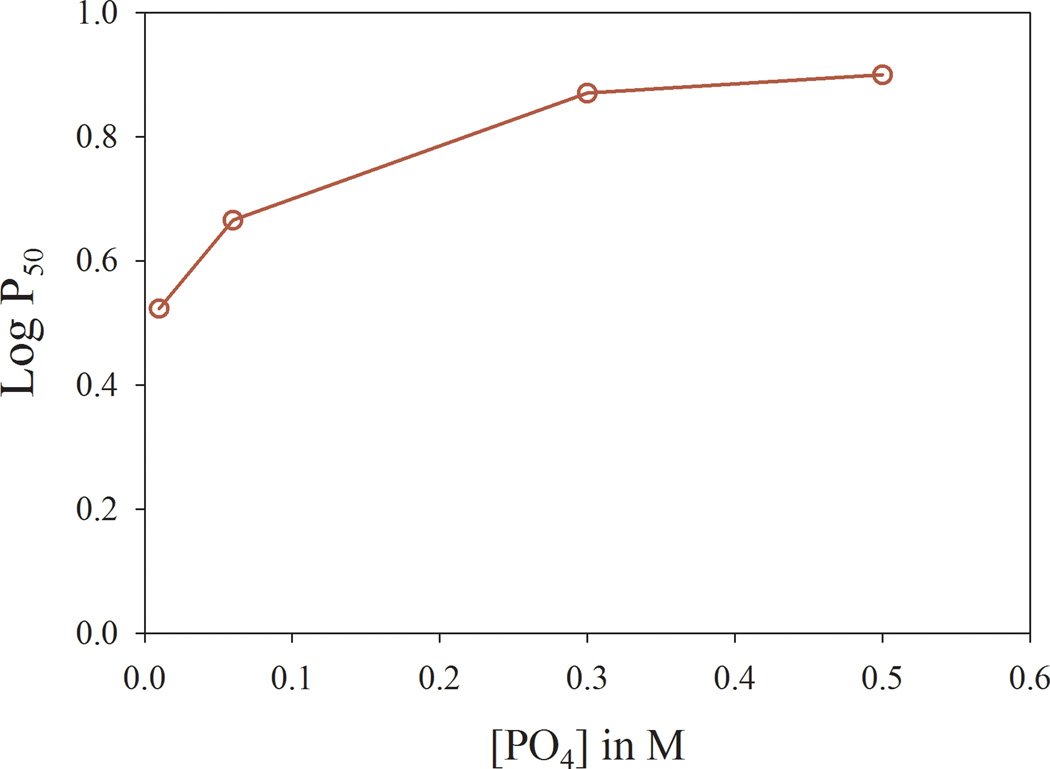
Inorganic phosphate (PO4) produced a rightward shift in the O2-binding curve in a concentration-dependent manner. In both the Soret (400–430 nm, not shown) and visible (500–650 nm; Fig. 2) regions, Hb in low-strength (0.01M) PO4 had the highest O2 affinity, followed by simultaneously studied samples in 0.06M, 0.3M and 0.5M PO4 (Fig. 2 and Table]. Our results resemble published PO4 concentration-response curves of HbA0, and P50 values obtained using glass tonometry methods.[18] These findings indicate that OECs and P50 values (Table) using the current method are highly similar to those from published methods, consistent with validation of our method.
Figure 2.
(a) Oxygen equilibrium curves (OECs) from human RBC sample replicates were scanned (500–600 nm) simultaneously after equilibration with varying [PO4]. (b) Mean P50 values as a function of [PO4].
Table 1.
P50 (PO2 (mm Hg) at 50% HbO2 saturation) for selected experimental series. Shown are the mean and SD from n=3 replicate experiments. Where no SD is given, a single experiment was performed.
| Figure 2 | Soret | Visible (A, B) |
|---|---|---|
| 0.01 M PO4 | 3.646(±0.196) | 3.352(±0.164) |
| 0.01 M PO4 | 4.809(±0.110) | 4.786 |
| 0.3 M PO4 | 7.413 | 7.413 |
| 0.5 M PO4 | 7.990(±0.195) | 7.886(±1.164) |
| Figure 3 | Visible (A, B) | |
| Human HbAo | n/a | 22.520(±0.550) |
| T. albacores | n/a | 23.634(±0.866) |
| M. nigricans | n/a | 33.709(±2.918) |
Non-human Hbs
Given our simultaneous interest in the biomedical research and clinical significance of our OEC work, and comparative physiological and biochemical bases for O2 binding, we have also included limited non-human Hb studies.
