Abstract
Human infants face the formidable challenge of learning the structure of their social environment. Previous research indicates that infants have early-developing representations of intentional agents, and of cooperative social interactions, that help meet that challenge. Here we report five studies with 144 infant participants showing that 10-to 13-month-old, but not 8-month-old, infants recognize when two novel agents have conflicting goals, and that they use the agents’ relative size to predict the outcome of the very first dominance contests between them. These results suggest that preverbal infants mentally represent social dominance and use a cue that covaries with it phylogenetically, and marks it metaphorically across human cultures and languages, to predict which of two agents is likely to prevail in a conflict of goals.
Because adequate inferences about social bonds and position confer adaptive advantages (1, 2), infants may posses early-developing mechanisms for representing elementary kinds of social relations, as well as perceptual input analyzers for identifying relevant instances in the social world (3–6). And indeed, young infants understand intentional action as goal-directed and rational (7–12). Further, infants represent social interactions among novel agents in terms of whether one helps or hinders the goals of the other, assigning positive valence to a helper and negative valence to a hinderer (13, 14). Slightly older toddlers are intrinsically motivated to help others achieve their goals (15), especially when primed with affiliation (16). Reflecting the evolved origins of these competencies (17), nonhuman primates also understand intentional action (18, 19), recruit the best collaborators (20), and sometimes help even human experimenters (15).
Affiliative and altruistic interactions reflect one important aspect of human social life. However, group living also entails conflicting goals (21) and competition for scarce resources (22). Dominance hierarchies that afford dominant animals privileged influence and access to food and mates are ubiquitous among animals, including humans. In fact, across cultures, social hierarchies are found within and between groups (4–6, 23). Even toddlers form dominance hierarchies that mirror those found among other primates (24, 25). Moreover, some cues for dominance appear to be (nearly) universal. In particular, dominance rank is associated with relative body size across the animal kingdom, and contestants in dominance fights typically assume postures that maximize their apparent body size until one party subordinates (6, 26–29). Increased access to resources as a result of dominance rank may itself lead to increased body size, and some species even show these morphological changes in response to experimental rank manipulations (27). Similarly, cultural practices and conceptual metaphors map size and relative height to social hierarchy (4–6, 29, 30). We may speak about a leader as the “big”man, place him on a throne above his subordinates, and kneel before our gods.
If hierarchy is a recurring feature of social environments, early representations of dominance may facilitate the learning process that makes a child a competent cultural member. Here we explore whether preverbal infants form representations of social dominance that mirror any of the evolutionary, cultural, and linguistically widespread features of this concept. Specifically, we established whether infants’ attention would be drawn more to unexpected events in which two agents block each other's path of motion and the bigger agent yields to the smaller one by bowing down and moving away, rather than vice versa. To ensure that we tapped general or abstract representations of dominance relations, the agents were novel and the goals minimally defined.
In the first Conflicting Goals experiment, 16 infants between 11 and 16 months of age were familiarized with a series of animations of two blocks of different sizes, each with an eye and a mouth (31) (fig. S1). During these familiarization trials, each agent was alone on the platform, bouncing gently from one side to the other, one from right to left and the other in the opposite direction (movies S1 and S2). This was to establish that each agent had the goal of moving to the opposite side of the platform from where it started. An intertrial consisted of both agents simultaneously starting from their habitual beginning positions so that they met in the middle, each blocking the other's habitual path. Then, the agents bumped into each other, backed up, and approached again for a total of three times before they each withdrew (movie S3). This served to highlight the conflict between the two agents’ goals being simultaneously realized and to acquaint infants with differences between the familiarization and test trials, such as the simultaneous presence of two agents on the stage and their new patterns of motion, that preceded the crucial experimental manipulations of the test events.
Two test events followed, beginning like the intertrial and with presentation order counterbalanced across participants. In the Expected Outcome test trial, the small agent bowed forward until it was lying down, and then scooted sideways out of the way (away from the viewer), upon which the large agent continued on its path to the end of the stage before the animation froze for 60 s (movie S5). In the otherwise identical Unexpected Outcome test trial, the large agent prostrated itself and yielded the way so that the small agent could complete its path to the end of the stage (movie S4). The time until the infant looked away from the test trials for more than 2 s was measured. In order to be included in the sample, infants must watch the screen during the bowing motion as each agent prostrated itself.
