Abstract
Background
Studies evaluating CYP2C19*2 and ABCB1-C3435T polymorphisms have shown conflicting results. We performed this meta-analysis to evaluate role of clinical testing for these polymorphisms in CAD patients on clopidogrel.
Methods
19,601 patients from 14 trials were analyzed. The endpoints were major adverse cardiovascular events (MACE), cardiovascular (CV) death, stent thrombosis (ST), myocardial infarction (MI), stroke and major bleeding. Combined relative risks (RR) with 95% confidence intervals (CI) were computed for each outcome by using standard methods of meta-analysis and test parameters were computed.
Results
CYP2C19*2 polymorphism was associated with higher risk of MACE [RR: 1.28, CI: 1.06–1.54; p = 0.009], CV death [RR: 3.21, CI: 1.65–6.23; p = 0.001], MI [RR: 1.36, CI: 1.12–1.65; p = 0.002], ST [RR: 2.41, CI: 1.69–3.41; p < 0.001]. No difference was seen in major bleeding events [RR: 1.02, CI: 0.86–1.20; p = 0.83]. Subgroup analysis showed similar results for elective PCI [RR: 1.34, CI: 1.01–1.76; p = 0.03], and PCI with DES [RR: 1.53, CI: 1.029–1.269; p = 0.03]. CYP2C19*2 polymorphism has very low sensitivity (28–58%), specificity (71–73%), positive predictive value (3–10%) but good negative predictive value (92–99%). ABCB1-C3435T polymorphism analysis revealed similar MACE [RR: 1.13, CI: 0.99–1.29; p = 0.06], ST [RR: 0.88, CI: 0.52–1.47; p = 0.63] and major bleeding [RR: 1.04, CI: 0.87–1.25; p = 0.62] in both groups.
Conclusion
In CAD patients on clopidogrel therapy, CYP2C19*2 polymorphism is associated with significantly increased adverse cardiovascular events. However, due to the low positive predictive value, routine genetic testing cannot be recommended at present.
Keywords: Genetic testing, CYP2C19*2 polymorphisms, ABCB1-C3435T polymorphisms
1. Background
Dual antiplatelet therapy of aspirin and clopidogrel is now established as a standard of care for patients undergoing percutaneous coronary intervention (PCI) or patient with acute coronary syndrome (ACS).1 In Percutaneous Coronary Intervention in the Clopidogrel in Unstable angina to prevent Recurrent Events (PCI-CURE) study, dual antiplatelet therapy (DAPT) in patients with non-ST elevation myocardial infarction (NSTEMI) lead to 31% reduction in cardiovascular death or myocardial infarction (MI).2 Similarly, in ClOpidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT), DAPT in patients with ST elevation myocardial infarction (STEMI) was shown to reduce all cause mortality, and major cardiovascular events (MACE) including reinfarction.3
However, despite the use of DAPT, nearly 10% patients still experience recurrent MACE.4–7 Persistent occurrence of MACE during DAPT may be partially explained by inter-individual variability of clopidogrel response, especially in patients undergoing PCI.8–10 One of the postulated non-modifiable factors for variability of response can be attributed to pharmacogenetics of clopidogrel metabolism.
Clopidogrel is a prodrug that requires to be converted in to active metabolites to irreversibly bind to the P2Y12 receptor. Clopidogrel metabolism is a two steps process dependent on cytochrome P450 (CYP), with contributions from the isoenzymes like: CYP2C19, CYP3A4 or CYP3A5, CYP2C9, CYP1A2, and CYP2B6.11–13 CYP2C19 is a key enzyme in this activation process. There are at least 9 LoF alleles in CYP2C19 gene: *2–*8 null-functioning, *9–*10 decreased functioning. The presence of carriers of the loss-of-function alleles of CYP2C19 polymorphism is associated with clopidogrel non-responsiveness in healthy people and in patients with coronary artery disease (CAD).14–16 In addition to CYP2C19 polymorphism, variations in the gene regulating clopidogrel absorption and efflux by encoding the P-glycoprotein a multi drug resistant-1 efflux transporter (called ABCB1),17 might also affect the rate of clinical events during treatment. According to ACC/AHA guidelines, current evidence base is insufficient to recommend either routine genetic or platelet function testing at the present time. There is no information that routine testing improves outcome in large sub-groups of patients and hence is not currently recommended.18
Individual studies evaluating role of CYP2C19*2 polymorphism and ABCB1-C3435T polymorphism have yielded mix results. Therefore we performed pooled analysis of prospective studies comparing clinical outcomes in patients with CYP2C19*2 and ABCB1 polymorphism on clopidogrel therapy to evaluate association and role of clinical testing for these polymorphisms in CAD patients on clopidogrel.
2. Methods
We performed this review in accordance with the Quality of Reporting of Meta-analysis (QUOROM) statement and the Consolidated Standards of Reporting Trials (CONSORT) Group recommendations.19 A protocol was prospectively developed, detailing the objectives, criteria for study selection and approach to assessing the study quality, primary outcome and methodology.
2.1. Literature search
We searched the National Library of Medicine Pub Med, National Institutes of Health clinical trials registry and the Cochrane Central Register of Controlled Trials for prospective studies of CAD patients on clopidogrel treatment comparing clinical outcomes based on CYP2C19*2 and ABCB1 polymorphism. Selection process was similar both polymorphism. We also searched Internet-based sources of information on the results of clinical trials in cardiology (www.cardiosource.com/clinicaltrials, www.theheart.org, www.clinicaltrialresults.com and www.tctmd.com), as well as conference proceedings from meetings of the American College of Cardiology, the American Heart Association, and the European Society of Cardiology. Searches were restricted to the period from January 2000 through May 2011. The key words used for search were: coronary artery disease, acute coronary syndrome, percutaneous coronary interventions, STEMI, NSTEMI, Unstable Angina, stable angina, clopidogrel, prasugrel, ticagrelor, CYP2C19, CYP3A4 or CYP3A5, CYP2C9, CYP1A2, and CYP2B6, ABCB1.