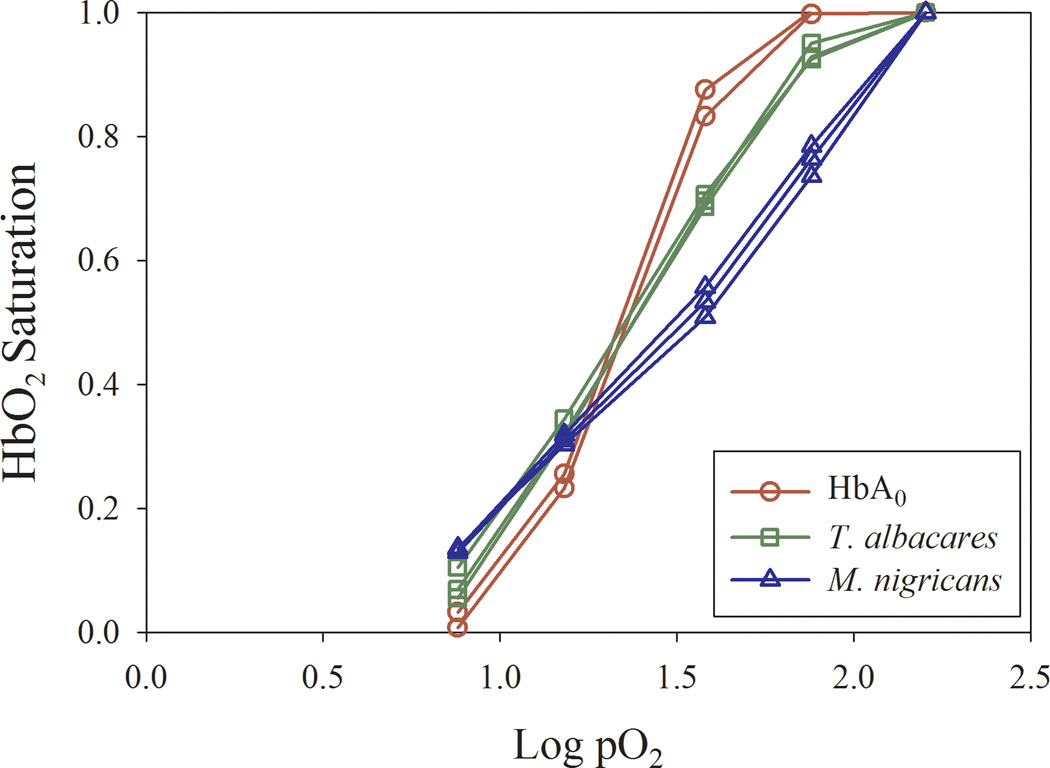
Non-human sampling produced results consistent with previous O2-binding results, and further demonstrates the ability of this machine to process blood from any organism. Comparison of blood from Thunnus albacares (yellowfin tuna), Makaira nigricans (Atlantic blue marlin) and HbA0 indicates excellent replicability of individual blood samples [Figure 3].
Figure 3.
Parallel comparison of replicate OECs from Hbs of human (HbA0), Thunnus albacares (yellowfin tuna), and Makaira nigricans (Atlantic blue marlin) at 25°C. The high similarity between replicate results highlights the reproducibility typical of this device and approach.
Hbs from Amphitrite ornata, a Terebellid polychaete common to North Carolina, were also studied (not shown). A. ornata Hb has a P50 of <1mm pO2, indicating the capability of this apparatus to handle very high-O2-affinity proteins.
DISCUSSION AND CONCLUSIONS
The device and methods presented here establish a new, efficient way to determine and compare O2-binding properties in numerous blood samples simultaneously and in parallel, reproducibly, and in remote locations. The ability to simultaneously examine multiple samples should allow researchers to acquire both higher result replicability and more accurate and direct comparisons between numerous different samples, by truly testing at the same time and under identical conditions. Our goal is to create a portable O2-binding instrument that can be used in sampling processes anywhere in the world, from hospitals and blood banks to remote island lagoons with heat-adapted fish, or mountain-dwelling hypoxia-tolerant species or strains, as examples. We have transported the instrument as checked airline luggage between laboratories in Durham and Beaufort, North Carolina; Mayaguez, Puerto Rico; and Monterey, California. The apparatus is typically ready to assay samples in less than an hour. The mobility, compact size, and ease of setup of this apparatus make it a practical option for portable field and laboratory research around the world.