To compare looking times across animations of slightly different lengths, in all experiments we analyzed and report here continued looking times once the animations had frozen to stills (31). A repeated-measures analysis of variance (ANOVA) examined the effects of outcome (expected versus unexpected) and presentation order (Big versus Small agent bows first) on looking times. As predicted, infants attended longer to the Unexpected Outcome, in which a big agent bowed and yielded to a small one [mean (M)= 20.0 T 4.2 (SEM)], than vice versa [M = 12.0 T 3.2 (SEM); F(1,14) = 9.4, P = 0.008; partial ratio of variance accounted for (h2) = 0.40; table S1] (31).
A second experiment explored the developmental course of coming to expect that a small, novel agent will yield to a larger one in their first right-of-way conflict, using stimuli identical to those in experiment 1. Participants were 8-month-old (n = 16), 9-month-old (n =16), 10-month-old (n = 16), and 12-to 13-monthold (n =16) infants(31).
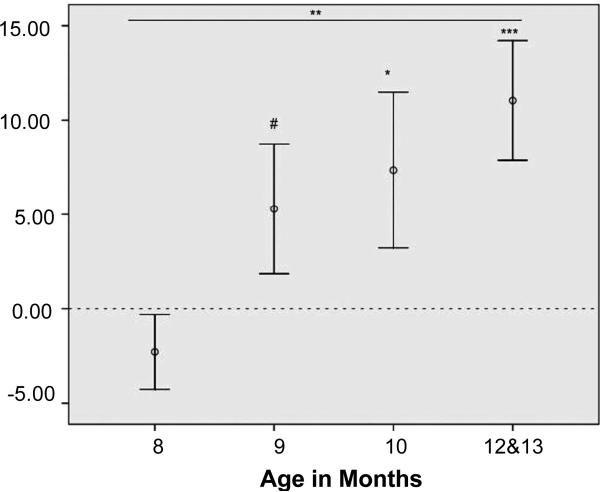
A repeated-measures ANOVA with test trials (expected or unexpected) varied within subjects and presentation order (expected first or unexpected first) and age category (8, 9, 10, and 12 to 13 months) varied between subjects again revealed that infants’ attention was drawn especially to those trials in which the larger agent made way for the smaller one [M= 17.2 T 1.6 (SEM)], rather than the reverse [M =11.8 T 1.2 (SEM); F(1, 56) = 11.40, P= 0.001, partial h2= 0.17]. This effect interacted with participant age (F(3, 56) = 3.14, P= 0.032, partial h2 = 0.14). Planned follow-up analyses (with one-tailed tests of the unidirectional hypothesis that the main effects of experiments 1 and 2 should replicate within each age group of the present experiment) demonstrated that 8-month-olds failed [MBigYields = 6.0 T 1.0 (SEM), MSmallYields =8.2 T 2.5 (SEM); paired-samples t(15) = –1.15, P(one-tailed) =0.13],and 9-month-olds marginally succeeded [MBigYields = 11.9 T 3.0 (SEM), MSmallYields =6.7 T 1.9 (SEM), paired-samples t(15) =1.54, P(one-tailed) =0.073], in differentiating between unexpected and expected test trials, whereas 10-month-olds [MBigYields = 21.7 T 4.1 (SEM), MSmallYields =14.4 T 2.6 (SEM); paired-samples t(15) =1.78, P(one-tailed) = 0.048] and 12-to 13-month-olds [MBigYields = 29.0 T 3.9 (SEM), MSmallYields = 18.0 T 2.9 (SEM), paired-samples t(15) = 3.49, P(one-tailed) = 0.0015] were able to do so (Fig. 1 and table S1) (31).
Fig. 1.
Developmental onset of the Conflicting Goals effect (experiment 2). Continued mean differential looking times (TSEM) at unexpected over expected test trials by categorical age, once the animations had frozen to stills (after 19.1 s), are shown. N =64 infant participants (8-, 9-, 10-, and 12to 13-month-olds). #P(one-tailed) = 0.073, *P(one-tailed) = 0.048, **P= 0.032, ***P(one-tailed) = 0.0015.