2.2. Study selection
Two independent authors reviewed all titles and abstracts from the results of our computerized search. We also went into the related links of all relevant articles. In addition to our computerized search, we manually reviewed the reference list of all retrieved articles to complete our search. Study selection process is outlined in Fig. 1.
Fig. 1.
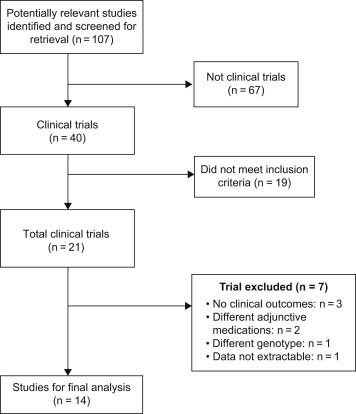
Study selection process for CYP2C19*2 polymorphism.
2.3. Inclusion criteria
We included in our analysis the results of randomized clinical trials or post hoc analysis of randomized control trial that compared clinical outcomes of patients with and without CYP2C19*2 and ABCB1 polymorphism in CAD patients on clopidogrel treatment.
All studies had to meet all the following criteria to be included in the analysis:
-
1.
Randomized controlled trial or post hoc analysis of randomized controlled trials.
-
2.
Include patients with coronary artery diseases mainly undergoing PCI.
-
3.
Compare CYP2C19*2 carrier (variant) versus non-carrier (normal) and ABCB1 polymorphism (CT/TT) versus normal (CC).
-
4.
Report at least one of the outcomes: MACE, CV death, ST, MI, stroke and major bleeding.
2.4. Exclusion criteria
Studies that did not meet the above criteria were excluded.
2.5. Data abstraction
After identifying all relevant articles, we extracted characteristics of the study like (author, year, design, duration, sample size, patient population, and genotype characteristics) and outcomes like (MACE, CV death, MI, ST, stroke, major bleeding complications and follow-up percentage). Two reviewers independently extracted data and assessed outcomes. The inter-rater agreement was 90%, and disagreements were resolved by consensus.
2.6. Quality assessment
All the trials reported adequate concealment of the randomized treatment sequence. In all studies, follow-up was more than 90% complete.
2.7. Statistical analysis
Combined relative risks (RR) across all the studies with 95% confidence intervals (CI) were computed for each endpoint by using the Comprehensive Meta-Analysis software package (version CM 2.2, Biostat, Englewood, NJ). Heterogeneity of the studies was assessed for each endpoint (Table 2). Those studies that were homogenous for an endpoint were analyzed by the Mantel–Haenszel fixed effect model, while those studies that were heterogeneous for an endpoint were analyzed by the random effect model. A two-sided alpha error of <0.05 was considered to be statistically significant. The inverse variance method was used for study weighting. Potential publication biases were assessed by the funnel plot method and Egers test. Sensitivity, specificity, and the positive and negative predictive values of genetic testing were computed for each endpoint.
Table 2.
Heterogeneity results for individual study outcomes and subgroup outcomes.
| Q-value | df (Q) | P-value | I-squared | Result | Publication bias (Funnel plots) | |
|---|---|---|---|---|---|---|
| All studies of CYP219*2 | ||||||
| MACE | 32.9 | 13 | 0.002 | 60.5 | Heterogenic | None |
| CV deaths | 2.0 | 4 | 0.730 | 0.0 | Homogenic | None |
| MI | 6.6 | 5 | 0.250 | 24.5 | Homogenic | Possible |
| Stroke | 1.7 | 2 | 0.425 | 0.0 | Homogenic | None |
| Major bleeding | 1.9 | 2 | 0.393 | 0.0 | Homogenic | None |
| Stent thrombosis | 7.3 | 5 | 0.199 | 31.5 | Homogenic | Possible |
| All studies ABCB1 | ||||||
| MACE | 7.2 | 2 | 0.027 | 72.4 | Heterogenic | None |
| Stent thrombosis | 0.2 | 1 | 0.692 | 0.0 | Homogenic | Cannot be evaluate – only 2 studies |
| Major bleeding | 1.2 | 1 | 0.269 | 18.0 | Homogenic | Cannot be evaluate – only 2 studies |
| Elective PCI sub-groups of CYP219*2 | ||||||
| MACE | 5.6 | 3 | 0.136 | 46.0 | Homogenic | No |
| ACS sub-groups of CYP219*2 | ||||||
| MACE | 20.6 | 6 | 0.002 | 70.9 | Heterogenic | No |
| CV deaths | 1.4 | 2 | 0.493 | 0.0 | Homogenic | No |
| MI | 4.7 | 2 | 0.095 | 57.4 | Heterogenic | No |
| Major bleeding | 1.9 | 2 | 0.393 | 0.0 | Homogenic | No |
| Stent thrombosis | 6.2 | 3 | 0.102 | 51.7 | Homogenic | Possible |
| DES sub-groups of CYP219*2 | ||||||
| MACE | 11.5 | 4 | 0.022 | 65.1 | Heterogenic | No |
| MI | 6.4 | 2 | 0.041 | 68.6 | Heterogenic | Possible |
| Stent thrombosis | 1.2 | 2 | 0.555 | 0.0 | Homogenic | Possible |
[PCI = Percutaneous Coronary Interventions, ACS = Acute Coronary Syndrome, CAD = Coronary artery diseases, MACE = Major Adverse Cardiovascular Event, CV = Cardiovascular and DES = Drug Eluting Stent.]