O2-binding studies to date have relied on evolving but cumbersome low-throughput technologies to acquire accurate measurements. The Riggs Tonometer, developed by Riggs and Wolbach [19], is one of the most precise and accurate for O2-binding studies, and uses a closed-chamber glass tonometer attached to a spectrophotometer cuvette. Following gasometric deoxygenation, precisely measured gas injections are made and the cumulative internal pO2 is calculated. The Riggs Tonometer can be used to measure individual O2-binding curves, but only after laborious hours of measurements and calculations. Stanley Gill[20] developed a thin-layer sample cell to hold a single Hb sample between a glass window and a gas-permeable membrane. The “Gill Cell” was used in many studies concerning the thermodynamics of O2-binding and allosteric modulation of Hb. A similar thin-film diffusion technique for determining OECs was successfully used by Clark and coworkers [21] in experiments with blood of southern blue fin tuna, Thunnus maccoyii. Imai [22] developed an elegant apparatus for precise O2 equilibrium measurements, using dilute samples of Hb similar to those used by Riggs. The absorption spectrum of Hb was measured as the pO2 changed, and pO2 was determined with an O2 electrode immersed in the Hb sample.
These methods represent important steps in the evolution of O2-binding studies, but all have handled only one sample at a time. The machine and methods we have developed provide a portable, rapid, method for analyzing in parallel O2-binding properties of multiple samples/replicates of blood and/or isolated hemoproteins ex vivo, including both Hb and myoglobin (Mb). This device can also be used to study other O2-binding proteins, such as hemocyanin, hemerythrin, synthetic O2 carriers, and tissue slices. In this apparatus, we present progress toward the goal of fabricating a multi-cuvette spectrophotometer-compatible device centered in a hermetically-sealed chamber equipped such that mixtures of O2 and an inert gas can be introduced. This new approach confers the significant advantage of ensuring that samples studied (e.g., sample replicates, samples from multiple donors, and/or samples under multiple conditions) experience, at the same time, the same absolute – and incremental changes in – pO2, pCO2, temperature, exposure time, and humidity (among other experimental variables) as do their comparators.
Determination of the O2 saturation of Hb and the pO2 can be accomplished by techniques other than absorbance measurements. In fact, other optical means of both pO2 and HbO2 measurement can also be used via this platform. For example, the well-known fluorescence differences between oxygenated and deoxygenated Hb can allow one to accurately gauge O2 saturation of the Hb, and O2-sensitive fluorophores can be used to measure gas phase pO2.
There are myriad potential variations on the design and application of this platform. The microplate-reading spectrophotometer, which forms the basis for the absorbance changes that allow for spectral determination of O2 saturation here, can be inexpensively replaced with LEDs and photodiodes. The gas-tight cuvette holder, if mass-produced, can be made by injection-molding or other polymer manufacturing systems. The double gas-permeable sample sandwich can similarly be made of molded parts and the sample preparation in those cuvettes can be made vastly simpler, allowing for more plausible scaling-up to more numerous simultaneous samples. Gas mixing systems, presently costly, can be replaced with simpler methods. The addition of a small number of stable “Hemoglobin O2-Affinity Standards”, each having an established O2-binding curve, would allow for internal standardization of every set of experiments.
In summary, we present a simple apparatus that shows great promise with respect to human basic, clinical and translational medicine. It is transportable and sufficiently robust for field deployment in extreme environments and gathering parallel data on the essential function of blood.
Supplementary Material
Supplemental Figure 1. Influence of equilibration time using a thin-film, air-injection method. For each oxygen equilibrium curves (OEC), successive scans were obtained either one or three minutes after each pO2 change. The overlap of the two OECs suggests that blood Hb-O2 equilibrium is as rapid as one minute following each pO2 change.
Supplemental Figure 2. Time to thermodynamic equilibrium following each stepwise pO2 (%O2) change. %O2s used were: 0.2%, 0.6%, 1%, 3%, and 15% O2. ≥ 97% of the total absorbance change from each stepwise pO2 change was visible within one minute, and was complete by three minutes. The results are typical of those from 3 similar, independent experiments.
