Abstract
The guanine nucleotide exchange factor (GEF) Vav1 synergizes with the adapter SLP-76 to support T cell development and activation. Here, we demonstrate that Vav1 controls the stability and movement T cell receptor-induced SLP-76 microclusters. The SH2 domain enables the recruitment of Vav1 into SLP-76 microclusters, whereas the SH3 domains of Vav1 cooperate to enhance microcluster stability and function. Although the amino-terminus of Vav1 is essential for downstream signaling, it possesses novel scaffolding functions that are unaffected by the inactivation of the Vav1 GEF or by the constitutive GEF activation that accompanies the mutation of the regulatory tyrosine residues 142, 160, and 174. In contrast, GEF-inactivating point mutations predicted to perturb the structural integrity of the Vav1 GEF abolish these scaffolding functions. Paradoxically, the excision of catalytic Dbl-homology (DH) / pleckstrin homology (PH) cassette produces a relatively mild scaffolding defect, indicating that the L213A and L278Q point mutations antagonize scaffolding functions mediated by adjacent domains. A deletion mutant lacking the CH domain potently inhibits calcium responses, but also exhibits mild scaffolding defects. We conclude multiple GEF-independent scaffolding functions contained within the amino-terminus of Vav1 contribute to T cell activation by acting synergistically to increase the stability and functionality of SLP-76 microclusters.
Introduction
Vav1 is a proto-oncogene that plays crucial roles in the signaling pathways initiated by the T cell receptor (TCR) (1). Vav1 regulates T cell proliferation and effector function through its impacts on GTPases, calcium responses, mitogen-activated protein kinases, and the cytoskeleton (2–5). Although recent studies have provided conflicting views regarding the role of the Vav1-intrinsic Rho-family GTPase guanine nucleotide exchange factor (GEF) activity in T cell development, Vav1 possesses both GEF-dependent and GEF-independent functions (6–8). The transforming activity of Vav1 is dependent upon the Vav1 GEF (9). In addition, Vav1 GEF is required for the activation of the Akt and JNK, the activation of AP-1, and the production of IL-2 downstream of the TCR (6, 9, 10). In contrast, the Vav1 GEF is dispensable for calcium and ERK responses, and for the polarization of the T cell cytoskeleton (6–8). The latter GEF-independent functions are commonly attributed to adapter-like functions that have not been fully characterized.
The Src-homology 2 (SH2) is required for the TCR-dependent phosphorylation of Vav1 and is required to support the synergistic activation of NF-AT by SLP-76 and Vav1 (11–14). In addition, the SH2 domain of Vav1 binds directly to tyrosines 113 and 128 in the N-terminus of human SLP-76 (14). The crucial impact of the interaction between Vav1 and SLP-76 is reflected in the parallel developmental and functional defects observed in the absence of either SLP-76 or Vav1, and in SLP-76 knock-in mice lacking the Vav1-binding tyrosines (15–19). Since Vav1 enhances the co-precipitation and co-localization of PLCγ1 with SLP-76, Vav1 is likely to influence the overall stability and functionality of signaling complexes containing SLP-76 (3, 4, 20).
Upon encountering antigenic surfaces, T cells generate signaling microclusters analogous to the ‘signalosomes’ defined by classical biochemical techniques. Two distinct complexes are assembled: TCR microclusters, which recruit the tyrosine kinase ZAP-70, and SLP-76 microclusters, which are nucleated in close proximity to TCR microclusters by LAT and Gads (21–27). Both TCR and SLP-76 microclusters are coupled to the actin cytoskeleton, and are thereby transported to the center of the stimulatory contact unless restrained by interactions with immobilized ligands (22, 26). Perturbations of the TCR-proximal signaling cascade that reduce the persistence of SLP-76 microclusters universally inhibit T cell activation (27). Crucially, the functionally inert Y3F mutant of SLP-76, which lacks docking sites for Vav1 and Itk, reduces the persistence and mobility of SLP-76 microclusters (27). This scaffolding defect has never been decisively attributed to the loss of Vav1; however, Vav1 could enhance microcluster stability by providing a localized GEF activity or by acting as a scaffold to sustain the interactions among microcluster-resident proteins (20).
Vav1 can be subdivided into two distinct regions. An adapter module at the C-terminus consists of the SH2 domain, a classical SH3 domain, and an atypical SH3 capable of binding SH3 domains within Grb2 or Nck (1, 28). However, this module, when expressed in isolation, is a potent inhibitor of antigen-dependent signals (11, 29). Therefore, any Vav1-mediated adapter functions must involve its N-terminus. This region consists of a calponin homology (CH) domain involved in the regulation of calcium entry, three major tyrosine phosphorylation sites (Y142, Y160, and Y174), and a catalytic core composed of the tightly associated Dbl homology (DH), pleckstrin homology (PH), and C1 domains. This region forms a compact auto-inhibited fold that opens in response to the phosphorylation of the regulatory tyrosines lying between the CH domain and the catalytic core (30, 31). At present there is no information regarding the contribution of any of these N-terminal domains to the stabilization of SLP-76 microclusters.
Using dynamic imaging analyses and functional studies, we demonstrate that the C-terminus of Vav1 controls the recruitment of Vav1 into SLP-76 microclusters and contributes to the stability of the resulting structures. The activity of the Vav1 GEF is required for optimal downstream signaling; however, the N-terminus of Vav1 also acts as a GEF-independent scaffold to promote the stabilization, movement, and function of SLP-76 microclusters. Unexpectedly, mutations impacting the core of the DH domain or the interface between the DH and C1 domains result in inert proteins that do not exhibit the scaffolding functions normally associated with the N-terminus of Vav1. In contrast, the deletion of either the CH domain or the DH-PH module results in a dysfunctional molecule that exhibits limited defects in microcluster stability and movement. We conclude that scaffolding interactions provided by the CH domain and the DH-PH-C1 module cooperate to promote the stabilization and movement of SLP-76 microclusters, and that these scaffolding functions are completely disrupted by the perturbation of the catalytic core. Our results indicate that Vav1 promotes T cell activation both through its GEF activity and through multiple scaffolding interactions that cooperate to increase the stability and functionality of SLP-76 microclusters.
Results
Vav1 Selectively Enters SLP-76 MC and Influences Their Persistence and Movement
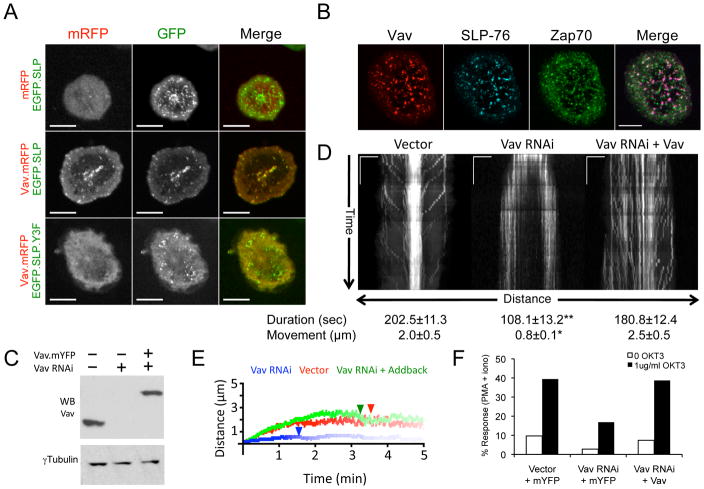
Because the SLP-76 Y3F (Y113F/Y128F/Y145F) mutation reduces the persistence of SLP-76 MC, we postulated that the adaptor functions of Vav1 enable it to enter and stabilize these essential signaling structures (27). To test this hypothesis, we evaluated the ability of wild-type or Y3F-mutant EGFP.SLP-76 chimeras to recruit Vav1.mRFP into SLP-76 MC. These studies were performed in the absence of endogenous SLP-76 using J14 T cells expressing matched levels of these chimeras. Although mRFP1 does not enter SLP-76 MC in the absence of a fusion partner (Figure 1A, upper row), Vav1.mRFP1 colocalizes with EGFP.SLP-76 in microclusters (Figure 1A, middle row). In contrast, Vav1.mRFP1 remains completely cytoplasmic in the EGFP.SLP-76.Y3F-expressing cell line (Figure 1A, lower row), indicating that the recruitment of Vav1 into microclusters is strictly controlled by the amino-terminal tyrosines of SLP-76. Since previous studies suggested that the Vav1 SH2 domain also binds ZAP-70, we also imaged Vav1.mCFP with respect to SLP-76.YFP and phosphorylated ZAP-70 (pY 319) (32). As we reported previously, ZAP-70 and SLP-76 enter distinct microclusters (22). Vav1 (Figure 1B, red) predominantly enters SLP-76 microclusters (blue, purple overlay), and does not colocalize with ZAP-70 in TCR microclusters (green). Thus, the amino-terminal tyrosines of SLP-76 play the decisive role in the recruitment of Vav1 into signaling microclusters following TCR ligation.
Figure 1. Vav1 enters SLP-76 microclusters and controls their persistence and movement.
(A) J14 cells stably expressing either EGFP-tagged WT or Y3F SLP-76 chimeras (green) were transiently transfected with either mRFP or Vav1.mRFP (red). Still images of cells expressing matched levels of Vav1 were acquired by confocal microscopy 5 minutes after stimulation on anti-TCR coated coverslips. (B) J14 cells stably reconstituted with SLP-76.mYFP (J14.SY) were transiently transfected with Vav1.mCFP and stimulated as in A. The cells were fixed and stained for ZAP70 pY319. Images in A and B are representative of 3 experiments; scale bars correspond to 10μm. (C–F) J14.SY cells were transfected with an empty vector control, a Vav1-specific shRNA expression vector, or the Vav1-targeting vector in conjunction with an shRNA-resistant Vav1.mCFP expression vector. (C) Total lysates were western blotted as indicated. (D) Vav sufficient, deficient, and reconstituted J14.SY cells were stimulated as in A and imaged continuously for 5 minutes. Representative kymographs depicting the directional movement of SLP-76 MC (x-axis) over time (y-axis) are shown. Scale bars represent 5μm x 60 seconds. Mean microcluster duration and movement were determined on a per cell basis by manually tracing MC paths in kymographs and are shown ± SEM (n=10 cells). Single and double asterisks indicate significant differences (p < 0.05 and p < 0.005, respectively) from the vector control. (E) Mean MC traces for each cell were averaged to yield composite kymographs depicting both MC movement (y-axis) and fractional persistence (line intensity) over time (x-axis). See Supplemental Table 1 for further analysis. (F) Vav1 sufficient, deficient, and reconstituted J14.SY cells were assayed for NF-AT activation. One of 4 representative experiments is shown.