3. Results
3.1. Literature search for CYP2C19*2 polymorphism
A total of 107 articles were identified of which 40 were potentially relevant studies and screened for retrieval. After title and abstract evaluation, 19 studies were excluded and 21 studies were retrieved for a more detailed screening. Out of these 21 studies 7 studies were excluded and fourteen trials were included for final analysis [Fig. 1]. Among excluded seven studies, 3 studies were excluded, as they have demonstrated genotype polymorphism co-relation with platelet reactivity and no clinical outcomes assessed,20–22 while other 3 studies were excluded as they have either compared the different genotype (CYP2C19*17)23 or no clinical outcomes were evaluated.24,25 Additionally, CLARITY TIMI 28 genomic study was excluded as data was not extractable.26 Thus, fourteen trials were included in the final analysis.26–40
3.2. Literature search for ABCB1 polymorphism
A total of three studies identified which also looked in to ABCB1 polymorphism data and included in our meta-analysis comparing the effect of clopidogrel in CAD patients with or without ABCB1 polymorphism.31,34,41
3.3. Overview of study and patient characteristics
Study design was either RCT or post hoc analysis of RCT, comparison of clinical outcomes between a CYP2C19*2 or ABCB1 carrier with non-carrier in CAD patients on clopidogrel treatment. The characteristics of included trials are mentioned in Table 1. For simplicity, patient population was categorized as CYP2C19*2 carrier (defined as a variant) group who are either heterozygous (has at least one loss of *2 functional allele) or homozygous (has two loss of *2 functional alleles). Patients were categorized in CYP2C19*2 non-carrier (defined as normal) who are either carrying wild type (*1/*1) or none *2 alleles. Similarly, for ABCB1 polymorphism patient population was categorized in ABCB1 carrier (CT/TT) and ABCB1 non-carrier (CC).
Table 1.
Study characteristics.
| Name of study (year) | Total no of patients | F/UP | Study population | Study type | Treatment protocol | CYP 2 C19 (1*/1*) | CYP 2 C19 (1*/2*) | CYP 2 C19 (1*/2*) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| SHULDINER (2009) | 225 | 12 | Elective PCI | Cohort | LD 300/600 mg MD 75 mg | 158 | 63 | 4 | MACE |
| MEGA TRINTON TIMI- 38 (2009) | 1459 | 15 | ACS undergoing PCI | Post hoc RCT | LD 600/300 mg MD 75 mg | 1064 | 357 | 38 | MACE, CV deaths, stroke, MI, ST and major bleeding |
| PARE CURE (2010) | 2537 | 12 | ACS undergoing PCI | Post hoc RCT | LD 300 mg MD 75 mg | NA | NA | NA | MACE and major bleeding |
| MALEK (2008) | 105 | 12 | ACS undergoing PCI | Cohort | LD 600/300 mg MD 75 mg | 84 | 21 | 1 | MACE, CV deaths, MI and ST |
| SIMON (2009) | 2178 | 12 | MI | Cohort | LD 300 mg MD 75 mg | 1561 | 564 | 53 | MACE |
| TRENK (2008) | 797 | 12 | Elective PCI | Cohort | LD 600 mg MD 75 mg | 552 | 228 | 17 | MACE |
| COLLETE (2009) | 259 | 6 | MI | Cohort | MD 75 mg | 186 | 64 | 9 | MACE, CV deaths, MI and ST |
| WALLENTINE (2010) | 4904 | 12 | ACS | Post hoc RCT | LD 600/300 mg MD 75 mg | NA | NA | NA | MACE, ST and major bleeding |
| BHATT (2009) | 2428 | 12 | Stable CAD | Post hoc RCT | LD 300 mg MD 75 mg | 950 | 490 | 58 | MACE |
| ANDERSON (2009) | 1250 | 12 | CAD undergoing PCI | Cohort | NA | NA | NA | NA | MACE and MI |
| WORRALL (2009) | 104 | 12 | ACS undergoing PCI | Post hoc RCT | NA | NA | NA | NA | MACE |
| SIBBING (2009) | 2485 | 12 | CAD undergoing PCI | Cohort | LD 600 mg MD 75 mg | 1805 | 633 | 47 | MACE, stroke, MI and ST |
| GIUSTI (2009) | 772 | 6 | CAD undergoing PCI | Cohort | LD 600 mg MD 75 mg | 525 | 221 | 26 | MACE, CV deaths and ST |
| Yamamoto (2011) | 98 | 12 | Elective PCI stable CAD | Cohort | LD 300 mg MD 75 mg | 47 | 51 | 25 | MACE, stroke, MI and CV deaths |
[PCI = Percutaneous Coronary Interventions, ACS = Acute Coronary Syndrome, CAD = Coronary artery diseases, LD = Loading Dosage, MD = Maintenance Dose, MACE = Major Adverse Cardiovascular Event, CV = Cardiovascular and ST = Stent Thrombosis.]
3.4. Endpoints
All fourteen studies included in meta-analysis had MACE as a primary endpoint. Out of fourteen, six studies28,30,33,34,38,39 included in the meta-analysis had stent thrombosis (ST) as an endpoint, while thee studies evaluated major bleeding28,29,34 and stroke28,38,40 as an endpoints. Outcomes for all studies included in our analysis are given in Table 1.
3.5. Heterogeneity testing
Results of heterogeneity testing for both CYP2C19*2 and ABCB1 polymorphisms are shown in Table 2. For heterogeneous outcomes random effect model and for homogenous outcomes fixed effect model was used. Publication bias analysis revealed no evidence of bias except for MI and ST (Table 2).
3.6. Clinical outcomes of CYP2C19*2 polymorphism
The trials included in this meta-analysis consisted of a total of 19,601 patients (CYP2C19*2 carrier group, n = 5540; non-carrier group, n = 14,061). The results of current meta-analysis are shown in Figs. 2–8.
Fig. 2.
