Abstract
Endocarditis due to brucellosis is considered a rare occurrence involving native, congenital and prosthetic valves. The diagnosis needs high degree of suspicion in culture negative endocarditis especially in those with history of exposure to farm animals. A positive culture in a susceptible patient confirms the diagnosis with 91% sensitivity. An early diagnosis and prompt treatment with appropriate antibiotics can restore the valve structural integrity with minimal damage. Here we present a series of five cases of culture proven Brucella endocarditis (four native valves, one prosthetic valve) and this report discusses the diagnostic and management issues involved.
Keywords: Brucella endocarditis, Prosthetic valve, Mitral stenosis, Aortic root abscess, Mitral valvotomy
1. Introduction
Brucellosis is a world-wide zoonosis. The causative organism is an intracellular α2 proteobacteria gram-negative bacillus of the genus Brucella. Human brucellosis is a multiorgan disease, transmitted via unpasteurized animal milk and cheese. It often presents with fever and non-specific symptoms. A renewed scientific interest in human brucellosis has been fueled by its recent re-emergence and due to its potential for use as a class-B bioterrorist agent.1,2
Endocarditis due to brucellosis is rare but about 80% of deaths in brucellosis are due to endocarditis.3 In a recent Greek study over 20 years from different centers reported an incidence up to 4%.4 During the period from 2008 to 2011 we admitted six cases of bacteriologically confirmed Brucella endocarditis and the following is an account of the diagnostic and management issues involved in managing them. We lost one case during early follow up.
1.1. Details of case reports
1.1.1. Case-1
A 32-year-old male who was a farmer by occupation presented with history of continuous fever since 2 months and congestive cardiac failure. Patient had a history of rheumatic heart disease with severe mitral stenosis, mild mitral regurgitation, severe pulmonary artery hypertension. He underwent PTMC 7 years ago. Patient was evaluated and found to have elevated white blood cell count. Serum creatinine 1.8 mmol/L. Electrocardiogram showed right bundle branch block. Echocardiogram showed moderate size vegetation on mitral leaflet of mitral valve (Fig. 1). The diagnosis of Brucella endocarditis is confirmed by positive blood culture (Fig. 2). He was successfully treated with medical management. After 3 months he underwent PBMV.
Fig. 1.
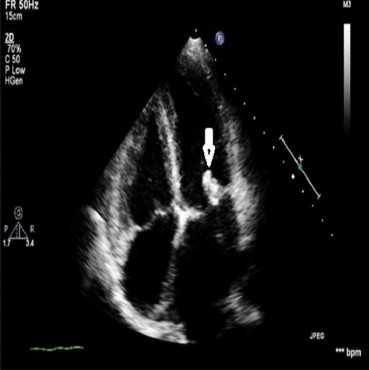
Moderate size vegetation pointed by white arrow on mitral valve in a rheumatic heart disease patient on 2nd day of antibiotic therapy.
Fig. 2.
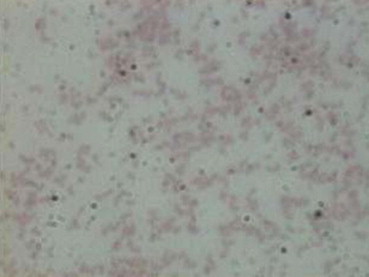
Gram's smear of culture showing gram negative Brucella melitensis bacillus.
1.1.2. Case-2
A 22-year-old male farmer who is a known case of acyanotic congenital heart disease, small restrictive ASD moderate size VSD and congenital mitral regurgitation underwent a patch closure and mitral valve replacement with Carbomedic prosthetic valve 3 years ago. He had a history of struck valve for which he took thrombolytic therapy 1 year ago. He presented with high grade fever and severe breathlessness. Echocardiogram showed elevated gradients across the prosthetic valve. Patient was found to have Brucella endocarditis on blood culture and started treatment similarly. He improved initially but he developed multiorgan dysfunction and developed shock and succumbed.
1.1.3. Case-3
A 44-year-old male a known chronic rheumatic heart disease with severe mitral stenosis presented with continuous high grade fever for 2 months. ECG showed right bundle branch block. Echocardiography showed large vegetation on mitral valve (Fig. 3). Patient was confirmed Brucella endocarditis after 5 days of subculture. He was successfully treated with antibiotics followed by elective mitral valve replacement after 3 months.
Fig. 3.