Replicating the results of experiment 1, which used children 11 months and older, experiment 2 thus demonstrates that the difference of attention when a large agent bows and yields to a smaller agent rather than vice versa develops between 8 and 10 months.
It is possible that the results of experiments 1 and 2 reflect merely that there is more mass in motion, and thus perhaps more salient motion catching the infants’ attention, when the larger rather than the smaller agent bows and scoots away for the other. As an initial control for this possibility, a third Isolated Motion Control experiment (fig. S2) exposed 11-to 16-month-olds (n = 16) to the same familiarization events used in experiments 1 and 2 (movies S1 and S2), but without conflicting goals in intertrials and test trials. Instead, either the large or small agent was alone on stage, performing the identical motions as in the Conflicting Goal trials. Two intertrials (movies S6 and S7) showed infants the actions of each agent immediately before it bowed and scooted back on subsequent test trials (movies S8 and S9), while not implying conflicting goals, because each agent was alone on the stage (31). Hence, if the results of the Conflicting Goals experiments had been driven by differences in the total mass in motion across the unexpected and expected test trials, then infants should also differentiate the Isolated Motion test trials. Conversely, if the results so far reflect infants’ use of relative size as a cue for dominance rank predicting the outcome of a conflict of goals, then infants should not differentiate the current Isolated Motion trials.
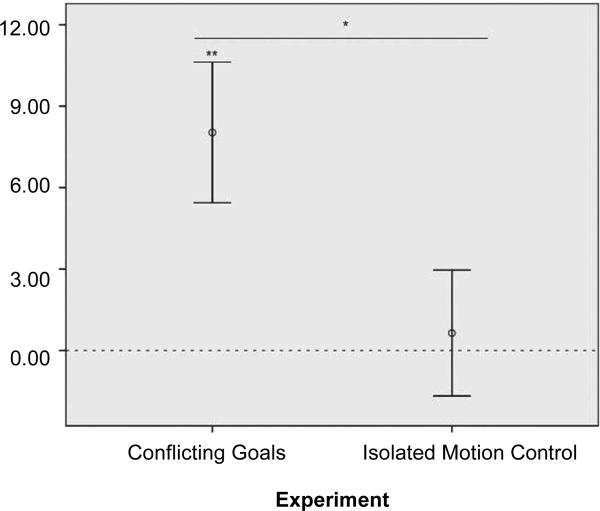
Here, a repeated-measures ANOVA with the test factor (expected or unexpected) varied within subjects and presentation order varied between subjects revealed that 11-to 16-month-olds no longer differentiated between a large [M =6.9 T 2.2 (SEM)] and small [M =6.3 T 2.0 (SEM)] agent bowing and scooting away [F (1,14) = 0.089, ns (not significant); table S1] (31). Pooling these data with those of experiment 1, the 11-to 16month-olds in these two experiments looked equally long at the identical familiarization events, ruling out preexisting group differences in attentiveness. Crucially, an interaction confirmed that infants’ differentiation of unexpected and expected test trials varied significantly across experiments in such a way that the size of the bowing agent mattered in the Conflicting Goals experiment but not in the Isolated Motion Control experiment (Test × Experiment: F (1,28) = 4.75, P= 0.038, partial h2 = 0.15) (Fig. 2) (31).
Fig. 2.
Conflicting Goals (experiment 1) versus Isolated Motion Control (experiment 3). Continued mean differential looking times (TSEM) at unexpected over expected test trials by experimental condition, once the animations had frozen to stills (after 19.1 and 13.2 s, respectively), are shown. N = 32 infant participants (11 to 16-month-olds). *P <0.05, **P(one-tailed) <0.01.
Still, it is possible that infants are only sensitive to the greater mass in motion when the large block prostrates itself if cues to relative size are also present. This was not the case in the Isolated Motion Control experiment, because infants never watched the two agents together. Thus, low-level perceptual factors might still have driven the results presented so far. Experiment 4 (Motion Behind Control, fig. S3) addressed this possibility and explored another alternative interpretation of the results of experiments 1 and 2: that young infants merely expect that smaller agents are more likely to fall over than are bigger ones.