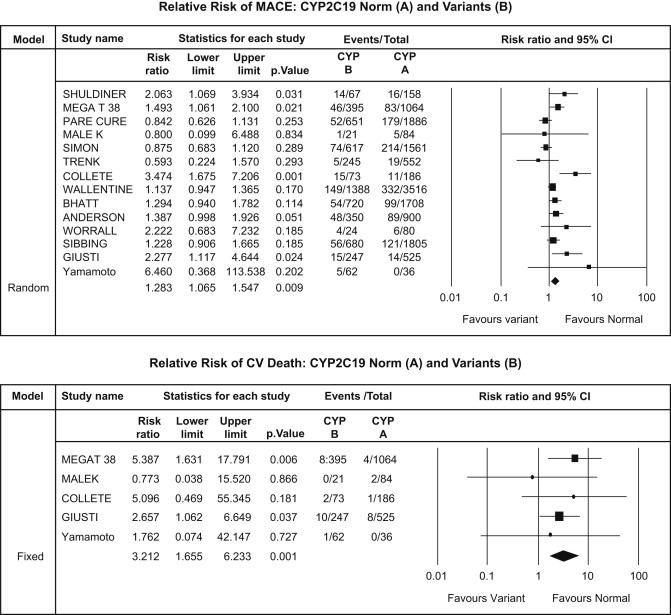
CYP2C19*2 analysis – major adverse cardiovascular events (upper panel) and cardiovascular death (lower panel).
Fig. 3.
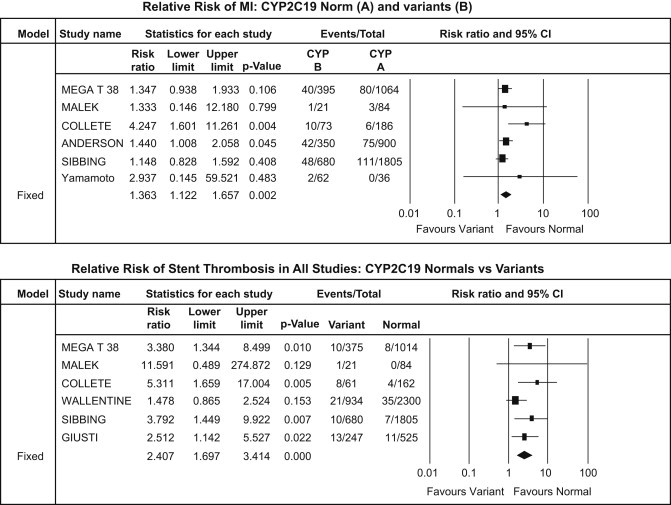
CYP2C19*2 analysis – myocardial infarction (upper panel) and stent thrombosis (lower panel).
Fig. 4.
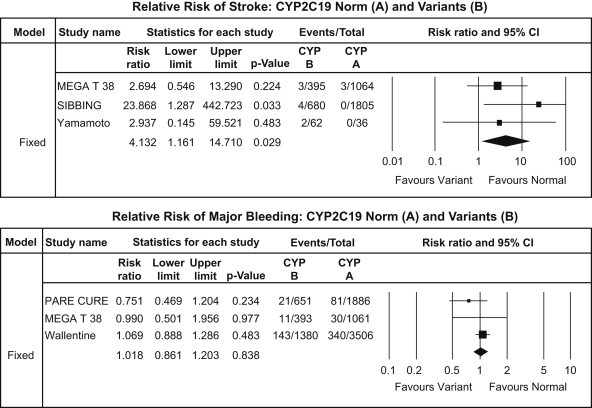
CYP2C19*2 analysis – stroke (upper panel) and major bleeding events (lower panel).
Fig. 5.
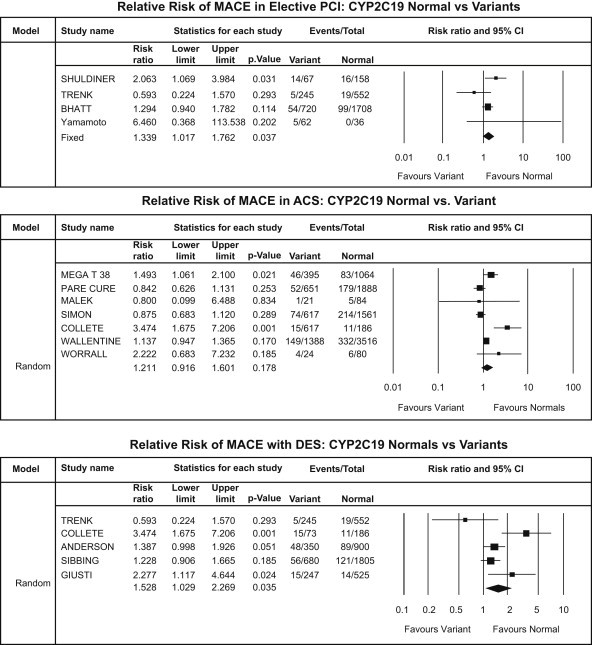
CYP2C19*2 subgroup analysis – MACE: elective PCI patients (upper panel), ACS patients (middle panel) and DES patients (lower panel).
Fig. 6.
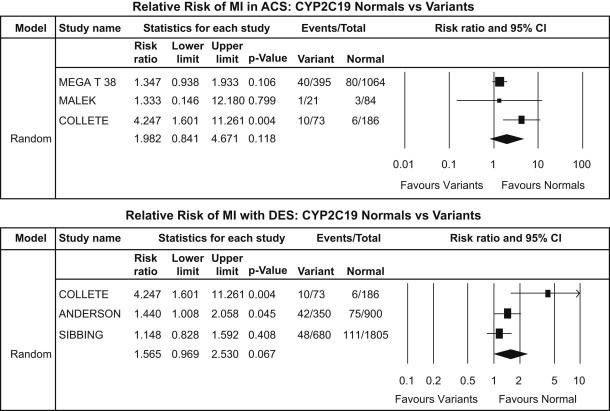
CYP2C19*2 subgroup analysis – MI: ACS patients (upper panel) and DES patients (lower panel).
Fig. 7.
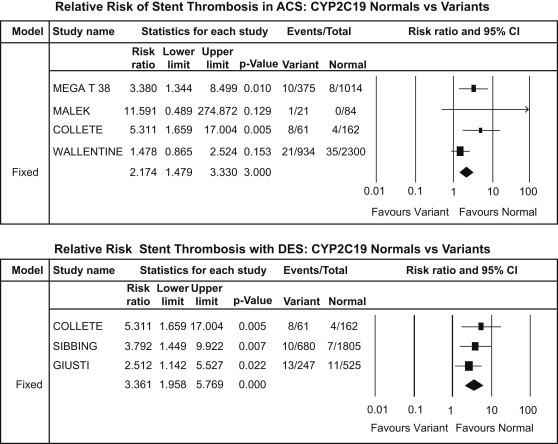
CYP2C19*2 subgroup analysis – ST: ACS patients (upper panel) and DES patients (lower panel).