To determine whether Vav1 influences the behavior of SLP-76 MC we transfected J14.SY cells with a vector encoding a Vav1-specific short hairpin RNA (shRNA) or an empty vector control, in conjunction with a vector encoding either mCFP or an shRNA-insensitive Vav1.mCFP chimera (Figure 1C). The resulting differences in the movement and persistence of SLP-76 MC are depicted in kymographs derived from diametrical regions of interest (Figure 1D). In control cells receiving the empty hairpin vector, SLP-76 MC form in the periphery of the contact and converge towards the center of the contact (peripheral diagonal traces), where they persist throughout the experiment (central vertical traces). In Vav1-deficient cells SLP-76 MC also form in the periphery, but are short lived and dissipate near their points of origin (short, vertical, peripheral traces). To quantitate these changes, manually generated SLP-76 MC traces were compiled to generate a mean trace for each cell. For each condition, these cell-specific traces were averaged, weighting the contribution of each cell equally (see Supplementary Table S1 for a more extensive analysis of SLP-76 microcluster properties in J14.SY cells). Composite kymographs (Figure 1E) depicting mean microcluster displacement (y-position), persistence (line intensity), and half-maximal survival (arrowheads) confirm that microcluster persistence and movement were significantly reduced in Vav1 knockdown cells (blue line) relative to control cells (red line). Reconstitution with Vav1.mCFP corrects the defect in microcluster persistence, and substantially corrects the defect in microcluster centralization (Figure 1D, right panel, note long vertical traces displaying episodic inward movement; Figure 1E, green line). The residual differences between add-back and control cells were not statistically significant (Supplementary Table 1). These data establish, for the first time, that Vav1 plays decisive roles in the persistence and movement of SLP-76 MC. As previously observed, microcluster persistence and movement were positively correlated with the TCR-mediated activation of a composite NF-AT/AP-1 element derived from the IL-2 promoter (Figure 1F) (27).
The Src Homology Domains of Vav1 Control Microcluster Behavior and Function
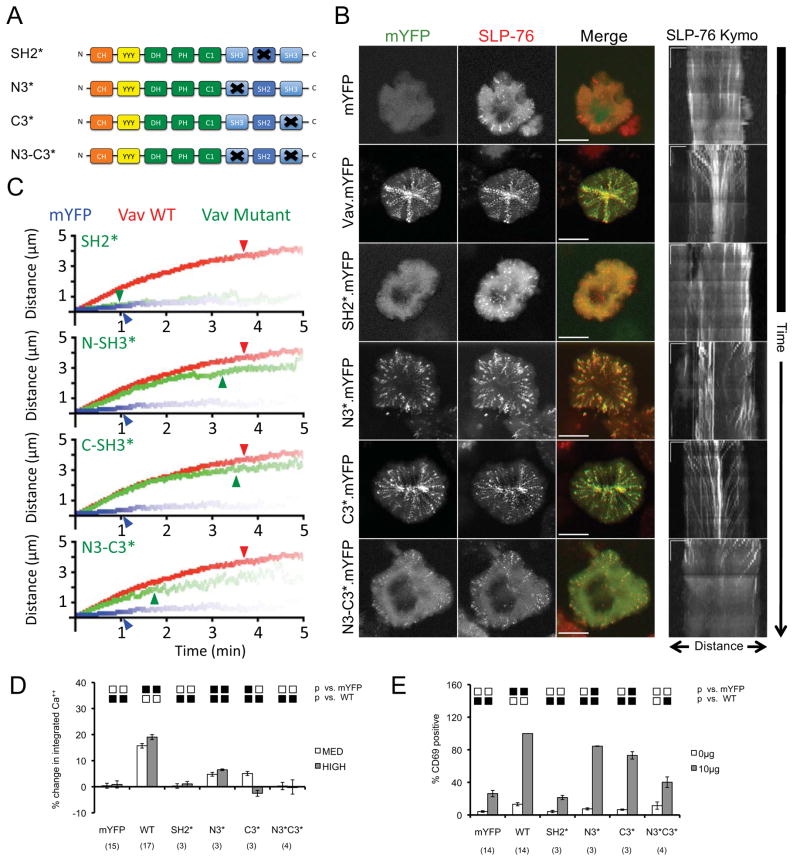
To evaluate SLP-76 MC in the presence of various Vav1 mutants (Figure 2A), we stably transfected the Vav-deficient Jurkat T cell line, J.Vav1, with a hemagglutinin-tagged SLP-76-mCFP chimera (2xHA.SLP-76.mCFP) (33). When transfected with vectors expressing mYFP alone, these J.Vav1-2xHA.SLP-76.mCFP (JV.SC) cells generate transient SLP-76 MC that dissipate without moving (Supplementary Movies S1 and S2), producing spots in maximum over time (MOT) images, and short vertical lines in kymographs (Figure 2B). In contrast, JV.SC cells expressing wild-type Vav1.mYFP generate persistent and mobile SLP-76 MC (Supplementary Movies S3 and S4), which produce radial ‘spokes’ in MOT images, and converging diagonal traces in kymographs (Figure 2B). These differences in behavior are significant and are readily apparent in composite kymographs (Figure 2C, blue and red lines; see Table 1 for a more extensive analysis of SLP-76 microcluster properties in JV.SC cells). In addition, reconstitution with Vav1.mYFP increased the fraction of SLP-76 found in microclusters by nearly 8-fold (Table 1; note the differences in the relative intensities of the diffuse cytoplasmic and clustered pools of SLP-76 in Figure 2B). These data confirm that Vav1 augments the recruitment of SLP-76 into microclusters and controls the persistence and movement of the resulting SLP-76 MC. Finally, Vav1.mYFP reconstituted defects in TCR-induced calcium entry and CD69 upregulation (Figures 2D and 2E), further validating the correlation between microcluster persistence and optimal T cell signaling.
Figure 2. The Src homology domains of Vav1 play distinct roles in microcluster entry, microcluster stabilization, and Vav1 function.
(A) The domain structure of Vav1 mutants used in this figure. (B) Left - J.Vav1 cells stably expressing SLP-76.mCFP (JV.SC cells) were transiently transfected with the indicated Vav1.mYFP constructs and continuously imaged on stimulatory coverslips for 5 minutes. One representative ‘maximum over time’ (MOT) image selected from 3 independent experiments is shown. Scale bars correspond to 10 μm. Right – Kymographs were generated as in Figure 1D; scale bars correspond to 5μm x 60 seconds. (C) Composite kymographs depicting SLP-76 MC movement and persistence were generated as in Figure 1E, using JV.SC cells transfected with mYFP (blue), WT Vav1 (red), or the indicated mutant (green). Arrowheads correspond to the half-life of SLP-76 MC for each condition. See Table 1 for further analysis. (D) TCR-induced intracellular calcium flux was measured in J.Vav1 cells transiently transfected with the indicated Vav1 mutants fused to mYFP. The percent change in the integrated calcium responses of moderate- and high-expressing populations is shown ± SEM, calculated relative to the calcium response of untransfected, mYFP-null cells in the same sample. The number of replicates is shown in parentheses. Significant differences (p < 0.05) from the indicated conditions are denoted by black boxes, unless dominant negative (below the mYFP response, red boxes) or supra-physiological (above the WT response, green boxes). Trends (0.05 < p < 0.10) are denoted similarly by gray, orange, and yellow boxes. (E) J.Vav1 cells were transiently transfected with the indicated Vav1 constructs fused to mYFP and stained for CD69. The fraction of cells expressing CD69 within a tightly gated population expressing moderate levels of mYFP was normalized to the response observed in Vav1.WT-reconstituted cells and is shown ± SEM. Replicates and significant differences are shown as above.
Table 1. Effect of Vav1 Mutations on SLP-76 Microcluster Dynamics.
SLP-76 microcluster dynamics were measured in JV.SC cells transfected with vectors expressing either mYFP or the indicated Vav1.mYFP chimeras. Individual SLP-76 microcluster paths were obtained by manual tracing, and average microcluster behaviors were calculated per cell. The values presented are the means of values obtained in the indicated number of cells.