Ten-month-old infants (n = 16) were familiarized with the two agents each walking in turn across the stage, both in the same direction, crossing it fully or halfway (movies S10 to S13). In the intertrials, one first traveled to the end of the stage and was then followed by the other, who stopped behind its back in the middle of the stage, in the same position in which bowing took place in experiments 1 through 3 (movies S14 and S15). Repeating this sequence on test trials, the agent who had stopped behind the other bowed and scooted away in a manner identical to that in previous experiments. For half of the test trials, the agent falling forward was the large one, and for the other half, it was the small one (movies S16 and S17) (31).
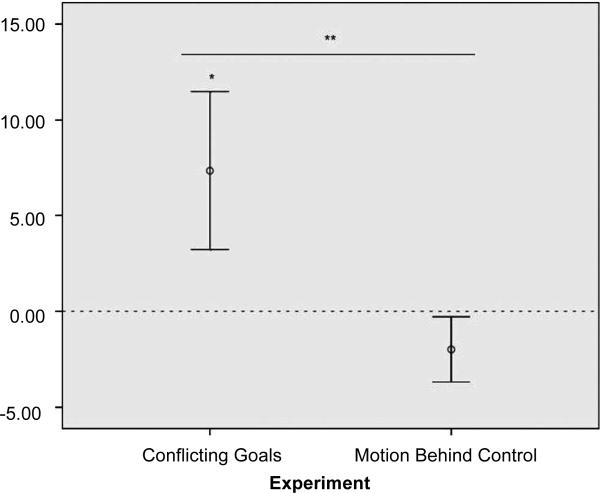
A repeated-measures ANOVA with the test factor (expected or unexpected) varied within subjects and presentation order of the test trials varied between subjects revealed that infants did not differentiate between these two test events [MBigBows =4.5 T 1.08 (SEM), MSmallBows=6.5 T 1.8 (SEM), F(1,14) = 1.31, ns]. Pooling these data with those of the 10-month-olds from experiment 2, a repeated-measures ANOVA varying the test factor (unexpected or expected) within subjects and presentation order and experimental condition (Conflicting Goals or Motion Behind) between subjects revealed an interaction (Test × Experiment: F(1,28) = 4.41, P= 0.045, partial h2 =0.14). This confirmed that infants’ differentiation of test trials varied across experiments in such a way that they were surprised when a larger agent bowed and scooted away from a smaller agent in a Conflicting Goals face-off, but not when it performed the exact same motion behind the back of a smaller agent (Fig. 3).
Fig. 3.
Conflicting Goals (experiment 2) versus Motion Behind Control (experiment 4). Continued mean differential looking times (TSEM) at unexpected over expected test trials by experimental condition, once the animations had frozen to stills (after 19.1 and 5.00 20.5 s, respectively), are shown. N = 32 infant participants (10month-olds). *P(one-tailed) <0.05, **P <0.05.
Thus, 10-month-olds’ increased attention to a large agent yielding to a smaller one rather than vice versa in experiments 1 and 2 is unlikely to reflect differences in the saliency of motion of the two agents, nor expectations that larger agents are unlikely to fall over. Such accounts would also predict that infants would differentiate the test trials in the Isolated Motion and Motion Behind experiments, but we found that infants did not do so, as predicted by a social dominance account of the results of the Conflicting Goals experiments.
The Conflicting Goals test trials confound full occlusion (when the small agent, behind, is passed by the large one) and partial occlusion (when the big agent, behind, is passed by the small one) with the expected and unexpected outcomes (fig. S1 and movies S4 and S5). To ensure that greater perceptual complexity of partial occlusion did not drive the results of the Conflicting Goals experiments, 10-month-old (n = 16) and 12-to 13month-old (n = 16) infants participated in a fifth Occlusion Control experiment (fig. S4) that matched experiments 1 and 2 in all aspects of occlusion during the test events, but removed cues to agency and conflicting goals from the animations.
The first two familiarization trials showed upright blocks, with no eyes or mouth, gliding smoothly across the front of the stage, one from left to right and one from right to left (movies S18 and S19). In the third and fourth familiarization trials, the blocks were lying down while gliding in a path in the back of the stage, before stopping in the middle, at the exact place where the yielding agent comes to a halt when scooting backward in the Conflicting Goals scenarios (movies S20 and S21). The first intertrial showed one block lying in the back of the stage and one standing to the side in the front, ready to begin gliding across the stage (movie S22). The second intertrial reversed the roles of the blocks (movie S23). In each test trial, the block in the foreground glided across the stage, fully occluding (the large block) or partially occluding (the small block) the one in the background (movies S24 and S25) (31). Hence, if perceptual differences of total versus partial occlusion had driven the differentiation of the Conflicting Goals trials, then infants should also differentiate the present Occlusion test trials. However, this should not be the case if the Conflicting Goals results reflect infants’ use of relative size as a cue for social dominance.