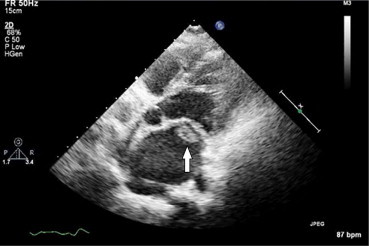
Large fluffy vegetation on mitral valve showed by arrow in a CRHD patient with severe mitral stenosis and Brucella endocarditis.
1.1.4. Case-4
A 36-year-old male presented with pyrexia of unknown origin for one month. Patient was subjected to all routine tests which did not yield any cause. Echocardiography showed aortic valve abscess (Fig. 4). Blood culture for fastidious organisms showed Brucella melitensis. Patient was treated appropriately with antibiotics and elective aortic valve replacement after 4 months.
Fig. 4.
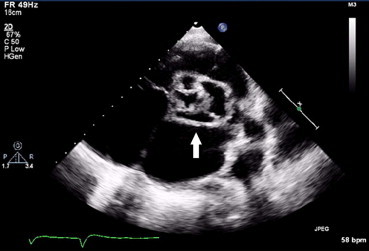
Aortic root abscess as pointed by arrow in a PUO patient.
1.1.5. Case-5
A 45-year-old male agricultural laborer, known bicuspid aortic valve presented with prolonged fever for 2 months. Patient was found to have severe aortic stenosis (Fig. 5). Blood culture showed B. melitensis. Patient was treated successfully with antibiotics and valve replacement at a later date.
Fig. 5.
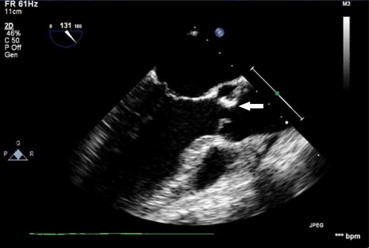
Moderate size vegetation showed by arrow on bicuspid aortic valve in a patient with Brucella endocarditis.
All were treated with empirical antibiotics (ceftriaxone 2 g and gentamicin 3 mg/kg) prior to culture report. As three of our patients had significant history of animal exposure we have asked our laboratory to look for Brucella species. In the other two cases, as there is no response with empirical therapy and initial cultures were negative, the search for fastidious organisms was made and Brucella was identified. All the five patients were found to have B. melitensis. Agglutination tests were positive with elevated antibody titers 1:640–1:1280.
Therapy with doxycycline 100 mg twice daily + gentamicin 3 mg/kg i.v. in 2 divided doses + rifampin 600 mg once daily for 6 weeks was started after confirmation of Brucella endocarditis in all patients.
The clinical and laboratory profiles of all above five cases studied are summarized in Table 1.
Table 1.
Brief summary of clinical and laboratory profiles of five cases studied.
| Profile | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age in years | 32 | 22 | 44 | 36 | 45 |
| Sex | M | M | M | M | M |
| Exposure to farm animals | – | + | + | + | – |
| C/P | Cont fever 2 months, CHF | Fever 3 months, LVF, shock | Fever, SOB | Fever, SOB | Fever 2 months |
| Heart lesions | CRHD, severe MS, mild MR, severe PAH, S/P PTMC | Acyanotic CHD, ASD, VSD S/P patch closure, S/P MVR Carbomedic, PVE | CRHD, severe MS | AV abscess | Bicuspid aortic valve, severe aortic stenosis |
| Valve involved | MV | MV | MV | AoV | AoV |
| Blood C/S | B. melitensis | B. melitensis | B. melitensis | B. melitensis | B. melitensis |
| Brucella abortus | + | + | + | + | + |
| Rx | MM followed by elective PBMV | MM (awaiting MVR) | MM followed by elective valve replacement MVR | MM followed by elective valve replacement AVR | MM followed by elective valve replacement AVR |
| Outcome | Discharged | Death | Discharged | Discharged | Discharged |
2. Discussion
2.1. Prevalence
Human brucellosis is a well controlled disease in the developed countries. Its exact prevalence in India and other developing nations is not clearly known. High prevalence is reported from the Mediterranean areas, the Arabian Peninsula, Mexico, and Central and South America. Endocarditis is a rare complication of brucellosis. Five series with 1500 patients of brucellosis reported only 12 (0.8%) cases with endocarditis.5–9 Dalrymple-Champneys observed only five cases of endocarditis among 1500 cases (0.3%) of human brucellosis in England over a period of 43 years.10 In our study, the observed incidence of Brucella endocarditis is relatively higher and it may be the reflection of selective study of only culture negative endocarditis. In a recent study, it was estimated that it could be as high as 4%.4
2.2. Presentation
Prolonged fever is the most common presentation. History of exposure to farm animals or consumption of unpasteurized animal milk or cheese may be present. Detection of vegetations on the valves on echocardiogram along with a positive culture is diagnostic. The aortic valve was predominantly affected (75%), and mitral valve, both mitral and aortic valves, and prosthetic valves were equally affected (8.3%).11,12 The infection of a previously healthy valve most often involves the aortic valve, whereas secondary infection of pre-damaged valve prefers the mitral valve.13 Though rarely mitral valve stenosis following an episode of endocarditis is reported to occur.14,15 Our series showed more of mitral valve involvement (3 out of 5, two are native valves, one is a prosthetic valve.) Brucella infection of the mechanical and bioprostheses has also been described.16,17
Electrocardiogram changes may reflect involvement of the cardiac conduction system (i.e. right or left bundle branch blocks or atrioventricular blocks).18 In the present case series, a complete right bundle branch block was found in two patients.