Fig. 8.
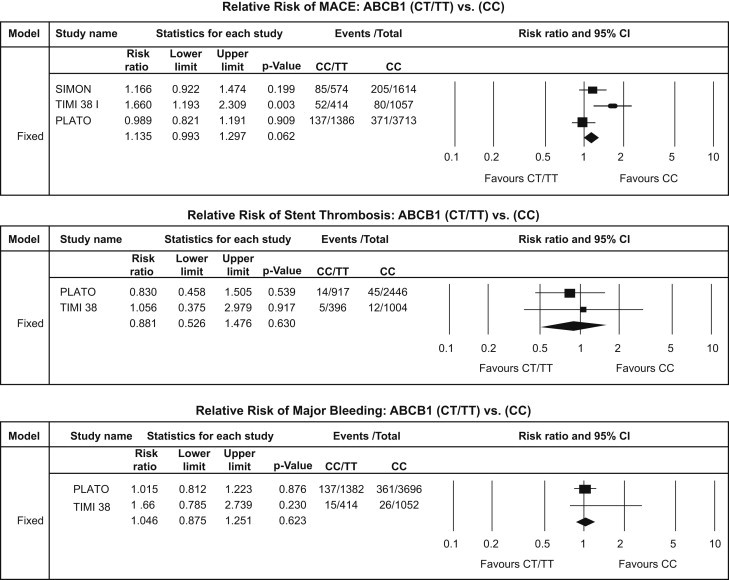
ABCB1-C3435T polymorphism analysis – MACE (upper panel), ST (middle panel) and major bleeding (lower panel).
3.6.1. Major adverse cardiovascular event (MACE)
Overall there were total of 1726 [8.8%] MACE of which 538 [9.71%] were in carrier group while 1188 [8.44%] in the non-carrier group. There was a significant increase of MACE in carrier group [RR: 1.28, CI: 1.06–1.54; p = 0.009] [Fig. 2].
3.6.2. Cardiovascular (CV) death
There were a total of 36 [17.54%] cardiovascular deaths of which 21 [2.63%] were in carrier group while 15 [0.79%] in the non-carrier group. The risk of cardiovascular mortality was higher in carrier groups than non-carrier group [RR: 3.21, CI: 1.65–6.23; p = 0.001] [Fig. 2].
3.6.3. Myocardial infarction (MI)
A total of 418 [7.39%] MI events occurred of which 143 [9.04%] were in carrier group while 275 [6.74%] in the non-carrier group. There was increase in MI events [RR: 1.36, CI: 1.12–1.65; p = 0.002] in carrier group compared to non-carrier group [Fig. 3].
3.6.4. Stent thrombosis (ST)
This includes a total of definite, probable and possible stent thrombosis. Incidence of ST was a total of 128 [1.56%]; out of which 63 [2.72%] were in carrier group while 65 [1.10%] in the non-carrier group. The risk of ST was significantly higher in carrier groups than non-carrier group [RR: 2.41, CI: 1.69–3.41; p < 0.001] [Fig. 3].
3.6.5. Stroke
Stroke outcome occurred only in 12 [0.29%] out of 4042 patients. The risk of stroke was also significantly higher in carrier group versus non-carrier group [RR: 4.13, CI: 1.16–14.71; p = 0.029] [Fig. 4].
3.6.6. Major bleeding
A total of 626 [5.54%] bleeding events occurred of which 175 [5.57%] were in Carrier group while 451 [5.53%] in the non-carrier group. Bleeding events did not differ between the two groups [RR: 1.02, CI: 0.86–1.20; p = 0.84] [Fig. 4].
3.7. Subgroup analysis of CYP2C19*2 polymorphism
3.7.1. Major adverse cardiovascular event (MACE)
There was a significant increase in incidence of MACE in carrier group in all patient populations irrespective of clinical sub-groups {stable CAD patients undergoing PCI [RR: 1.34, CI: 1.02–1.76; p = 0.037], ACS patients [RR: 1.21, CI: 0.91–1.60; p = 0.178] and patients undergoing PCI with DES [RR: 1.53, CI: 1.03–2.27; p = 0.035]} [Fig. 5].
3.7.2. Myocardial infarction (MI)
There was a significant increase of MI events in carrier group in ACS patients [RR: 1.98, CI: 0.84–4.67; p = 0.118] but no significant increase in MI events in carrier group in patients undergoing PCI with DES [RR: 1.56, CI: 0.97–2.53; p = 0.067] [Fig. 6].
3.7.3. Stent thrombosis (ST)
Stent thrombosis was significantly higher in carrier group of patients undergoing PCI with DES [RR: 3.36, CI: 1.96–5.77; p < 0.001] and ACS patients [RR: 2.17, CI: 1.42–3.33; p < 0.001] [Fig. 7].
3.8. Test parameters of CYP2C19*2 polymorphism
Test parameter analysis was done using 2 × 2 table. Genetic assay for CYP2C19*2 polymorphism was found to have sensitivity of (28–58%), specificity of (71–73%), negative predictive value of (92–99%) and positive predictive value of (3–10%) for various outcomes studied in this meta-analysis. Test parameters for individual outcomes are given in Table 3.
Table 3.
Test parameters of CYP2C19*2 polymorphism.
| All studies of CYP219*2 | MACE | CVS deaths | MI | ST | Major bleeding |
|---|---|---|---|---|---|
| Sensitivity % | 31 | 58 | 34 | 43 | 28 |
| Specificity % | 72 | 71 | 73 | 73 | 72 |
| NPV % | 92 | 99 | 93 | 98 | 94 |
| PPV % | 10 | 3 | 9 | 4 | 6 |
[MACE = Major Adverse Cardiovascular Event, CV = Cardiovascular and MI = Myocardial Infarction, ST = Stent Thrombosis.]