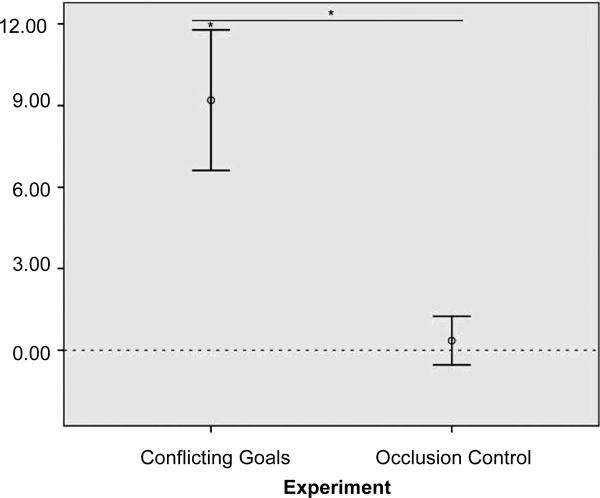
A repeated-measures ANOVA varying the test factor (expected or unexpected) within subjects and the presentation order of the two test trials and categorical participant age (10 or 12 to 13 months) between subjects revealed that infants failed to differentiate these test trials [MPartial Occlusion =2.7 T 0.6 (SEM), MFull Occlusion = 2.4 T 0.8 (SEM) F(1,28) = 0.15, ns; table S1] and that infant age did not moderate this null effect (Test × Age: F(1,28) = 0.98, ns) (31). Pooling these data with those from the 10-and 12-to 13-montholds from experiment 2, a repeated-measures ANOVA that varied the test factor within subjects and the presentation order of test trials, categorical age (10 or 12 to 13 months), and experimental condition (Conflicting Goals or Occlusion) between subjects again confirmed that infants’ differentiation of test trials significantly differed across experiments (Test × Experiment: F(1,56) = 11.69, P= 0.001, partial h2 = 0.17): Whether the large or small agent was passed, lying head-down, by the other one mattered only in the context of zero-sum conflict, not when identical occlusion aspects were presented without this context (Fig. 4) (31). This rules out the possibility that the greater complexity of the occlusion events in the unexpected Conflicting Goals test trials drove the infants’ interest to them.
Fig. 4.
Conflicting Goals (experiment 2) versus Occlusion Control (experiment 4). Continued mean differential looking times (T SEM) to Unexpected9.00 over Expected test trials by experimental condition, once the animations had frozen to 6.00 stills (after 19.1 and 9.9 s, respectively). N =64infant participants (10-and 12-to 3.00 13-month-olds). *P < 0.001.
Together, the five experiments presented here show that preverbal infants use relative size to predict which of two novel agents has the right of way. This effect is not driven by increased saliency of the greater area in motion when the large agent bows; nor by expectations that small, rather than large, agents or objects fall over; nor by differential attention to partial versus full occlusion when the agents pass each other. In addition to the results from the three quite different control conditions, the fact that 8-month-olds fail, 9-month-olds marginally succeed, and 10-and 12-to 13-month-olds robustly succeed at differentiating the Conflicting Goals outcomes speaks further against low-level perceptual saliency accounts that would predict no such age differences. One final detail of our data provides additional support for the interpretation that our findings in experiments 1 and 2 depend on the representations of dominance ranks for predicting the outcomes of conflicting goals. What the qualitatively different control experiments have in common is that they do not establish conflicting goals, and infants were far less interested in all of them than in the Conflicting Goals test trials (31).