Echocardiography showed vegetations on mitral valve in 2 cases, and on bicuspid aortic valve in one patient, prosthetic valve in one case. All the vegetations are of moderate to large size (0.6–0.8 cm). The gradients were increased across the prosthetic mitral valve. One case showed aortic root abscess. All patients had moderate pulmonary hypertension. Many of the earlier case series showed that regurgitant lesions are more common than stenotic lesion. But our series showed that stenotic lesions are not uncommon.
2.3. The organism
Brucella abortus and B. melitensis are reported to be the most frequently isolated species. Brucella suis is rarely found (5% of cases). B. melitensis is known to cause more severe disease associated with disabling complications.19 The present series showed that all 5 cases were caused by B. melitensis. Blood culture is positive in 40–90% in acute cases and 5–20% in chronic and complicated cases of brucellosis. In such cases bone marrow culture is a better and recommended specimen. In our laboratory, the time to detection for Brucella species is 5–7 days on an average. Any negative blood culture bottles, in a clinically suspected case of brucellosis, is unloaded from the system after 5 days and further incubated at ambient air in the incubator at 37 °C for another 7–10 days. A terminal subculture is done on 5% sheep blood agar incubated at 37 °C under 5% CO2 for 48 h. All positive cultures are identified using the VITEK2 system (Biomerieux) and the ID GN panel. Antibiotic susceptibility is not routinely indicated, due to lack of resistance plasmids and thereby rare development of antibiotic resistance.
Serology using febrile agglutinins has a high rate of false negativity in complicated and chronic cases. However, ELISA for brucellosis is highly sensitive and specific. High Brucella titer in a course of Brucella endocarditis has been reported by Cohen et al.20 Titers are useful for monitoring the treatment. We adopted tube agglutination test where 1:160 is considered to be the cut off. All 5 patients had elevated titers which were in diagnostic range.
Algorithm for diagnosing Brucella endocarditis is mentioned (Fig. 6).
Fig. 6.
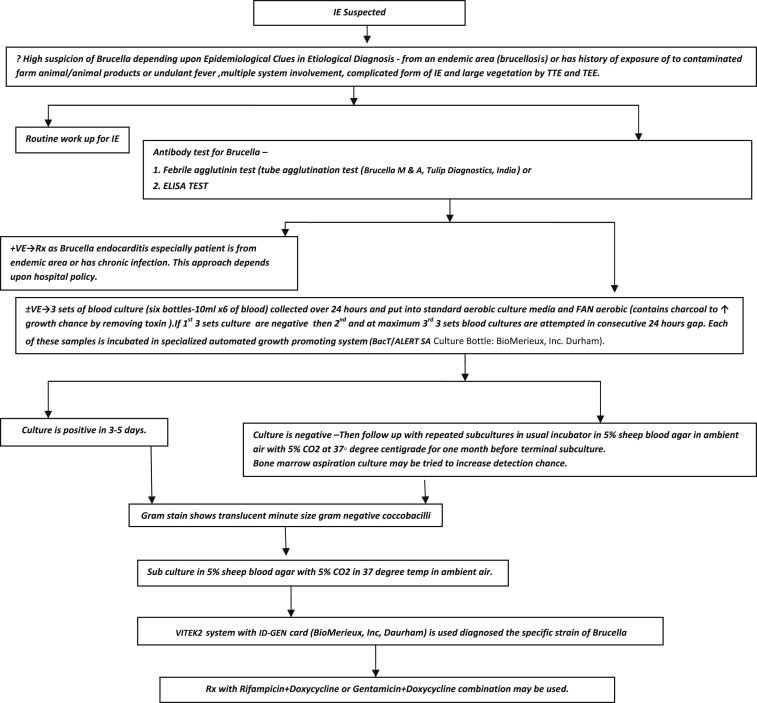
Making a decision for Brucella endocarditis diagnosis through an easy algorithm followed in our institute. IE: infective endocarditis; TTE: trans thoracic echocardiography; TEE: transesophageal echocardiograhy = Brucella melitensis; A = Brucella abortus; Rx: treatment.