| Persistence (sec) | p. | Movement (μm) | p. | Speed (nm/sec) | p. | % SLP in Clusters | p. | Clusters per cell | p. | N (Cells) | N (Expts) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mYFP | 68.5 ± 3.92 | †† | 0.6 ± 0.03 | †† | 56.6 ± 2.12 | †† | 5.9 ± 0.66 | †† | 10.8 ± 0.83 | †† | 45 | 13 |
| WT | 180.5 ± 6.94 | ** | 3.3 ± 0.19 | ** | 102.9 ± 3.25 | ** | 43.5 ± 2.83 | ** | 19.1 ± 0.8 | ** | 46 | 13 |
| N-SH3* | 164.9 ± 11.6 | ** | 2.6 ± 0.26 | ** | 99.6 ± 4.79 | ** | 33.9 ± 3.68 | ** | 20.6 ± 1.51 | ** | 15 | 3 |
| C-SH3* | 181.6 ± 10.94 | ** | 3.1 ± 0.28 | ** | 104.8 ± 4.54 | ** | 40.8 ± 4.44 | ** | 22.6 ± 1.17 | **,† | 15 | 3 |
| SH2* | 74.2 ± 8.5 | †† | 0.6 ± 0.05 | †† | 52.7 ± 4.35 | †† | 7.4 ± 1.34 | †† | 13.6 ± 1.81 | †† | 15 | 3 |
| N3*C3* | 98.3 ± 11.83 | **,†† | 1.9 ± 0.33 | **,†† | 85.4 ± 8.04 | **,† | 24.5 ± 5.98 | **,† | 16 ± 1.86 | * | 10 | 3 |
| Δ323 | 62.1 ± 5.11 | †† | 0.7 ± 0.07 | †† | 55.5 ± 5.04 | †† | 6.5 ± 1.29 | †† | 12.4 ± 1.97 | †† | 10 | 3 |
| SH323 | 91.4 ± 12.91 | *,†† | 0.8 ± 0.1 | †† | 70.8 ± 8.75 | *,†† | 11.2 ± 3.4 | *,†† | 14.2 ± 1.65 | † | 10 | 3 |
| Y174F | 165.6 ± 10.14 | ** | 3 ± 0.3 | ** | 98.1 ± 5.07 | ** | 25.6 ± 2.75 | **,†† | 15.7 ± 0.95 | **,† | 15 | 3 |
| Y3F | 158.4 ± 15.11 | ** | 2.9 ± 0.41 | ** | 99.4 ± 8.65 | ** | 22.1 ± 3.34 | **,†† | 13.6 ± 0.98 | †† | 11 | 3 |
| L213A | 101.6 ± 11.99 | **,†† | 0.9 ± 0.1 | *,†† | 66.4 ± 3.64 | *,†† | 12.2 ± 2.3 | **,†† | 15.1 ± 1.83 | †† | 12 | 3 |
| L278Q | 93.9 ± 15.04 | *,†† | 1.4 ± 0.3 | **,†† | 73.3 ± 8.8 | *,†† | 11.6 ± 4.45 | *,†† | 10.5 ± 1.38 | †† | 11 | 3 |
| LK-AA | 170.8 ± 7.34 | ** | 2.9 ± 0.31 | ** | 101.2 ± 5.13 | ** | 32.2 ± 3.26 | **,† | 18.8 ± 1.44 | ** | 15 | 3 |
| ΔCH | 153 ± 10.09 | **,† | 2.1 ± 0.22 | **,†† | 90.2 ± 3.84 | **,† | 23.6 ± 3.49 | **,†† | 20.6 ± 1.4 | ** | 15 | 3 |
| ΔDH-PH | 166.6 ± 13.41 | ** | 2.2 ± 0.33 | **,† | 82.3 ± 4.72 | **,† | 28.9 ± 3.57 | **,† | 17.4 ± 1.66 | ** | 10 | 3 |
Significant differences from the control JV.SC cells transfected with an mYFP expression vector are indicated with asterisks:
for p < 0.05,
for p < 0.005.
Significant differences from JV.SC cells reconstituted with wild-type Vav1.mYFP are indicated with daggers:
for p<0.05,
for p<0.005.
To determine how the C-terminal Src homology domains of Vav1 influence the behavior and function of SLP-76 MC, we transiently expressed mutant Vav1.mYFP chimeras in JV.SC cells (Figure 2A). The SH2 domain of Vav1 binds directly to SLP-76, is indispensible for the function of Vav1 in T cells, and has been implicated in the recruitment of Vav1 into mast cell-receptor nucleated signaling complexes (11, 29). As anticipated, the inactivation of the Vav1 SH2 domain (R696A, SH2*) precluded the recruitment of Vav1 into SLP-76 MC and abolished both TCR-initiated calcium entry and CD69 upregulation (Figures 2B, 2D, and 2E). In addition, the SH2* mutation prevented the enhanced recruitment of SLP-76 into microclusters, the stabilization of SLP-76 MC, and the movement of SLP-76 MC (Figures 2B and 2C; Table 1; Supplementary Movie S5). Thus, the SH2 domain of Vav1 plays a pivotal role in the recruitment of Vav1 into SLP-76 MC, the stabilization of SLP-76 MC, and all Vav1-dependent effector functions evaluated to date,
The SH3 domains of Vav1 each mediate protein-protein interactions that could contribute to the overall avidity of SLP-76 MC (28, 34–36). Nevertheless, Vav1 mutants lacking the atypical SH3 domain-binding surface of the N-terminal SH3 domain (P657A, N-SH3*) or the classical ligand-binding pocket of the C-terminal SH3 domain (WW820/821YY, C-SH3*) were recruited into SLP-76 MC (Figure 2B). Both SH3 domain mutants significantly increase the stoichiometry of SLP-76 recruitment, the persistence of SLP-76 MC, and the movement of SLP-76 MC relative to JV.SC cells expressing mYFP alone (Figure 2C; Table 1; Supplementary Movies S6 and S7). By all of these measures, the N-SH3* mutant performs slightly less well than the C-SH3* mutant and wild-type constructs, but differs significantly from wild-type Vav1 only in its ability to support the recruitment of SLP-76 into microclusters (Figure 2B; Table 1) and the movement of SLP-76 MC (Figure 2C; Table 1). In contrast to these relatively mild defects in microcluster behavior, the Vav1 SH3 domain mutants display moderate, but significant defects in CD69 upregulation and substantial defects in calcium entry (Figures 2D and 2E; see Supplemetary Figure 1 for representative calcium traces). Thus the individual SH3 domains of Vav1, in contrast to the SH2 domain, play limited roles in cluster entry, cluster stabilization, and CD69 upregulation, but are essential for optimal TCR-induced calcium responses.
Multivalent interactions often contribute to overall avidity of signaling complexes (20, 27, 37). Hypothesizing that the Vav1 SH3 domains possess redundant functions, we examined the behavior of a double SH3 domain mutant (N3*C3*). This mutant significantly exacerbates the subtle defects in microcluster behavior observed with the N-SH3* mutant (Figures 2B and 2C; Table 1; Supplementary Movie S8). However, the N3*C3* mutant is not a null; this mutant enters SLP-76 MC, and the properties of the resulting SLP-76 MC differ dramatically from those of SLP-76 MC formed in the absence of Vav1. In particular, the initial rate of microcluster movement is largely unaffected by the N3*C3* mutant (Figures 2B and 2C). This dissociation of SLP-76 MC movement and persistence is unique, and indicates that domains other than the SH3 domains of Vav1 contribute to microcluster movement. The defect in net movement reflects the frequent failure of N-SH3* and N3*C3* SLP-76 MC reach the center of the contact (Figure 2B, MOT images, contrast WT/C-SH3* with N-SH3*/N3*C3*). The point at which SH3-mutant SLP-76 MC slow (Figure 2C, note divergence of red and green lines) is closely related to the mean persistence of these microclusters (Table 1), suggesting that the instability of the mutant SLP-76 MC results in the premature dissociation of movement-promoting factors. Despite the intermediate impact of the Vav1 N3*C3* mutant on the behavior of SLP-76 MC, this mutation eliminates the ability of Vav1 to upregulate CD69 or to augment calcium responses (Figures 2D and 2E; Supplemetary Figure 1). Thus, the SH3 domains of Vav1 play redundant and important roles in the stability and signaling functions of SLP-76 microclusters, but are largely dispensable for microcluster movement.
The N-terminus of Vav1 Contributes to the Stabilization of SLP-76 MC
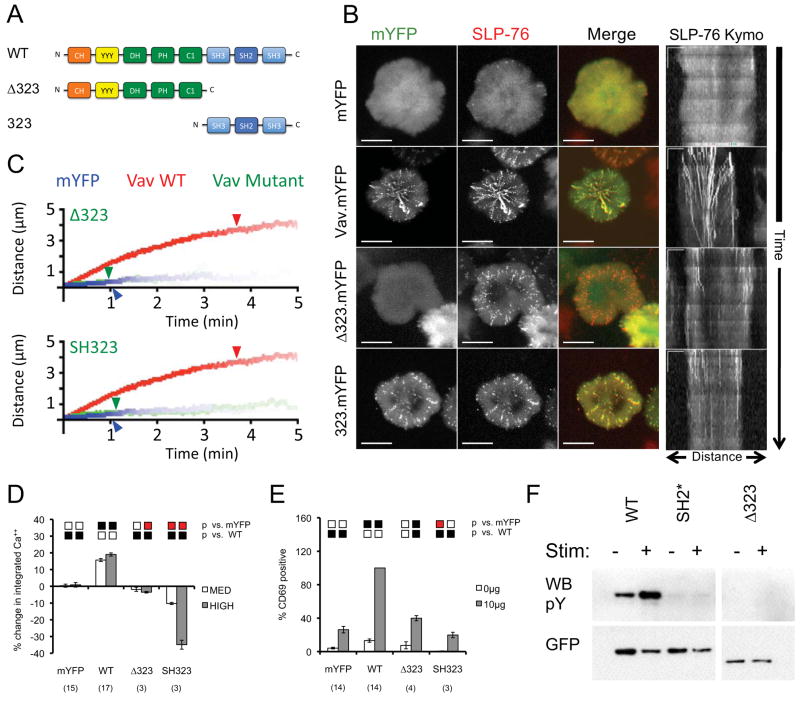
To determine whether previously uncharacterized scaffolding interactions mediated by the C-terminus of Vav1 enhance the persistence and movement of SLP-76 MC, we created deletion mutants that separate the C-terminal Src homology domains from the regulatory and catalytic N-terminus of Vav1 (SH323 and Δ323, respectively; Figure 3A). In JV.SC cells, the Vav1 Δ323 mutant failed to enter SLP-76 MC (Figure 3B, red SLP-76 MC in MOT overlay). The SLP-76 MC that do form in these cells are short-lived and immobile (Figure 3B, lack of radial spokes in MOT, short vertical traces in kymograph; Figure 3C; Table 1, Supplementary Movie S9). Furthermore, the fraction of SLP-76 recruited into these SLP-76 MC remains very low (Table 1). In contrast, the Vav1 SH323 mutant enters SLP-76 MC (Figure 3B). Although this mutant significantly increases the persistence and stoichiometry of SLP-76 MC, the magnitude of each of these effects is quite small, and the behavior of SH323 mutant SLP-76 MC is grossly similar to that of Vav1-null and Δ323 mutant SLP-76 MC (Figures 3B and 3C; Table 1; Supplementary Movie S10). These data show that the C-terminal Src homology domains are both necessary and sufficient for the recruitment of Vav1 to SLP-76 MC, but also show that these domains are insufficient to stabilize or to promote the movement of SLP-76 MC. We conclude that these functions must be provided by the N-terminus of Vav, even though the isolated N-terminus of Vav1 has no impact on microcluster properties.