3.9. Clinical outcomes of ABCB1 polymorphism
The trials included in this meta-analysis consisted of a total of 8758 patients (non-carrier group, n = 6384; carrier group, n = 2374). The results of ABCB1-C3435T polymorphism summarized in Fig. 5. Overall there were a total of 930 [10.61%] MACE of which 274 [11.54%] were in carrier (CT/TT) group while 656 [10.27%] in the non-carrier (CC) group. This difference between the two groups was not statistically significant [RR: 1.13, CI: 0.99–1.29; p = 0.06] [Fig. 8]. Similar results were obtained for ST [RR: 0.88, CI: 0.52–1.47; p = 0.63] and major bleeding events [RR: 1.04, CI: 0.87–1.25; p = 0.62] [Fig. 8].
4. Discussion
The present meta-analysis included nearly 19,600 patients from 14 prospective clinical trials conducted through May, 2011. This pooled analysis is different from previous meta-analysis,42–45 as we assessed not only the association but also the CYP2C19*2 genetic testing parameters for individual outcomes. We also analyzed the role of ABCB1 polymorphism in clopidogrel non-responsiveness and included separate sub-analysis of three different patient populations to identify the population at risk.
Results of our analysis show that CYP2C19*2 polymorphism is associated with significantly increased relative risk of MACE (1.28 fold), MI (1.3 fold), CVS death (3 fold), ST (2.4 fold) and stroke (4 fold) without any decrease or increase in the incidence of bleeding events. Moreover, stable patients undergoing elective PCI also have significantly increased risk for MACE (1.3 fold) with CYP2C19*2 polymorphism carrier state. Additionally, it shows ABCB1 polymorphism is not associated with increased incidence of MACE or ST.
Majority of stent thrombosis occur early (<30 days) and according to previous reports incidences are approximately 1% that causes serious consequences like MI, CVS deaths and strokes.46 According to our analysis, compared to normal genotype, CYP2C19*2 polymorphism was associated with higher incidence of stent thrombosis, MI, CVS deaths and strokes. Overall, we found higher risk of ST (2.4 fold) as compared to MACE (1.28 fold). This could be due to the fact that MACE was reported in 14 studies while ST was reported by only six studies with wide confidence intervals.
Based on our data, CYP2C19*2 polymorphism genotyping has around 10% positive predictive value for almost each adverse outcomes (MI, MACE, CVS deaths etc.) which suggest that genotyping has no predictive value to detect studied outcomes. On the other hand, normal CYP2C19 had a very high (90–100%) negative predictive value for almost each adverse outcome. Thus, patients without CYP2C19*2 polymorphism are at very low risk of having an adverse event while in case of polymorphisms, the negative outcome does not seem to be predictable. This is true despite that the overall RR values indicate higher risks in this patient group. Thus, it is reasonable to think that there are other (unknown?) factors that play the major role in the negative outcomes in CYP2C19*2 polymorphism group of patients. This finding indicates the potential role genotype testing may play in evaluating genetically non-modifiable factors responsible for clopidogrel non-responsiveness in patient with recurrent ischemic events. However, due to the poor sensitivity and low positive predictive value, routine genetic testing cannot be recommended at present.
Our data also shows that ABCB1 polymorphism has no clinical role in clopidogrel non-responsiveness in patient undergoing PCI or patients with ACS. Although, this data only compares, the 3435C → T heterozygous/homozygous variant (includes CT/TT) to normal (CC) while data comparing homozygous variant (TT) to normal (CC) is not known and is a subject to be evaluated in future study.
Subgroup analysis of stable CAD patients undergoing elective PCI shows higher incidence of MACE (1.3 fold) in variant group compared with normal group, which contradicts the conclusion drawn from CHARISMA genomic sub-study that indicated no association with ischemic outcomes in CYP2C19*2 heterozygotes.35 As previously mentioned, high on treatment platelet reactivity (HTPR) is the patho-physiologic phenomenon behind the development of recurrent ischemic events in ACS patients or patients undergoing PCI.47 Apart from pharmacogenetic polymorphism, other factors also contributes to HTPR are non-compliance, under-dosing, drug–drug interactions, co-morbidities (diabetes mellitus, abnormal renal function, hyperlipidemia, obesity) active smoking, clinical presentation, and procedural complexities.7 According to initial data published in GRAVITAS trial (Price et al) shows that even after adjusting clopidogrel therapy (maintenance dose 150 mg), based on persistent HTPR assessed by platelet function test, did not result in change in clinical outcomes and persistent HTPR was attributed to non-modifiable risk factors like clinical presentation, procedural characteristics and genetic polymorphism.48 Therefore, combining platelet function testing to identify the HTPR followed by genotyping to identify CYP2C19*2 polymorphism may give us guidance to use alternate anti-platelets like prasugrel49 or ticagrelor.33
Overall, the prevalence of laboratory-defined clopidogrel non-responsiveness has been estimated at 21–26%.50 Clinical implications to clopidogrel non-responsiveness of CYP2C19*2 polymorphism are obvious since significant number of population has at least one loss-of-function CYP2C19 allele: ≈30–50% of Asians, 11–16% of Caucasians, and 14–25% of African-Americans.51 Recently, FDA issued black box warning regarding use of clopidogrel in poor metabolizers but recommended against routine genotyping in patients on clopidogrel therapy.52 Our meta-analysis indicates that CYP2C19*2 polymorphism results in significantly increased risk of cardiovascular events like MI, ST and CV deaths. Therefore it is imperative to have clinical trial designed to evaluate clinical benefit of personalizing antiplatelet therapy based on genotyping and platelet function test.