Other lean interpretations that make no reference to goal-directed agents and social dominance cannot account for our data. The animations were designed so that they would not invoke mechanistic physics. One agent did not knock over the other one; instead the two agents moved back from each other and paused, before one of them lay down forwardly, opposite to what billiard-ball causality would entail. Finally, a lean account might argue that infants’ attention could simply be drawn to relative (nonsocial) size difference. However, relative size difference is maximized in the expected Conflicting Goals test trials (fig. S1 and movies S4 and S5), whereas we found that infants attend more to the unexpected trials, and infants did not differentiate the exact same relative size differences in the Motion Behind test trials (fig. S3 and movies S16 and S17).
Social relations are irreducible to features of individual agents. Although a social interaction gives evidence that intentional agents are present, the presence of several intentional agents alone does not predict the kind of social relationship they have with one another. Previous research has documented that young infants represent interactions among others in terms of affiliative and cooperative relations (13–16). The present studies show that preverbal infants also represent dominance between competing agents. Moreover, consistent with existing research on infants’ representations of intentional agency (7–12), our evidence suggests that this conceptual understanding develops between 8 and 10 months of age.
Nine-and 10-month-old infants are too young to have actively participated in dominance fights, and American infants are unlikely to have watched small agents bow and prostrate in subordination to others of more formidable physical size, such as their parents. They may have experienced older siblings taking their toys or observed older siblings struggle with each other, and learned that the bigger one often gets his or her way over the little one. If infants use these experiences to predict what will happen in our animations, they must see the similarity between previous experiences and the present animations, even though the spatiotemporal parameters of the agents and their physical movements do not exactly match those they have experienced. Hence, representations of conflicting goals and social dominance, rather than spatiotemporal primitives, must underpin the relevant similarity metric that allows infants to apply their experiences to the situations depicted in our animations.
We cannot know, at present, what aspects of our stimuli led the children to encode the familiarization and intertrial events in experiments 1 and 2 in terms of conflicting goals between two agents. It is likely that the presence of face-like features contributed to infants’ categorization of the blue and green blocks as agents (13, 14). But equifinality of motion is also sufficient to establish goals for novel agents with no facial features (9). It is also uncertain whether infants would have extrapolated that the agents’ goals conflict had the agents not bumped into each other when physically blocking each other's way (indeed, the infants might have thought that the agents could pass or jump over each other so that both could complete their paths to the end of the stage). These results also leave open just what about the unexpected conflicting goals event is unexpected. Infants’ attention may be drawn when the smaller agent prevails, or when the larger agent displays features of subordination (by prostrating itself), or both. Importantly, our results do not yet address which other conflicting goals and features of dominance infants can represent, nor whether infants expect the dominance relation between two agents to be stable across different contexts of conflicting goals, even when the physical dominance cue of relative size is absent.
A crucial task for the developing child is to learn the social structure of his or her world, in order to interact appropriately with kin and non-kin, friend and foe, superiors, inferiors, and peers. Constraints on infants’ mental representations of social relations may direct infants’ attention to the relevant features among the myriad stimuli present in any social scenario and assist them in interpreting social interactions (3–6). Our finding that preverbal infants mentally represent conflicting goals and social dominance between two agents suggests that just as infants possess early-developing mechanisms for learning about the physical world and the world of individual intentional agents (3), they also have early-developing representational resources tailored to understanding the social world, allowing infants to understand and learn the dominance structures that surround them.
Supplementary Material
Acknowledgments
This research formed part of L.T.'s doctoral dissertation. It was supported by Harvard University and Winkler Foundation dissertation fellowships, together with a postdoctoral fellowship and an early-career award from the National Danish Science Foundation to L.T., and a NIH/NICHD grant (2 ROI HD038338) to S.C. We thank L. Schultz and the MIT Play Lab in the Boston Children's Museum for lending us their lab space; J. Cotton, M. Renno, and B. Walker-Meade for help in data collection; S. Grum-Thomsen for help with graphics; and A. Fiske, J. Sidanius, T. Teasdale, and two anonymous reviewers for helpful feedback on an earlier draft.