Figure 3. The C-terminal homology domains of Vav1 are necessary and sufficient for microcluster entry, but are insufficient for microcluster movement and Vav1-dependent signaling.
(A) The domain structure of Vav mutants used in this figure. (B) JV.SC cells transiently transfected with the indicated Vav1.mYFP constructs were stimulated and imaged as in Figure 2B. Representative maximum-over-time projections (left) and kymographs (right) were selected from 3 independent experiments. Scale bars as in Figure 2B. (C) Composite kymographs depicting SLP-76 MC movement and persistence were prepared as in Figure 2C. Arrowheads correspond to the half-life of SLP-76 MC for each condition. See Table 1 for further analysis. (D) TCR-induced CD69 expression was determined in J.Vav1 cells reconstituted with the indicated Vav1.mYFP chimeras. Presentation and statistics are as in Figure 2E. (E) TCR-induced intracellular calcium responses were measured in J.Vav1 cells reconstituted with the indicated Vav1.mYFP chimeras. Presentation and statistics are as in Figure 2D. (F) The indicated Vav1.mYFP chimeras were expressed in Jurkat E6.1 cells and immunoprecipitated from unstimulated and TCR-stimulated lysates using GFP-specific antibodies. Membranes were sequentially western blotted for phosphotyrosine and GFP. Images are representative of 3 independent experiments.
The C-terminus of Vav1 Controls the Recruitment, Phosphorylation, and Function of Vav1
The N-terminus of Vav1 possesses two distinct effector functions. First, the DH, PH, and C1 domains comprise a Rho-family GEF that is required for the transforming activity of onco-Vav and the activation of JNK, which contributes to the activation of AP-1 and the upregulation CD69 (9, 33, 38). Second, the CH domain plays a lymphocyte-specific and GEF-independent role in antigen receptor-initiated calcium entry (8, 33, 39). The SH323 fragment of Vav1, which lacks both of these effector modules, does not increase TCR-dependent CD69 upregulation and potently suppresses TCR-dependent calcium responses in a dose-dependent manner (Figures 3D and 3E; Supplementary Figure 1). In contrast, the Δ323 fragment of Vav1, which contains both effector modules, has limited impacts on the upregulation of CD69 and on cytoplasmic calcium (Figures 3D and 3E; Supplementary Figure 1). These data indicate that the recruitment of the effector domains of Vav1 into SLP-76 MC is a prerequisite for their function. Since the N-terminus of Vav1 adopts an autoinhibitory fold that opens in response to tyrosine phosphorylation (30, 31, 40), the Δ323 fragment could remain inert if not appropriately tyrosine phosphorylated in response to TCR ligation. In fact, the deletion of the C-terminal Src homology domains or the mutation of the SH2 domain abolishes the basal and TCR-induced tyrosine phosphorylation of Vav1 (Figure 3F). These data confirm that the defects in microcluster stability and downstream signaling observed in the SH2* and Δ323 mutants could result from secondary defects in the accessibility of the N-terminal domains of Vav1 or the recruitment of Vav1 phosphotyrosine-binding proteins (11, 41).
Regulatory Phosphorylation Sites Are Dispensable for Vav1 Scaffolding and Effector Functions
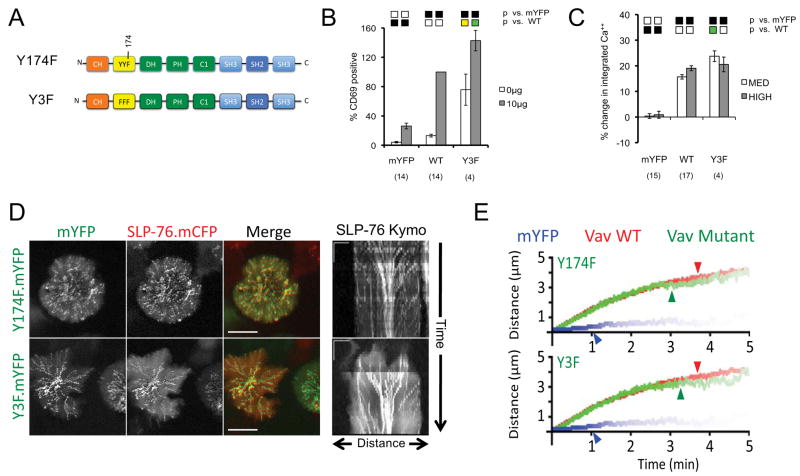
In response to TCR ligation, the N-terminal acidic region tyrosines of Vav1 (Y142, Y160, and Y174) are processively phosphorylated, destabilizing the auto-inhibited conformation of the Vav1 N-terminus and increasing the activity of the Vav1 GEF (9, 30, 31, 40, 42). Since the Vav1 GEF contributes to the activation of JNK and AP-1, which promote the upregulation of CD69, we used CD69 expression as a surrogate marker of GEF activity (9, 33, 38). As anticipated, the Vav1 triple tyrosine mutant (Y3F) mutation increases CD69 expression on resting J.Vav1 cells and augments TCR-dependent increases in CD69 expression (Figure 4A and 4B). As previously reported, the Vav1 Y3F mutant also supports supra-physiological calcium responses when expressed at moderate levels in J.Vav1 cells (Figure 4C; Supplementary Figure 1) (39). These data are consistent with a model in which the Y3F mutation perturbs the mutual sequestration of the calcium entry-promoting CH domain and the GTPase-activating DH-PH-C1 module (30). Although tyrosines 142, 160, and 174 have been proposed to act as SH2 domain docking sites (41), the Vav1 Y3F mutant was effectively recruited into SLP-76 MC and significantly increased the fraction of SLP-76 found in microclusters. Furthermore, the Y3F mutant enabled wild-type microcluster persistence and centralization (Figures 4D and 4E; Table 1; Supplementary Movie S11). Nearly identical microcluster behaviors were observed with the Y174F mutant of Vav1, which has also been shown to perturb the regulatory fold within Vav1 (Figures 4D and 4E; Table 1). In neither case did the resulting deregulation of the Vav1 GEF alter the ability of Vav1 to support normal microcluster behavior. Taken together, these data indicate that these three tyrosine residues function primarily in a regulatory capacity, and are largely dispensable for the adaptor functions of Vav1.
Figure 4. Constitutively active Vav1 tyrosine mutants support normal microcluster behavior and augment Vav1-dependent signals.
(A) The domain structure of Vav mutants used in this figure. (B–C) J.Vav cells were transiently transfected with the indicated constructs. CD69 upregulation (B) and calcium responses (C) are presented as in Figure 2. (D) JV.SC cells transiently transfected with the indicated Vav1.mYFP constructs were stimulated and imaged as in Figure 2B. Representative maximum-over-time projections (left) and kymographs (right) were selected from 3 independent experiments. Scale bars as in Figure 2B. (E) Composite kymographs depicting SLP-76 MC movement and persistence were prepared as in Figure 2C. Arrowheads correspond to the half-life of SLP-76 MC for each condition. See Table 1 for further analysis.
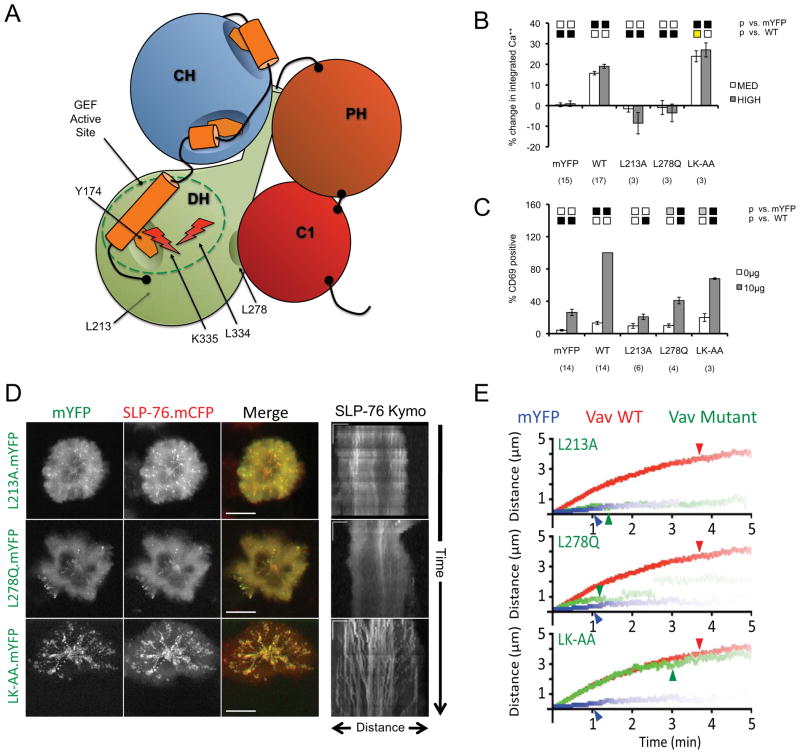
Divergent Consequences of the Disruption and the Catalytic Inactivation of the Vav1 GEF
Although recent studies in Vav1-null genetic backgrounds have conclusively identified GEF-independent functions for Vav1 in T cell activation, these studies yielded divergent results with respect to T cell development and proliferation (6, 7). Due to the use of distinct developmental models and GEF mutations (L278Q versus L334A/K335A), the role of the catalytic activity of Vav1 GEF in primary T cells remains controversial. To clarify this issue, we examined the impacts of three distinct GEF-dead alleles on T cell function and on the properties of SLP-76 microclusters (Figure 5A; see Supplementary Figure 2 for structural details). The L213A mutation, which impacts the hydrophobic core of the DH domain beneath the active site, precludes Vav1-dependent increases in TCR-induced CD69 expression and cytoplasmic calcium (Figures 5B and 5C). Similarly, the L278Q mutation, which affects the hydrophobic interface linking the DH and C1 domains, is a weak inducer of CD69 and fails to support TCR-dependent increases in cytoplasmic calcium. In contrast, the L334A/K335A mutation, which perturbs surface-exposed residues contributing to Rac1 binding and nucleotide exchange, displays an intermediate CD69 phenotype and supports normal to enhanced calcium responses, depending on the level of Vav1 expression. The intermediate CD69 response associated with the L334A/K335A mutant is quite striking, as CD69 responses normally reflect the activity of the Vav1 GEF (4, 33, 38). We suggest that the calcium responses observed are indicative of second messenger generation via PLCγ1, and that the residual CD69 upregulation is driven by an alternate mechanism involving diacylglycerol-dependent ERK and AP-1 activation. However, this explanation cannot account for the residual capacity of the calcium-dead L278Q mutant to upregulate CD69.