2.4. Complications
Certain complications are seen in the course of Brucella endocarditis like – myocardial abscesses; left ventricular failure; disseminated intravascular coagulation; and embolic phenomena (i.e., mycotic aneurysms transient ischemic attacks, myocardial and other organ infarctions). Septic emboli dislodged from valve vegetations may cause infected infarcts of any organ, leading to relapse. One of our cases with PVE developed multiorgan dysfunction and shock which lead to the death of the patient. In a series of 44 necropsies on cases of fatal brucellosis, Peery and Belter3 found myocardial abscesses in 43%. Abscess formation is more common in endocarditis caused by Brucella than other organisms.21 Our series also had reported a case of aortic valve abscess.
2.5. Treatment
Because endocarditis is a rare focal complication of brucellosis, most cases described in the literature are single reports or small series. No treatment recommendations exist specific to this entity. The treatment recommended by WHO in acute brucellosis in adults is rifampicin 600 mg and doxycycline 100 mg for a minimum of 6 weeks.22 Gentamicin 3 mg/kg in 3 divided doses can be added in cases of endocarditis for 2 weeks. The indication for surgery or its timing has not yet been defined. Jacobs et al recommended a combination of antibiotic administration with valve replacement as the most effective therapy.23 Fifteen well documented patients were cured by medical treatment alone in another study.24 In our study, one case with mitral valve involvement was proven bacteriologically and cured with medical management alone. Definitive valve therapy is decided on residual valve lesion as shown in our patients where one patient underwent balloon mitral valvotomy while 3 others had valve replacement.
2.6. Mortality
Although overall mortality due to brucellosis is low (<1%), endocarditis is responsible for the majority of deaths (80%) related to this disease.3 Congestive heart failure is responsible for the majority of deaths in Brucella endocarditis.
3. Conclusions
The relative higher incidence of Brucella endocarditis (5%) in this study implies that culture negative endocarditis cases mandate search for evidence of Brucella species. A high degree of suspicion is needed especially in young male patients and with history of exposure to farm animals. B. melitensis was the offending species in all our cases. No valve native, congenitally abnormal or prosthetic, is immune to Brucella endocarditis. Though Brucella endocarditis is known for large vegetations and surgery is the treatment of choice, our series showed that early treatment besides life saving can restore the affected valve anatomy to minimal stigmata.
Conflicts of interest
All authors have none to declare.
Abbreviations
- CRHD
chronic rheumatic heart disease
- PTMC
percutaneous transluminal mitral commissurotomy
- PBMV
percutaneous balloon mitral valvotomy
- ASD
atrial septal defect
- VSD
ventricular septal defect
- MVR
mitral valve replacement
- AVR
aortic valve replacement
- PVE
prosthetic valve endocarditis
- ELISA
enzyme linked immune sorbent assay
- CHF
Congestive Heart Failure
- LVF
Left ventricular failure
- MS
mitral stenosis
- MR
mitral regurgitation
- PAH
pulmonary artery hypertension
- MM
medical management
- AoV
aortic valve
References
- 1.Pappas G., Papadimitriou P., Akritidis N. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 2.Greenfield R.A., Drevets D.A., Machado L.J. Bacterial pathogens as biological weapons and agents of bioterrorism. Am J Med Sci. 2002;323:299–315. doi: 10.1097/00000441-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Peery T.M., Belter L.F. Brucellosis and heart disease. II. Fatal brucellosis: a review of the literature and report of new cases. Am J Pathol. 1960;36:673–697. [PMC free article] [PubMed] [Google Scholar]
- 4.Hadjinikolaou L., Triposkiadis F., Zairis M. Successful management of Brucella melitensis endocarditis with combined medical and surgical approach. Eur J Cardiothorac Surg. 2001;19:806–810. doi: 10.1016/s1010-7940(01)00696-0. [DOI] [PubMed] [Google Scholar]