References and Notes
- 1.Richerson PJ, Boyd R. Not By Genes Alone. Univ. of Chicago Press; Chicago: 2006. [Google Scholar]
- 2.Silk JB. Science. 2007;317:1347. doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- 3.Carey S. The Origin of Concepts. Oxford Univ. Press; Oxford: 2009. [Google Scholar]
- 4.Fiske AP. Structures of Social Life. Free Press; New York: 1991. [Google Scholar]
- 5.Fiske AP. Pers. Soc. Psychol. Rev. 2000;4:76. doi: 10.1207/S15327957PSPR0401_7. [DOI] [PubMed] [Google Scholar]
- 6.Fiske AP. In: Relational Models Theory. Haslam N, editor. Erlbaum; Mahwah, NJ: 2004. pp. 61–146. [Google Scholar]
- 7.Gergely G, Nadasdy Z, Csibra G, Biro S. Cognition. 1995;56:165. doi: 10.1016/0010-0277(95)00661-h. [DOI] [PubMed] [Google Scholar]
- 8.Gergely G, Bekkering H, Kiraly I. Nature. 2002;415:755. doi: 10.1038/415755a. [DOI] [PubMed] [Google Scholar]
- 9.Csibra G, Biro S, Koos O, Gergely G. Cogn. Sci. 2003;27:111. [Google Scholar]
- 10.Woodward AL. Cognition. 1998;69:1. doi: 10.1016/s0010-0277(98)00058-4. [DOI] [PubMed] [Google Scholar]
- 11.Saxe R, Tenenbaum JB, Carey S. Psychol. Sci. 2005;16:995. doi: 10.1111/j.1467-9280.2005.01649.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson SC. Philos. Trans. R. Soc. London Ser. B. 2003;358:549. doi: 10.1098/rstb.2002.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhlmeier V, Wynn K, Bloom P. Psychol. Sci. 2003;14:402. doi: 10.1111/1467-9280.01454. [DOI] [PubMed] [Google Scholar]
- 14.Hamlin K, Wynn K, Bloom P. Nature. 2007;450:557. doi: 10.1038/nature06288. [DOI] [PubMed] [Google Scholar]
- 15.Warneken F, Tomasello M. Science. 2006;311:1301. doi: 10.1126/science.1121448. [DOI] [PubMed] [Google Scholar]
- 16.Over H, Carpenter M. Psychol. Sci. 2009;20:1189. doi: 10.1111/j.1467-9280.2009.02419.x. [DOI] [PubMed] [Google Scholar]
- 17.Silk J. Philos. Trans. R. Soc. London Ser. B. 2009;364:3243. doi: 10.1098/rstb.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Call J, Hare B, Carpenter M, Tomasello M. Dev. Sci. 2004;7:488. doi: 10.1111/j.1467-7687.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- 19.Phillips W, Barnes JL, Mahajan N, Yamaguchi M, Santos LR. Dev. Sci. 2009;12:938. doi: 10.1111/j.1467-7687.2009.00840.x. [DOI] [PubMed] [Google Scholar]
- 20.Melis AP, Hare B, Tomasello M. Science. 2006;311:1297. doi: 10.1126/science.1123007. [DOI] [PubMed] [Google Scholar]
- 21.Aureli F, de Waal F. Natural Conflict Resolution. Univ. of California Press; Berkeley, CA: 2000. [Google Scholar]
- 22.Darwin C. The Origin of Species. Vol. 1982. Penguin Classics; Berkeley, CA: 1859. [Google Scholar]
- 23.Sidanius J, Pratto F. Social Dominance. Cambridge Univ. Press; Cambridge: 1999. [Google Scholar]
- 24.McGrew WC. An Ethological Study of Children's Behavior. Academic Press; New York: 1972. [Google Scholar]
- 25.Sluckin AM, Smith PK. Child Dev. 1977;48:917. [Google Scholar]
- 26.Brown JH, Maurer BA. Nature. 1986;324:248. [Google Scholar]
- 27.Buston P. Nature. 2003;424:145. doi: 10.1038/424145a. [DOI] [PubMed] [Google Scholar]
- 28.Clutton-Brock TH, et al. Nature. 2006;444:1065. doi: 10.1038/nature05386. [DOI] [PubMed] [Google Scholar]
- 29.Schubert T, Waldzus S, Seibt B. In: Embodied Grounding. Smith E, editor. Cambridge Univ. Press; New York: 2008. pp. 160–185. [Google Scholar]
- 30.Lakoff G, Johnson M. Metaphors We Live By. Univ. of Chicago Press; Chicago: 2003. [Google Scholar]
- 31.See supporting material on Science Online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.