Figure 5. The selective inactivation of the Vav1 GEF active site does not alter microcluster persistence or movement.
(A) The domain structure of Vav mutants used in this figure. (B–C) J.Vav cells were transiently transfected with the indicated constructs. CD69 upregulation (B) and calcium responses (C) were measured as in Figure 2. (D) JV.SC cells transiently transfected with the indicated Vav1.mYFP constructs were stimulated and imaged as in Figure 2B. Representative maximum-over-time projections (left) and kymographs (right) were selected from 3 independent experiments. Scale bars as in Figure 2B. (E) Composite kymographs depicting SLP-76 MC movement and persistence were prepared as in Figure 2C. Arrowheads correspond to the half-life of SLP-76 MC for each condition. See Table 1 for further analysis.
The differences in the abilities of the domain-disrupting mutants and the catalytically inactive L334A/K335A mutant to support TCR-induced calcium responses appear to reflect the abilities of these mutants to support normal microcluster function (Figures 5D and 5E; Table 1). In this regard, the L213A (Table 1; Supplementary Movie S12) mutant resembles the functionally dead SH323 fragment, in that it drives limited increases in microcluster persistence and stoichiomtery and does not support microcluster movement. The L278Q mutant (Table 1; Supplementary Movie S13) is very similar to the L213A mutant, but produces slightly more mobile microclusters. This difference in microcluster movement is largely dependent on a small fraction of microclusters that persist and move normally (Figure 5E, green line, note the pale extension between 3 and 5 minutes). We postulate that the residual CD69 upregulation mediated by the L278Q mutant is associated with these rare, but persistent, microclusters. In contrast, the L334A/K335A mutant supports the formation of SLP-76 microclusters that are nearly indistinguishable from those containing wild-type Vav1; the only significant difference noted was a minor, but significant, reduction in the stoichiometry of SLP-76 recruitment into microclusters (Table 1; Supplementary Movie S14). Our data indicate that the mutants in which the folding and/or packing of the domains of catalytic core is perturbed are functionally dead because they disrupt the microcluster stabilizing and movement-promoting adaptor functions mediated by the Vav1 N-terminus, whereas the catalytically dead L334A/K335A mutant leaves these adaptor functions intact.
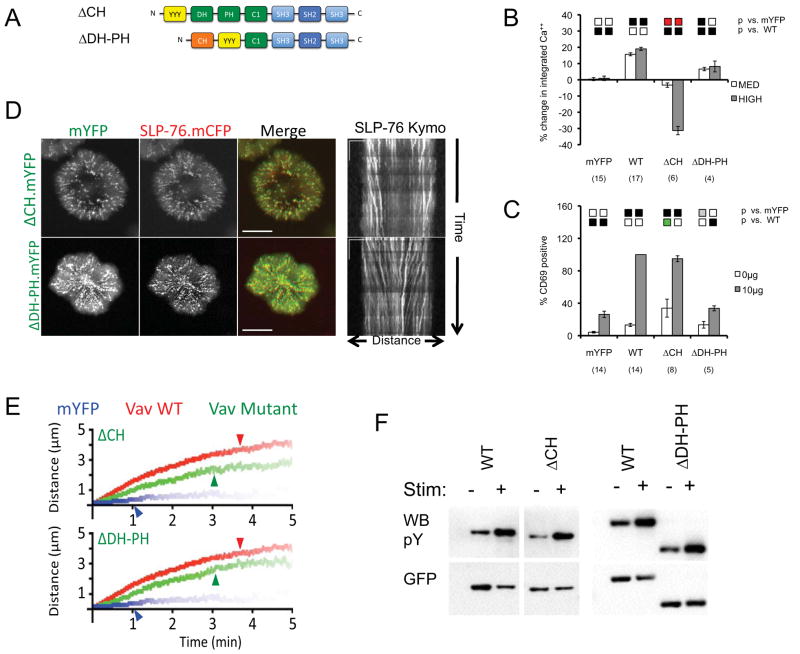
Mapping the Adaptor Functions Mediated by the N-Terminus of Vav1
To clarify how the N-terminus of Vav1 facilitates microcluster stabilization and movement we independently deleted the CH domain (ΔCH) and the catalytic DH-PH (ΔDH-PH) module (Figure 6A). We expected both deletions to perturb the GEF-inhibitory fold and to ensure the availability of the remaining domains in the N-terminus of Vav1. In agreement with published results, the deletion of the CH domain prevents Vav1-mediated increases in TCR-induced calcium entry (Figure 6B) (33). Furthermore, this mutant profoundly inhibits calcium responses when highly expressed. As with the Y3F mutant, the ΔCH mutant upregulates CD69 in resting JV.SC cells, most likely reflecting the constitutive activation of the GEF module (Figure 6C) (9, 30). However, in contrast to the Y3F mutant, the ΔCH mutant does not support TCR-dependent increases in CD69 expression greater than those observed with wild-type Vav1 (compare Figures 4B and 6C). This impairment is likely to reflect the loss of a CH domain-dependent pathway contributing to CD69 upregulation via PLCγ1, ERK, and AP-1. Despite the absolute defect in Vav1-mediated calcium entry, the ΔCH mutant exerts intermediate, but significant, positive effects on almost all aspects of microcluster behavior (Figures 6D, 6E; Table 1; Supplementary Movie S15). Despite the fact that SLP-76 MC containing the ΔCH mutant form normally, in the cell periphery, and begin to migrate toward the center of the contact (Figure 6D, radial streaks in MOT images, diagonal lines in kymographs), these SLP-76 MC never accumulate in the center of the contact. These changes are correlated with significant reductions in the persistence, movement, and stoichiomtery of SLP-76 MC relative to wild-type Vav1 (Figure 6E; Table 1). Thus, in addition to its well-established role in mediating calcium flux, the Vav1 CH domain contributes to the stabilization and movement of SLP-76 MC.
Figure 6. Multiple domains within the N-terminus of Vav1 contribute to microcluster localization, persistence, movement, and function.
(A) The domain structure of Vav mutants used in this figure. (B–C) J.Vav cells were transiently transfected with the indicated constructs. Calcium responses (B) and CD69 upregulation (C) were measured as in Figure 2. (D) JV.SC cells transiently transfected with the indicated Vav1.mYFP constructs were stimulated and imaged as in Figure 2B. Representative maximum-over-time projections (left) and kymographs (right) were selected from 3 independent experiments. Scale bars as in Figure 2B. (E) Composite kymographs depicting SLP-76 MC movement and persistence were prepared as in Figure 2C. Arrowheads correspond to the half-life of SLP-76 MC for each condition. See Table 1 for further analysis. (F) TCR-induced Vav1 phosphorylation was determined as in Figure 3F. Images are representative of 3 independent experiments.
The consequences of the DH-PH deletion are also intermediate, falling between the effects observed with the GEF-disrupting L213A and L278Q mutants and the GEF-inactivating L334A/K335A mutant. Our ΔDH-PH mutant augmented the calcium responses observed in J.Vav1 cells, confirming that Vav1 controls TCR-mediated calcium responses via its non-catalytic functions (Figure 6B). However, the ΔDH-PH mutant was significantly less effective than either wild-type Vav1 or the L334A/K335A mutant, suggesting that either the overall topology of the N-terminus or non-catalytic surfaces present within the DH-PH module contribute to Vav1-dependent calcium responses. In addition, the ΔDH-PH mutant abolished the Vav1-dependent upregulation of CD69 following TCR ligation (Figure 6C), as would be expected given the elimination of the GEF cassette and the reduced efficacy of the ΔDH-PH mutant in promoting calcium responses. Despite the serious functional defects associated with the deletion of the DH-PH cassette, the ΔDH-PH mutant exerts significant positive effects on the behavior of SLP-76 MC (Figures 6D, 6E; Table 1; Supplementary Movie S16), indicating that the non-catalytic scaffolding functions of the L334A/K335A mutant are largely retained in the ΔDH-PH mutant. Nevertheless, the microclusters observed in the presence of the ΔDH-PH mutant are somewhat less mobile and contain significantly less SLP-76 than the microclusters observed in the presence of wild-type Vav1.
Notably, neither the ΔCH nor the ΔDH-PH deletion mutant reduce the phosphorylation of Vav1 in response to TCR ligation (Figure 6F), indicating that secondary defects in the recruitment of effectors or scaffolds to these tyrosine residues cannot explain the impacts of these mutations on T cell function or on microcluster stability and movement.
Discussion
Although Vav1 has been reported to interact with ZAP-70, we find that Vav1 is selectively recruited into SLP-76 microclusters via its SH2 domain, and is excluded from ZAP-70 microclusters, suggesting that Vav1 plays a unique role within SLP-76 microclusters (32). Consistent with this hypothesis, Vav1 mutants that fail to enter SLP-76 MC are not phosphorylated (SH2*, Δ323), and the elimination the Vav1-binding tyrosines within SLP-76 suffices to prevent Vav1 phosphorylation (19). Since the phosphorylation of Vav1 reverses the inhibitory fold within the N-terminus of Vav1, the Vav1 GEF and N-terminal scaffolding functions may be selectively activated within SLP-76 microclusters (30, 40, 43).