- 5.Mousa A.R., Elhag K.M., Khogali M. The nature of human brucellosis in Kuwait: study of 379 cases. Rev Infect Dis. 1988;10:211–217. doi: 10.1093/clinids/10.1.211. [DOI] [PubMed] [Google Scholar]
- 6.Ariza J., Gudiol F., Pallares R. Treatment of human brucellosis with doxycycline plus rifampin or doxycycline plus streptomycin. Ann Intern Med. 1992;117:25–30. doi: 10.7326/0003-4819-117-1-25. [DOI] [PubMed] [Google Scholar]
- 7.Montejo J.M., Alberola I., Zarate P.G. Open, randomized therapeutic trial of six antimicrobial regiments in the treatment of human brucellosis. Clin Infect Dis. 1993;16:671–676. doi: 10.1093/clind/16.5.671. [DOI] [PubMed] [Google Scholar]
- 8.Colmenero J.D., Reguera J.M., Martos F. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore) 1996;75:195–211. doi: 10.1097/00005792-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Memish Z., Mah M.W., Mahmoud S.A. Brucella bacteremia: clinical and laboratory observations in 160 patients. J Infect. 2000;40:59–63. doi: 10.1053/jinf.1999.0586. [DOI] [PubMed] [Google Scholar]
- 10.Dalrymple-Champneys W. Oxford University Press; London: 1960. Brucella Infection and Undulant Fever in Man. 1. [Google Scholar]
- 11.Reguera J.M., Alarcon A., Miralles F. Brucella endocarditis: clinical, diagnostic, and therapeutic approach. Eur J Clin Microbiol Infect Dis. 2003;22:647–650. doi: 10.1007/s10096-003-1026-z. [DOI] [PubMed] [Google Scholar]
- 12.Jeroudi M.O., Halim M.A., Harder E.J. Brucella endocarditis. Br Heart J. 1987;58:279–283. doi: 10.1136/hrt.58.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Meara J.B., Eykyn S., Jenkins B.S. Brucella melitensis endocarditis: successful treatment of an infected prosthetic mitral valve. Thorax. 1974;29:377–381. doi: 10.1136/thx.29.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Güray Y., Oztürk S., Boyaci A. A rare complication of brucellosis: mitral valve endocarditis. Turk Kardiyol Dern Ars. 2008;36:329–331. [PubMed] [Google Scholar]
- 15.Yavuz T., Ozaydin M., Ulusan V. A case of mitral stenosis complicated with seronegative Brucella endocarditis. Jpn Heart J. 2004;45:353–358. doi: 10.1536/jhj.45.353. [DOI] [PubMed] [Google Scholar]
- 16.Arslan H., Korkmaz M.E., Kart H. Management of Brucella endocarditis of a prosthetic valve. J Infect. 1998;37:70–71. doi: 10.1016/s0163-4453(98)90819-2. [DOI] [PubMed] [Google Scholar]
- 17.Cakalagaoglu C., Keser N., Alhan C. Brucella-mediated prosthetic valve endocarditis with brachial artery mycotic aneurysm. J Heart Valve Dis. 1999;8:586–590. [PubMed] [Google Scholar]
- 18.Quiroga J., Miralles A., Farinola T. Surgical treatment of Brucella endocarditis. Cardiovasc Surg. 1996;4:227–230. doi: 10.1016/0967-2109(96)82321-0. [DOI] [PubMed] [Google Scholar]
- 19.Haleem S.A. Brucella endocarditis. Heart Views. 2006;24:286–288. [Google Scholar]
- 20.Cohen P.S., Maguire J.H., Weinstein L. Infective endocarditis caused by gram negative bacteria: a review of the literature 1945–1977. Prog Cardiovasc Dis. 1980;22:205–242. doi: 10.1016/0033-0620(80)90010-9. [DOI] [PubMed] [Google Scholar]
- 21.Uddin M.J., Sanyal S.C., Mustafa A.S. The role of aggressive medical therapy along with early surgical intervention in the cure of Brucella endocarditis. Ann Thorac Cardiovasc Surg. 1998;4:209–213. [PubMed] [Google Scholar]
- 22.Joint FAO/WHO Expert Committee on Brucellosis . World Health Organisation; Geneva: 1986. Sixth Report. World Health Organ Tech Rep Ser No.740. [PubMed] [Google Scholar]
- 23.Jacobs F., Abramowicz D., Vereerstraeten P., Le Clerc J.L., Zech F., Thys J.P. Brucella endocarditis: the role of combined medical and surgical treatment. Rev Infect Dis. 1990;12:740–744. doi: 10.1093/clinids/12.5.740. [DOI] [PubMed] [Google Scholar]
- 24.Mert Ali, Kocak Funda, Ozaras Resat. The role of antibiotic treatment alone for the management of Brucella endocarditis in adults: a case report and literature review. Ann Thorac Cardiovasc Surg. 2002;8:381–385. [PubMed] [Google Scholar]