As with any meta-analysis, one of the limitations of our study is the difference in the definitions of the endpoints in the component trials, such as the definition of MACE, MI and CV death was different in various studies. Also, there was also heterogeneity in the study population, follows up duration, clopidogrel therapy protocol. Similarly, baseline characteristics between the two groups cannot be compared completely in most meta-analyses because of differences in the study protocols across the component trials. Also, there is a potential for publication bias but the trials in our analysis had different results and it should reduce this potential risk. Moreover, publication bias analysis was negative for all outcomes except for MI and ST indicating robustness of our results.
5. Conclusion
In CAD patients on clopidogrel therapy, CYP2C19*2 polymorphism is associated with significantly increased adverse cardiovascular events. However, due to the low positive predictive value, routine genetic testing cannot be recommended at present and should not be performed.
Funding/Support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
All authors have none to declare.
References
- 1.Smith S.C., Jr., Allen J., Blair S.N., AHA/ACC; National Heart, Lung, and Blood Institute AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113(19):2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 2.Mehta S.R., Yusuf S., Peters R.J. Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z.M., Jiang L.X., Chen Y.P., COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomized placebo-controlled trial. Lancet. 2005;366(9497):1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 4.Kuliczkowski W., Witkowski A., Polonski L. Interindividual variability in the response to oral antiplatelet drugs; a position paper of the Working Group on antiplatelet drugs resistance appointed by the Section of Cardiovascular Interventions of the Polish Cardiac Society, endorsed by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2009;30:426–435. doi: 10.1093/eurheartj/ehn562. [DOI] [PubMed] [Google Scholar]
- 5.Sharma R.K., Reddy H.K., Singh V.N. Aspirin and clopidogrel hypo responsiveness and nonresponsiveness in patients with coronary artery stenting. Vasc Health Risk Manag. 2009;5:965–972. doi: 10.2147/vhrm.s6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurbel P.A., Antonio M.J., Tantry U.S. Recent developments in clopidogrel pharmacology and their relation to clinical outcomes. Expert Opin Drug Metab Toxicol. 2009;5:989–1004. doi: 10.1517/17425250903107772. [DOI] [PubMed] [Google Scholar]
- 7.Yukhanyan Liana, Freynhofer Matthias K., Siller-Matula Jolanta, Schrör Karsten, Huber Kurt. Genetic variability in response to clopidogrel therapy and its clinical implications. Thromb Haemost. 2011;105(suppl 1):S55–S59. doi: 10.1160/THS10-11-0747. [DOI] [PubMed] [Google Scholar]
- 8.Campo G., Fileti L., Valgimigli M. Poor response to clopidogrel: current and future options for its management. J Thromb Thrombolysis. 2010;30:319–331. doi: 10.1007/s11239-010-0457-5. [DOI] [PubMed] [Google Scholar]
- 9.Sibbing D., Kastrati A. Risk of combining PPIs with thienopyridines: fact or fiction? Lancet. 2009;374:952–953. doi: 10.1016/S0140-6736(09)61562-2. [DOI] [PubMed] [Google Scholar]
- 10.Sibbing D., Morath T., Braun S. Clopidogrel response status assessed with multiplate point-of-care analysis and the incidence and timing of stent thrombosis over six months following coronary stenting. Thromb Haemost. 2010;103:151–159. doi: 10.1160/TH09-05-0284. [DOI] [PubMed] [Google Scholar]
- 11.Hulot J.S., Bura A., Villard E. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2008;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 12.Savi P., Pereillo J.M., Uzabiaga M.F. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84:891–896. [PubMed] [Google Scholar]
- 13.Savi P., Herbert J.M. Clopidogrel and ticlopidine: P2Y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis. Semin Thromb Hemost. 2005;31:174–183. doi: 10.1055/s-2005-869523. [DOI] [PubMed] [Google Scholar]
- 14.Fontana P., Hulot J.S., De Moerloose P., Gaussem P. Influence of CYP2C19 and CYP3A4 gene polymorphisms on clopidogrel responsiveness in healthy subjects. J Thromb Haemost. 2007;5:2153–2155. doi: 10.1111/j.1538-7836.2007.02722.x. [DOI] [PubMed] [Google Scholar]
- 15.Frere C., Cuisset T., Morange P.E. Effect of cytochrome P450 polymorphisms on platelet reactivity after treatment with clopidogrel in acute coronary syndrome. Am J Cardiol. 2008;101:1088–1093. doi: 10.1016/j.amjcard.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 16.Giusti B., Gori A.M., Marcucci R. Cytochrome P450 2C19 loss-of-function polymorphism, but not CYP3A4 IVS10 + 12G/A and P2Y12 T744C polymorphisms, is associated with response variability to dual antiplatelet treatment in high-risk vascular patients. Pharmacogenet Genomics. 2007;17:1057–1064. doi: 10.1097/FPC.0b013e3282f1b2be. [DOI] [PubMed] [Google Scholar]
- 17.Taubert D., von Beckerath N., Grimberg G. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80:486–501. doi: 10.1016/j.clpt.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Holmes D.R., Jr., Dehmer G.J., Kaul S., Leifer D., O'Gara P.T., Stein C.M. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “Boxed Warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation. 2010;122:537–557. doi: 10.1161/CIR.0b013e3181ee08ed. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Cook D.J., Eastwood S., Olkin I., Rennie D., Stroup D.F. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354(9193):1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 20.Bonello L., Armero S., Ait Mokhtar O. Clopidogrel loading dose adjustment according to platelet reactivity monitoring in patients carrying the 2C19*2 loss of function polymorphism. J Am Coll Cardiol. 2010;56(20):1630–1636. doi: 10.1016/j.jacc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Gurbel P.A., Bliden K.P., Antonino M.J. The effect of elinogrel on high platelet reactivity during dual antiplatelet therapy and the relation to CYP2C19*2 genotype: first experience in patients. J Thromb Haemost. 2010;8(1):43–53. doi: 10.1111/j.1538-7836.2009.03648.x. [DOI] [PubMed] [Google Scholar]