We observe a significant role for the Vav1 GEF in the upregulation of CD69, and are in agreement with prior studies indicating that this upregulation is controlled, at least in part, by Rac1, c-jun N-terminal kinase (JNK), and AP-1 (9, 10, 33, 38). In this regard, our studies are compatible with those of Saveliev et al., who observed defects in multiple JNK and AP-1 dependent events in their L334A/K335A knock-in mice (6, 44–46). However, we find no evidence that the catalytic activity of the Vav1 GEF influences the persistence or movement of SLP-76 microclusters in a significant manner. This observation is applicable to the L334A/K335A mutant, which inactivates the Vav1 GEF, and to the Y174F and Y3F mutants, which both constitutively activate the Vav1 GEF. With respect to the impact of the Vav1 GEF on SLP-76 microclusters, we were unable to replicate a previously reported defect in microcluster persistence in J.Vav1 cells stably reconstituted with the constitutively active Y174F mutant (41). The primary difference that we identified in the two studies involves our use of transient transfections. We feel that our approach is more robust, as it obviates any line-specific changes arising as artifacts of selection, and incorporates within each experiment both positive and negative controls derived from an identical pool of JV.SC cells. Nevertheless, it remains conceivable that the Vav1 GEF could positively influence microcluster stability in primary cells, which may display a greater dependence on Vav1-dependent phosphotidylinositol-3,4,5-trisphosphate (PIP3) production (see Figures 7A and 7B) (3).
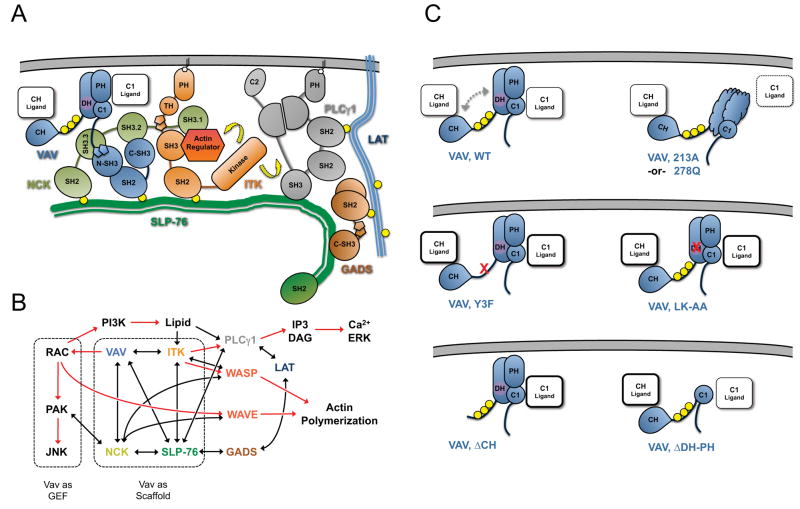
Figure 7. Model of Vav1-mediated scaffolding interactions within SLP-76 microclusters.
(A) Interactions within the signaling complex nucleated on the N-terminal tyrosines of SLP-76. Vav1, Nck, and Itk are recruited to the phosphorylated tyrosines (yellow circles) of SLP-76 where they participate in multivalent scaffolding interactions. The interactions conformationally activate and optimally position Itk, enabling it to activate (yellow arrows) known substrates, such as PLC γ1 and the actin regulator WASP. (B) Involvement of Vav1 in signaling pathways leading to T cell activation. Known biochemical interactions are represented by black lines and postulated interactions by dotted lines. The activation of downstream effectors is indicated by red arrows. Vav1 GEF-dependent and GEF-independent pathways are highlighted with dashed boxes. (C) The predicted conformation of and scaffolding interactions mediated by the N-terminus of Vav1 under diverse conditions, based on the crystal structure of the auto-inhibited N-terminus and the functional and imaging data presented here. In wild-type Vav1, phosphorylation (yellow circles) relieves the autoinhibitory interaction of the CH with the GEF cassette (dashed arrows), permitting ligand binding by the CH, PH and C1 domains. The L213A and L278Q mutations are predicted to disrupt domain folding and to occlude a novel binding surface on the C1 domain. The remaining mutations are expected to perturb the auto-inhibitory fold and may increase binding of ligands to the remaining domains.
The catalytically inactive L334A/K335A mutant supports normal TCR-dependent calcium responses, indicating that the adapter functions mediated by Vav1 influence microcluster function as well as microcluster structure. These adapter functions require several distinct modules within Vav1. The first module, consisting of the C-terminal Src homology domains, is necessary and sufficient for the recruitment of Vav1 into SLP-76 microclusters, and is essential for the stabilization of SLP-76 microclusters and for Vav1-mediated increases in calcium entry. Within this module, the SH2 domain plays the dominant role in Vav1 recruitment and phosphorylation, but is not sufficient for microcluster stabilization or for optimal calcium entry. Although the SH3 domains of Vav1 are both required for normal calcium responses, the contributions of these domains are much more evident in the double SH3 domain mutant, which is significantly impaired with respect to microcluster stabilization and CD69 expression. We propose that the SH2 domain enables i) the initial recruitment of Vav1 to SLP-76, and ii) the phosphorylation and activation of Vav1 by microcluster-proximal kinases, whereas the SH3 domains contribute to iii) the trapping of Vav1 within a nascent SLP-76 microcluster via a network of protein-protein interactions that contributes to the overall avidity of the SLP-76 microcluster (Figures 7A and 7B). Consistent with this hypothesis, Vav1 binds to both of its immediate neighbors within the SLP-76 microcluster: Nck and Vav1 interact via the atypical SH3 domain of Vav1, whereas Itk and Vav1 interact via a proline-rich motif within Itk (28, 47).
The scaffolding interactions mediated by the C-terminal SH323 fragment of Vav1 enhance microcluster persistence on a Vav1-null background, but are not sufficient to support normal microcluster persistence. Although the N-terminus of Vav1 cannot be recruited or phosphorylated in the absence of the C-terminal SH323 module, the adaptor functions of this fragment control: i) the stabilization of SLP-76 microclusters, ii) the movement of SLP-76 microclusters, and iii) Vav1-mediated increases in intracellular calcium. We predict that these poorly understood scaffolding functions are modulated by the inhibitory fold that regulates the Vav1 GEF and only become available following the phosphorylation of Vav1 within SLP-76 microclusters. Based on this and prior work, we postulate that the N-terminus of Vav1 assists in coupling SLP-76 to the underlying actin cytoskeleton, which directs microcluster movement (22). Although the precise surfaces and binding partners involved in these scaffolding functions remain unknown, their identification will contribute significantly to our understanding of T cell development and activation.
Our data confirm that the primary function of the N-terminal tyrosine phosphorylation sites in Vav1 acidic region is regulatory (30). Although these tyrosines are capable of binding the SH2 domains of various effector proteins implicated in T cell activation in vitro (41), we were unable to identify any significant requirement for these tyrosines in microcluster stabilization, microcluster centralization, or calcium release. In fact, our Y3F mutant significantly increases the peak calcium levels observed following to TCR ligation, consistent with prior studies (39).
This effect may involve the generation of an ‘open’ conformation that enables constitutive access to the CH domain, which has a well-established role in Vav1-dependent calcium entry (Figure 7C). Structural analyses suggest that conformational ‘opening’ may also account for the tendency of the L334A/K335A mutant to support supra-normal calcium responses when expressed at moderate levels, as the K335A substitution perturbs the binding pocket that accommodates the inhibitory tyrosine 174 (Figure 7C; Supplementary Figure 2).
Recent studies have arrived at divergent conclusions regarding the role of the Vav1 GEF in T cell development and activation (6, 7). These results have not yet been reconciled, as distinct GEF mutations were used in each study, and the levels of Vav1 expression may have differed in each case. In our model system, we have compared the mutations used in these studies while carefully controlling for the level of Vav1 expression in both flow cytometric functional assays and during live cell imaging. In our hands, the catalytically inactive L213A mutation and the L278Q mutation both exhibit significantly reduced CD69 responses and resemble the SH323 fragment in their inability to support wild-type microcluster persistence, microcluster movement, or calcium responses. We hypothesize that these mutations, which impact surfaces buried within the catalytic DH-PH-C1 module (Supplementary Figure 2), disrupt this module and thereby eliminate scaffolding functions responsible for microcluster stabilization, microcluster movement, and calcium release (Figure 7C). In contrast, the L334A/K335A mutant supports wild-type microcluster persistence, microcluster movement, and calcium responses, while producing a partial defect in CD69 expression. This discrepancy strongly suggests the existence a novel binding surface that accounts for the scaffolding functions mediated by the catalytically dead DH-PH-C1 module (Figure 7C). Paradoxically, the excision of the DH-PH cassette generates a protein that is incapable of upregulating CD69 and is relatively ineffective at generating calcium responses, but nonetheless supports nearly wild-type levels of microcluster persistence and microcluster movement. The low to intermediate calcium responses observed with this deletion mutant may reflect the loss of the inositide-binding function of the PH domain (Figure 7C) (39, 48). However, in order to explain how the scaffolding functions of this deletion mutant could be less severely affected than the L213A and L278Q point mutants, we postulate that the destabilized domains resulting from these mutations occlude a critical, but uncharacterized, binding surface incorporating the adjacent C1 domain (Figure 7C). To the best of our knowledge, this is the first time that an exhaustive comparison of distinct GEF-inactivating mutations has been carried out in a model system that permits the quantitative evaluation of the underlying biochemical structures required for optimal GEF function.
Structural and functional studies have demonstrated that the deletion of the CH domain increases the availability of the Vav1 GEF. However, the levels of CD69 expression supported by the CH mutant do not match those supported by the Y3F mutant. We postulate that this discrepancy arises because the CH domain contributes to CD69 upregulation via the same PLCγ1-dependent pathways that drive TCR-induced calcium responses, as noted above. To date, the mechanism by which the CH domain contributes to TCR-induced calcium responses remains unresolved, barring one study implicating an interaction between the Vav1 CH domain and calmodulin in the regulation of calcium release from internal stores (49). Our data indicate that the CH domain may also influence calcium responses by modulating the overall stability of SLP-76 microclusters, but have not yet shed light on the nature of the binding partners involved.