ACKNOWLEDGEMENTS
We acknowledge funding support from NIH (R21 HL-113943 to TJM), Department of Veterans Affairs (BX-000281 to TJM), Duke University Bookhout Scholarship Program and Stanford University Undergraduate Research Grants (to LEL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Antonini E, Brunori M. Hemoglobin. Annu Rev Biochem. 1970;39:977–1042. doi: 10.1146/annurev.bi.39.070170.004553. [DOI] [PubMed] [Google Scholar]
- 2.Secomb TW, Hsu R, Dewhirst MW. Models for oxygen exchange between microvascular networks and surrounding tissue. American Society of Mechanical Engineers, Heat Transfer Division. 1992;231:121–127. [Google Scholar]
- 3.Krogh A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol. 1919;52:457–474. doi: 10.1113/jphysiol.1919.sp001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon TJ, Exton Stone A, Bonaventura J, Singel DJ, Solomon Stamler J. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J Biol Chem. 2000;275:16738–16745. doi: 10.1074/jbc.M000532200. [DOI] [PubMed] [Google Scholar]
- 5.Root RW. The respiratory function of the blood of marine fishes. Biological Bulletin. 1931;61:427–456. [Google Scholar]
- 6.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaventura J. Clinical implications of the loss of vasoactive nitric oxide during red blood cell storage. Proc Natl Acad Sci U S A. 2007;104:19165–19166. doi: 10.1073/pnas.0708871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 10.Spinella PC, Doctor A, Blumberg N, Holcomb JB. Does the storage duration of blood products affect outcomes in critically ill patients? Transfusion. 2011;51:1644–1650. doi: 10.1111/j.1537-2995.2011.03245.x. [DOI] [PubMed] [Google Scholar]
- 11.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 12.Portner HO, Farrell AP. Ecology. Physiology and climate change. Science. 2008;322:690–692. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- 13.Bonaventura J, Bonaventura C, Sullivan B. Hemoglobins and hemocyanins: comparative aspects of structure and function. Journal of Experimental Zoology. 1975;194:155–174. doi: 10.1002/jez.1401940110. [DOI] [PubMed] [Google Scholar]
- 14.Lapennas GN, Colacino JM, Bonaventura J. Thin-layer methods for determination of oxygen binding curves of hemoglobin solutions and blood. Methods Enzymol. 1981;76:449–470. doi: 10.1016/0076-6879(81)76136-6. [DOI] [PubMed] [Google Scholar]
- 15.Wyman JaSJG. Binding and Linkage: Functional Chemistry of Biological Macromolecules. University Science Books; Mill Valley, CA: 1990. [Google Scholar]
- 16.Chiancone E, Ferruzzi G, Bonaventura C, Bonaventura J. Amphitrite ornata erythrocruorin. II. Molecular controls of function. Biochim Biophys Acta. 1981;670:84–92. doi: 10.1016/0005-2795(81)90052-0. [DOI] [PubMed] [Google Scholar]
- 17.Bonaventura C, Sullivan B, Bonaventura J. Spot hemoglobin. Studies on the Root effect hemoglobin of a marine teleost. J Biol Chem. 1976;251:1871–1876. [PubMed] [Google Scholar]
- 18.Bonaventura C, Bonaventura J. Anionic control of hemoglobin function. In: Caughey WS, editor. Biochemical and Clinical Aspects of Hemoglobin Abnormalities. Academic Press; New York: 1978. p. 725. [Google Scholar]
- 19.Riggs AF, Wolbach RA. Sulfhydryl groups and the structure of hemoglobin. J Gen Physiol. 1956;39:585–605. doi: 10.1085/jgp.39.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolman D, Gill SJ. Membrane-covered thin-layer optical cell for gas-reaction studies of hemoglobin. Anal Biochem. 1978;87:127–134. doi: 10.1016/0003-2697(78)90576-6. [DOI] [PubMed] [Google Scholar]
- 21.Clark TD, Seymour RS, Wells RM, Frappell PB. Thermal effects on the blood respiratory properties of southern bluefin tuna, Thunnus maccoyii. Comp Biochem Physiol A Mol Integr Physiol. 2008;150:239–246. doi: 10.1016/j.cbpa.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Imai K. Measurement of accurate oxygen equilibrium curves by an automatic oxygenation apparatus. Methods Enzymol. 1981;76:438–449. doi: 10.1016/0076-6879(81)76135-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Influence of equilibration time using a thin-film, air-injection method. For each oxygen equilibrium curves (OEC), successive scans were obtained either one or three minutes after each pO2 change. The overlap of the two OECs suggests that blood Hb-O2 equilibrium is as rapid as one minute following each pO2 change.
Supplemental Figure 2. Time to thermodynamic equilibrium following each stepwise pO2 (%O2) change. %O2s used were: 0.2%, 0.6%, 1%, 3%, and 15% O2. ≥ 97% of the total absorbance change from each stepwise pO2 change was visible within one minute, and was complete by three minutes. The results are typical of those from 3 similar, independent experiments.