- 22.Gladding P., Webster M., Zeng I. The pharmacogenetics and pharmacodynamics of clopidogrel response: an analysis from the PRINC (Plavix Response in Coronary Intervention) trial. JACC Cardiovasc Interv. 2008;1(6):620–627. doi: 10.1016/j.jcin.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Sibbing D., Koch W., Gebhard D. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121(4):512–518. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- 24.Hwang S.J., Jeong Y.H., Kim I.S. Cytochrome 2C19 polymorphism and response to adjunctive cilostazol versus high maintenance-dose clopidogrel in patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2010;3(5):450–459. doi: 10.1161/CIRCINTERVENTIONS.110.949859. [DOI] [PubMed] [Google Scholar]
- 25.Brandt J.T., Close S.L., Iturria S.J. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007 Dec;5(12):2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 26.Mega J.L., Thakuria J.V., Cannon C.P., Sabatine M.S. Sequence variations in CYP metabolism genes and cardiovascular outcomes following treatment with clopidogrel: insights from the CLARITY-TIMI 28 genomic study [abstract 1017–69, American College of Cardiology 57th Annual Scientific Session, Chicago, IL, March 29-April 1, 2008] J Am Coll Cardiol. 2008;51(suppl A 10):A178–A235. [Google Scholar]
- 27.Shuldiner A.R., O'Connell J.R., Bliden K.P. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mega J.L., Close S.L., Wiviott S.D. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 29.Paré G., Mehta S.R., Yusuf S. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. 2010;363(18):1704–1714. doi: 10.1056/NEJMoa1008410. [DOI] [PubMed] [Google Scholar]
- 30.Malek L.A., Kisiel B., Spiewak M. Coexisting polymorphisms of P2Y12 and CYP2C19 genes as a risk factor for persistent platelet activation with clopidogrel. Circ J. 2008;72:1165–1169. doi: 10.1253/circj.72.1165. [DOI] [PubMed] [Google Scholar]
- 31.Simon T., Verstuyft C., Mary-Krause M., French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 32.Trenk D., Hochholzer W., Fromm M.F. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008;51:1925–1934. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 33.Collet J.P., Hulot J.S., Pena A. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 34.Wallentin L., James S., Storey R.F., PLATO investigators Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic sub-study of the PLATO trial. Lancet. 2010;376(9749):1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 35.Bhatt D.L., Paré G., Eikelboom J.W., On behalf of the CHARISMA Investigators The relationship between CYP2C19 polymorphisms and ischaemic and bleeding outcomes in stable outpatients: the CHARISMA genetics study. Eur Heart J. 2012 Mar 26 doi: 10.1093/eurheartj/ehs059. [DOI] [PubMed] [Google Scholar]
- 36.Anderson J.L., Mower C.P., Horne B.D. Carriage of the CYP2C19*2 allele increases one-year risk of myocardial infarction among recipients of drug-eluting stents treated with clopidogrel (abstr) J Am Coll Cardiol. 2009;53(suppl A):A27. [Google Scholar]
- 37.Worrall A., Armesilla A., Norell M. The presence of the CYP p450 C19*2 allele is associated with impaired response to clopidogrel as measured by the VerifyNow P2Y12 near-patient testing device in patients undergoing coronary angiography (abstr) Eur Heart J. 2009;30(suppl):327. [Google Scholar]
- 38.Sibbing D., Stegherr J., Latz W. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. 2009;30:916–922. doi: 10.1093/eurheartj/ehp041. [DOI] [PubMed] [Google Scholar]
- 39.Giusti B., Gori A.M., Marcucci R. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am J Cardiol. 2009;103:806–811. doi: 10.1016/j.amjcard.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto K., Hokimoto S., Chitose T. Impact of CYP2C19 polymorphism on residual platelet reactivity in patients with coronary heart disease during antiplatelet therapy. J Cardiol. 2011;57(2):194–201. doi: 10.1016/j.jjcc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Mega J.L., Close S.L., Wiviott S.D. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376(9749):1312–1319. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sofi F., Giusti B., Marcucci R., Gori A.M., Abbate R., Gensini G.F. Cytochrome P450 2C19*2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: a meta-analysis. Pharmacogenomics J. 2011;11(3):199–206. doi: 10.1038/tpj.2010.21. [DOI] [PubMed] [Google Scholar]
- 43.Jin B., Ni H.C., Shen W., Li J., Shi H.M., Li Y. Cytochrome P450 2C19 polymorphism is associated with poor clinical outcomes in coronary artery disease patients treated with clopidogrel. Mol Biol Rep. 2011;38(3):1697–1702. doi: 10.1007/s11033-010-0282-0. [DOI] [PubMed] [Google Scholar]
- 44.Hulot J.S., Collet J.P., Silvain J. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J Am Coll Cardiol. 2010;56(2):134–143. doi: 10.1016/j.jacc.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 45.Mega J.L., Simon T., Collet J.P. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iakovou I., Schmidt T., Bonizzoni E. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 47.Bonello L., Tantry U.S., Marcucci R., Working Group on High On-Treatment Platelet Reactivity Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010 Sep 14;56(12):919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 48.Price M.J., Berger P.B., Teirstein P.S., GRAVITAS Investigators Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011 Mar 16;305(11):1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 49.Trenk D., Stone G.W., Gawaz M. A Randomized Trial of Prasugrel Versus Clopidogrel in Patients With High Platelet Reactivity on Clopidogrel After Elective Percutaneous Coronary Intervention With Implantation of Drug-Eluting Stents: Results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) Study. J Am Coll Cardiol. 2012 Apr 9 doi: 10.1016/j.jacc.2012.02.026. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Snoep J.D., Hovens M.M., Eikenboom J.C., van der Bom J.G., Jukema J.W., Huisman M.V. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007 Aug;154(2):221–231. doi: 10.1016/j.ahj.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Rosemary J., Adithan C. The pharmacogenetics of CYP2C9 and CYP2C19: ethnic variation and clinical significance. Curr Clin Pharmacol. 2007 Jan;2(1):93–109. doi: 10.2174/157488407779422302. [DOI] [PubMed] [Google Scholar]
- 52.FDA Drug Safety Communication: reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm; Accessed 12.03.10.