Our data are consistent with a model in which the primary non-catalytic function of Vav1 is to stabilize SLP-76 microclusters through adapter functions mediated by its conventional C-terminal Src homology domains and by novel interaction surfaces within its N-terminus (Figure 7). This stabilization is likely to increase the efficacy of signaling by these structures. This hypothesis is consistent with steadily accumulating evidence indicating that microcluster components, including LAT, Gads, SLP-76, SOS, PLCγ1, Itk, Vav1, and c-Cbl, participate in multivalent interactions within these structures (3, 4, 20, 27, 29, 37, 47, 50–53). The use of multivalent low-affinity interactions in the assembly of a signaling complex may be advantageous because it permits the assembly of a specific complex from modular components, and facilitates the rapid disassembly of these complexes upon the termination of the relevant initiating signal. In the context of a SLP-76 microcluster, the Vav1-dependent network of multivalent interactions may promote the phosphorylation of PLCγ1 by Itk, the hydrolysis of phopatidylinositol-4,5-bisphosphate by PLCγ1, and the action of Vav1 and SOS upon their GTPase substrates. Furthermore, multi-point interactions within the SLP-76 microcluster may activate signaling molecules by altering their quaternary structures, as has been proposed for Itk (54, 55). In addition, the impact of Vav1 on the overall avidity of the microcluster is likely to increase the dwell time of individual proteins within the dynamically evolving microcluster. We anticipate that Vav1-dependent calcium responses are more susceptible to perturbation by individual SH3 domain mutations because a more elaborate complex involving Nck, Itk, and PLCγ1 is involved, and requires a greater dwell time than the signals that drive CD69 upregulation.
In conclusion, our data indicate that Vav1 plays an integral role in the assembly of SLP-76 microclusters and in their transmission of signals initiated by the TCR. Furthermore, our data indicate that novel scaffolding domains within the N-terminus of Vav1 contribute to the multivalent interactions that increase the overall avidity of SLP-76 microclusters, and enable them to act as efficient platforms for downstream signaling.
Materials and Methods
Plasmid Constructs
Wild-type Vav1 and the point mutations inactivating the atypical N-terminal SH3 (P657A), C-terminal SH3 (WW820/821YY), and SH2 domain (R696A) were obtained from Dan Billadeau. The GEF-inactivating L213A mutant was obtained from M. Leinhard Schmitz. All Vav1 chimeras were tagged at the C-terminus by subcloning into a variant form of pEYFP-n1 (Clontech) containing the monomerizing A206K mutation and a modified EF1α promoter. The Vav1-specific short hairpin RNA targets the sequence GAAGGACTGTACCGGATCA, which was selected using the siDESIGN Center (Dharmacon). The hairpin vector and all additional Vav1 mutants were generated as described in the Supplementary Materials and Methods. SLP-76 deficient Jurkat T cells reconstituted with EGFP-tagged wild-type or Y3F mutant SLP-76 have been described previously (27). Vectors encoding mRFP1 and mStrawberry fluorescent proteins were provided by Roger Tsien (56); red fluorescent proteins were amplified by PCR and subcloned into the parental Vav1.mYFP expression vector using AgeI and NotI.
Cell lines and transfections
Jurkat T cells were maintained and transfected as previously described [1; 2; 10]. The SLP-76 deficient Jurkat T cell line reconstituted with YFP-tagged SLP-76 (J14.SY) has been described previously (27). Vav1-deficient Jurkat T cells (J.Vav1) were the gift of Robert T. Abraham (33). Transfections were performed as described (27). Vav1-deficient cells expressing SLP-76.mCFP (JV.SC cells) were generated by transfecting J.Vav1 cells with a SLP-76.mCFP expression vector, selecting in hygromycin, and sorting for TCR and SLP-76.mCFP expression.
Antibodies and Western Blotting
Jurkat T cells were stimulated with the anti CD3ε-specific antibody OKT3 (Bio-Express). Western blotting was performed using antibodies for GFP (JL-8, Clontech), Vav1 (Upstate), or phosphotyrosine (4G.10, Millipore). For immunoprecipitations, Jurkat E6.1 cells were transfected with Vav1.mYFP chimeras. Two days post transfection, bulk populations were stimulated with 1:500 soluble C305 for 5 minutes at 37°C. Stimulation was stopped by a quick rinse in ice-cold PBS containing 10 mM NaF and 1 mM Na3VO4. Cells were then lysed in buffer containing 20 mM Tris-HCl pH 8.0, 100 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10 mM NaF, 1 mM Na3VO4, and Complete Protease Inhibitor Cocktail (Roche). Vav constructs were immunoprecipitated for 16 hours at 4°C using anti-GFP antibody (ab290, Abcam) bound to Protein A beads (Pierce).
Imaging, Image Processing, and Statistical Analysis
Cells were stimulated in glass-bottom 96-well plates coated with 0.01% poly-L lysine and 3ug/ml OKT3, then blocked with 1% BSA in PBS. All imaging runs continued for at least 5 minutes and were performed on a spinning disk confocal microscope as previously described (22). Image analysis was performed using iVision software (BioVision Technologies). For quantitative microcluster analysis, two kymographs were generated per cell. The fraction of SLP-76 in microclusters was calculated from these kymographs by subtracting the average background found outside the cell, determining the average background intensity remaining within the cell, and then determining the fraction of the image area that exceeded four times this average background. Using these kymographs, paths were manually generated for all MC formed within the first two minutes of the imaging assay. The following parameters were calculated for each path: persistence, movement (total displacement over time), and maximum speed (sustained over a 24 second window). Microcluster paths were then aligned and average microcluster paths were derived for each cell. These cell-specific paths were compiled to derive the composite kymographs paths shown for each condition. Microcluster half-life for each condition was determined by identifying the point at which the intensity of these weighted average paths declines by 50 percent. Standard error and statistical significance was calculated using Microsoft Excel, using the student’s t-test for unpaired samples.
Functional Assays
Both CD69 upregulation and calcium flux analyses were performed by FACS. In all cases, cells were gated on populations expressing equal amounts of Vav1.mYFP chimeras. For CD69 assays, 2×105 Jurkat T cells were stimulated with 10 ng/ml OKT3 or 50 ng/ml PMA for 16 hours at 37°C. Cells were stained with anti-CD69 antibodies conjugated with PE-Cy5 (BD Pharmingen) for 30 minutes on ice. CD69 upregulation is expressed as the percentage cells expressing CD69 relative to a positive control consisting of J.Vav1 cells reconstituted with wild-type Vav1.mYFP. For calcium assays, 1×106 cells were stained with 10 μM Indo-1 for 1 hour at 37°C in HBSS supplemented with 1.25 mM CaCl2, 0.9 mM MgCl2 and 0.05% BSA. Basal calcium was read for 2 minutes at which point OKT3 was added to a final concentration of 30 ng/ml and calcium flux was measured over 10 minutes. After this time, ionomycin was added to a final concentration of 10 μM and peak calcium was read for 3 minutes. Null, moderate, and high subpopulations were gated based on their mYFP expression. Calcium responses were calculated as the ratio of the bound and unbound indo-1 signals (420nm/ 460nm) and normalized by the ionomycin-induced peak. Comparisons between subpopulations and across samples were made determining the percentage change in the area under the curve relative to the internal null population. Calcium data was collected on a BD LSRII FACS machine and analyzed using FlowJo (Version 8.5.3) and Microsoft Excel software. NF-AT activation was carried out as described previously (22).
Supplementary Material
Effects of Vav Mutants on TCR-Induced Calcium Entry
Mutations Impacting the Catalytic Core of the Vav1 GEF
Dynamic Visualization of SLP-76 and Vav1 Microclusters
Effect of Vav Knockdown on SLP-76 Microcluster Dynamics
Summary.
The amino-terminus of Vav1 regulates the persistence and function of SLP-76 microclusters through GEF-independent scaffolding interactions.
Acknowledgments
We thank M.C. Seminario for helpful discussions and critical reading of this manuscript.
Funding: This work was supported by the American Heart Association (Scientist Development Grant 0635546T), the Dana Foundation (Brain and Immuno-Imaging Award), the National Institutes of Health (R01 AI076575-01A1), the W.M. Keck Foundation, and the Eshe Fund.
Footnotes
Author Contributions: N.R.S and K.N. performed experiments and developed functional assays; N.R.S performed all imaging studies; N.R.S and S.C.B. performed computational work, developed analytical scripts, and wrote the paper.
Competing Interests: None.
References
- 1.Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Costello PS, Walters AE, Mee PJ, Turner M, Reynolds LF, Prisco A, Sarner N, Zamoyska R, Tybulewicz VL. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kappaB pathways. Proc Natl Acad Sci U S A. 1999;96:3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J Exp Med. 2002;195:1103–1114. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds LF, de Bettignies C, Norton T, Beeser A, Chernoff J, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J Biol Chem. 2004;279:18239–18246. doi: 10.1074/jbc.M400257200. [DOI] [PubMed] [Google Scholar]
- 5.Fischer KD, Kong YY, Nishina H, Tedford K, Marengere LE, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem MP, Bouchard D, Barbacid M, Bernstein A, Penninger JM. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 6.Saveliev A, Vanes L, Ksionda O, Rapley J, Smerdon SJ, Rittinger K, Tybulewicz VL. Function of the nucleotide exchange activity of vav1 in T cell development and activation. Sci Signal. 2009;2:ra83. doi: 10.1126/scisignal.2000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miletic AV, Graham DB, Sakata-Sogawa K, Hiroshima M, Hamann MJ, Cemerski S, Kloeppel T, Billadeau DD, Kanagawa O, Tokunaga M, Swat W. Vav links the T cell antigen receptor to the actin cytoskeleton and T cell activation independently of intrinsic Guanine nucleotide exchange activity. PLoS One. 2009;4:e6599. doi: 10.1371/journal.pone.0006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhne MR, Ku G, Weiss A. A guanine nucleotide exchange factor-independent function of Vav1 in transcriptional activation. J Biol Chem. 2000;275:2185–2190. doi: 10.1074/jbc.275.3.2185. [DOI] [PubMed] [Google Scholar]
- 9.Zugaza JL, Lopez-Lago MA, Caloca MJ, Dosil M, Movilla N, Bustelo XR. Structural determinants for the biological activity of Vav proteins. J Biol Chem. 2002;277:45377–45392. doi: 10.1074/jbc.M208039200. [DOI] [PubMed] [Google Scholar]
- 10.Kaminuma O, Deckert M, Elly C, Liu YC, Altman A. Vav-Rac1-mediated activation of the c-Jun N-terminal kinase/c-Jun/AP-1 pathway plays a major role in stimulation of the distal NFAT site in the interleukin-2 gene promoter. Mol Cell Biol. 2001;21:3126–3136. doi: 10.1128/MCB.21.9.3126-3136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Motto DG, Koretzky GA, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- 12.Tuosto L, Michel F, Acuto O. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen-stimulated T cells. J Exp Med. 1996;184:1161–1166. doi: 10.1084/jem.184.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang N, Motto DG, Ross SE, Koretzky GA. Tyrosines 113, 128, and 145 of SLP-76 are required for optimal augmentation of NFAT promoter activity. J Immunol. 1996;157:3769–3773. [PubMed] [Google Scholar]
- 14.Raab M, da Silva AJ, Findell PR, Rudd CE. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCR zeta/CD3 induction of interleukin-2. Immunity. 1997;6:155–164. doi: 10.1016/s1074-7613(00)80422-7. [DOI] [PubMed] [Google Scholar]
- 15.Clements JL, Yang B, Ross-Barta SE, Eliason SL, Hrstka RF, Williamson RA, Koretzky GA. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 16.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt FW, Geha RS. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 17.Myung PS, Derimanov GS, Jordan MS, Punt JA, Liu QH, Judd BA, Meyers EE, Sigmund CD, Freedman BD, Koretzky GA. Differential requirement for SLP-76 domains in T cell development and function. Immunity. 2001;15:1011–1026. doi: 10.1016/s1074-7613(01)00253-9. [DOI] [PubMed] [Google Scholar]
- 18.Kumar L, Pivniouk V, de la Fuente MA, Laouini D, Geha RS. Differential role of SLP-76 domains in T cell development and function. Proc Natl Acad Sci U S A. 2002;99:884–889. doi: 10.1073/pnas.022619199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, Aschenbrenner AC, Koretzky GA. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28:359–369. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braiman A, Barda-Saad M, Sommers CL, Samelson LE. Recruitment and activation of PLCgamma1 in T cells: a new insight into old domains. Embo J. 2006;25:774–784. doi: 10.1038/sj.emboj.7600978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunnell SC. Multiple microclusters: diverse compartments within the immune synapse. Curr Top Microbiol Immunol. 2010;340:123–154. doi: 10.1007/978-3-642-03858-7_7. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity. 2008;28:810–821. doi: 10.1016/j.immuni.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 26.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunnell SC, Singer AL, Hong DI, Jacque BH, Jordan MS, Seminario MC, Barr VA, Koretzky GA, Samelson LE. Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol Cell Biol. 2006;26:7155–7166. doi: 10.1128/MCB.00507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barda-Saad M, Shirasu N, Pauker MH, Hassan N, Perl O, Balbo A, Yamaguchi H, Houtman JC, Appella E, Schuck P, Samelson LE. Cooperative interactions at the SLP-76 complex are critical for actin polymerization. Embo J. 2010;29:2315–2328. doi: 10.1038/emboj.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arudchandran R, Brown MJ, Peirce MJ, Song JS, Zhang J, Siraganian RP, Blank U, Rivera J. The Src homology 2 domain of Vav is required for its compartmentation to the plasma membrane and activation of c-Jun NH(2)-terminal kinase 1. J Exp Med. 2000;191:47–60. doi: 10.1084/jem.191.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu B, I, Martins R, Li P, Amarasinghe GK, Umetani J, Fernandez-Zapico ME, Billadeau DD, Machius M, Tomchick DR, Rosen MK. Structural and energetic mechanisms of cooperative autoinhibition and activation of Vav1. Cell. 2010;140:246–256. doi: 10.1016/j.cell.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amarasinghe GK, Rosen MK. Acidic region tyrosines provide access points for allosteric activation of the autoinhibited Vav1 Dbl homology domain. Biochemistry. 2005;44:15257–15268. doi: 10.1021/bi051126h. [DOI] [PubMed] [Google Scholar]
- 32.Katzav S, Sutherland M, Packham G, Yi T, Weiss A. The protein tyrosine kinase ZAP-70 can associate with the SH2 domain of proto-Vav. J Biol Chem. 1994;269:32579–32585. [PubMed] [Google Scholar]
- 33.Cao Y, Janssen EM, Duncan AW, Altman A, Billadeau DD, Abraham RT. Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. Embo J. 2002;21:4809–4819. doi: 10.1093/emboj/cdf499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazer G, Pe’er L, Schapira V, Richard S, Katzav S. The association of Sam68 with Vav1 contributes to tumorigenesis. Cell Signal. 2007;19:2479–2486. doi: 10.1016/j.cellsig.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Hobert O, Jallal B, Schlessinger J, Ullrich A. Novel signaling pathway suggested by SH3 domain-mediated p95vav/heterogeneous ribonucleoprotein K interaction. J Biol Chem. 1994;269:20225–20228. [PubMed] [Google Scholar]
- 36.Ye ZS, Baltimore D. Binding of Vav to Grb2 through dimerization of Src homology 3 domains. Proc Natl Acad Sci U S A. 1994;91:12629–12633. doi: 10.1073/pnas.91.26.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houtman JC, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, Appella E, Schuck P, Samelson LE. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol. 2006;13:798–805. doi: 10.1038/nsmb1133. [DOI] [PubMed] [Google Scholar]
- 38.Castellanos MC, Munoz C, Montoya MC, Lara-Pezzi E, Lopez-Cabrera M, de Landazuri MO. Expression of the leukocyte early activation antigen CD69 is regulated by the transcription factor AP-1. J Immunol. 1997;159:5463–5473. [PubMed] [Google Scholar]
- 39.Billadeau DD. Specific Subdomains of Vav Differentially Affect T Cell and NK Cell Activation. J Immunol. 2000:3971–3981. doi: 10.4049/jimmunol.164.8.3971. [DOI] [PubMed] [Google Scholar]
- 40.Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. doi: 10.1016/s0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 41.Miletic AV, Sakata-Sogawa K, Hiroshima M, Hamann MJ, Gomez TS, Ota N, Kloeppel T, Kanagawa O, Tokunaga M, Billadeau DD, Swat W. Vav1 acidic region tyrosine 174 is required for the formation of T cell receptor-induced microclusters and is essential in T cell development and activation. J Biol Chem. 2006;281:38257–38265. doi: 10.1074/jbc.M608913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Lago M, Lee H, Cruz C, Movilla N, Bustelo XR. Tyrosine phosphorylation mediates both activation and downmodulation of the biological activity of Vav. Mol Cell Biol. 2000;20:1678–1691. doi: 10.1128/mcb.20.5.1678-1691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapley J, V, Tybulewicz L, Rittinger K. Crucial structural role for the PH and C1 domains of the Vav1 exchange factor. EMBO Rep. 2008;9:655–661. doi: 10.1038/embor.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabapathy K, Hu Y, Kallunki T, Schreiber M, David JP, Jochum W, Wagner EF, Karin M. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr Biol. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 45.Chen CY, Gherzi R, Andersen JS, Gaietta G, Jurchott K, Royer HD, Mann M, Karin M. Nucleolin and YB-1 are required for JNK-mediated interleukin-2 mRNA stabilization during T-cell activation. Genes Dev. 2000;14:1236–1248. [PMC free article] [PubMed] [Google Scholar]
- 46.Conze D, Krahl T, Kennedy N, Weiss L, Lumsden J, Hess P, Flavell RA, Le Gros G, Davis RJ, Rincon M. c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 have distinct roles in CD8(+) T cell activation. J Exp Med. 2002;195:811–823. doi: 10.1084/jem.20011508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 48.Prisco A, Vanes L, Ruf S, Trigueros C, Tybulewicz VL. Lineage-specific requirement for the PH domain of Vav1 in the activation of CD4+ but not CD8+ T cells. Immunity. 2005;23:263–274. doi: 10.1016/j.immuni.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Cao Y. The Calponin Homology Domain of Vav1 Associates with Calmodulin and Is Prerequisite to T cell Antigen Receptor-induced Calcium Release in Jurkat T Lymphocytes. J Biol Chem. 2007;282:23737–23744. doi: 10.1074/jbc.M702975200. [DOI] [PubMed] [Google Scholar]
- 50.Lin J, Weiss A. Identification of the minimal tyrosine residues required for linker for activation of T cell function. J Biol Chem. 2001;276:29588–29595. doi: 10.1074/jbc.M102221200. [DOI] [PubMed] [Google Scholar]
- 51.Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, Caplen NJ, Billadeau DD, Wange RL, Burkhardt JK, Schwartzberg PL. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol. 2005;174:1385–1392. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 52.Barr VA, Balagopalan L, Barda-Saad M, Polishchuk R, Boukari H, Bunnell SC, Bernot KM, Toda Y, Nossal R, Samelson LE. T-cell antigen receptor-induced signaling complexes: internalization via a cholesterol-dependent endocytic pathway. Traffic. 2006;7:1143–1162. doi: 10.1111/j.1600-0854.2006.00464.x. [DOI] [PubMed] [Google Scholar]
- 53.Balagopalan L, V, Barr A, Sommers CL, Barda-Saad M, Goyal A, Isakowitz MS, Samelson LE. c-Cbl-mediated regulation of LAT-nucleated signaling complexes. Mol Cell Biol. 2007;27:8622–8636. doi: 10.1128/MCB.00467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature. 1997;385:93–97. doi: 10.1038/385093a0. [DOI] [PubMed] [Google Scholar]
- 55.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci U S A. 2007;104:6638–6643. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of Vav Mutants on TCR-Induced Calcium Entry
Mutations Impacting the Catalytic Core of the Vav1 GEF
Dynamic Visualization of SLP-76 and Vav1 Microclusters
Effect of Vav Knockdown on SLP-76 Microcluster Dynamics