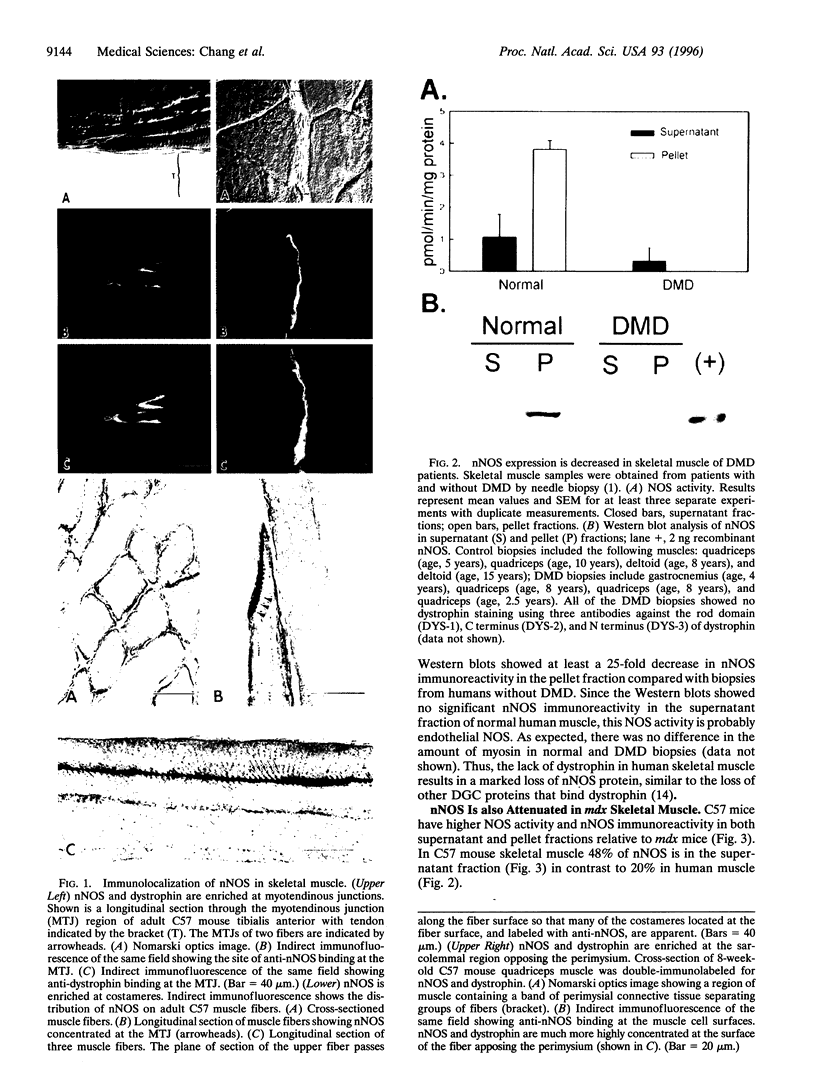
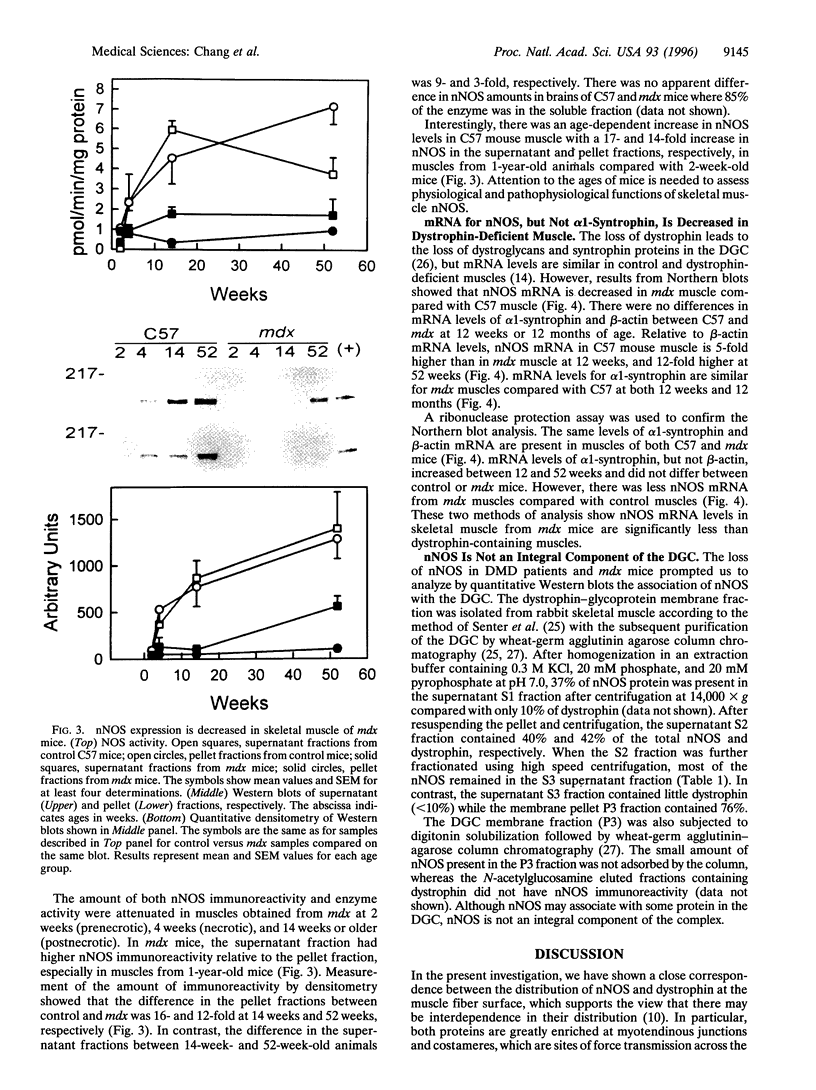
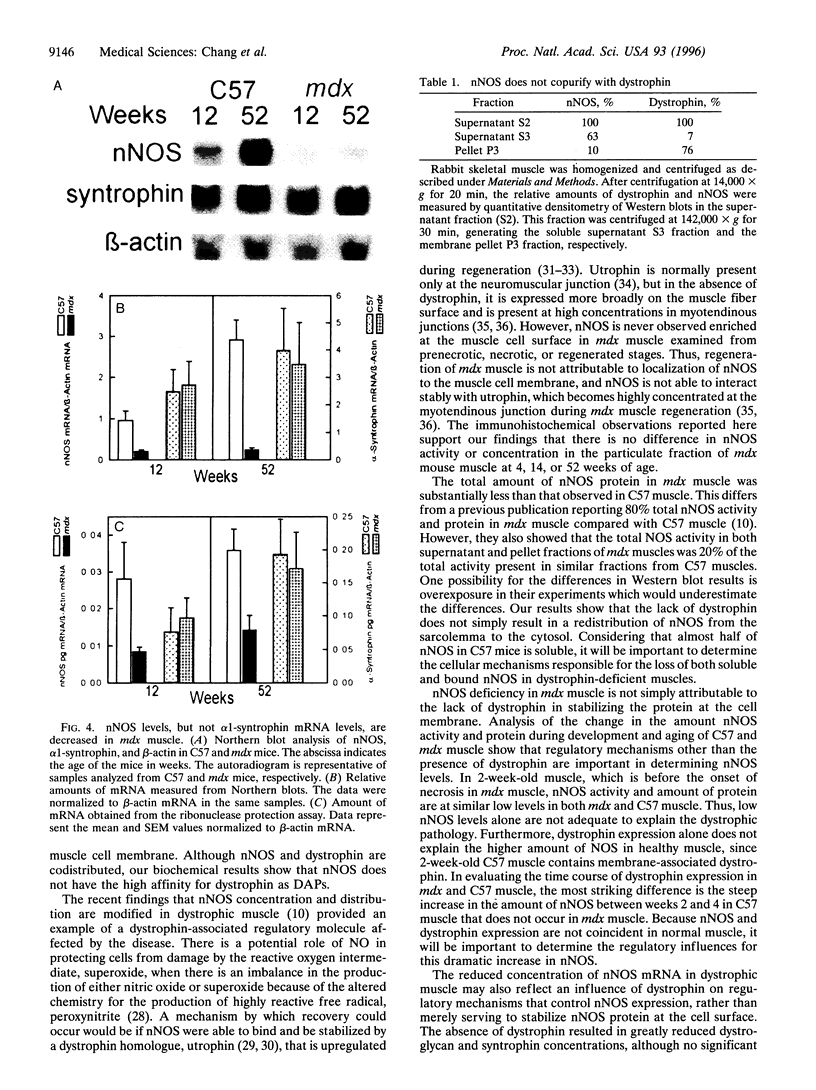
Abstract
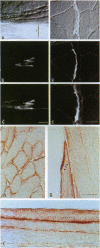
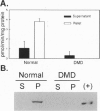
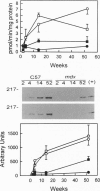
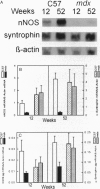
Neuronal nitric oxide synthase (nNOS) in fast-twitch skeletal muscle fibers is primarily particulate in contrast to its greater solubility in brain. Immunohistochemistry shows nNOS localized to the sarcolemma, with enrichment at force transmitting sites, the myotendinous junctions, and costameres. Because this distribution is similar to dystrophin, we determined if nNOS expression was affected by the loss of dystrophin. Significant nNOS immunoreactivity and enzyme activity was absent in skeletal muscle tissues from patients with Duchenne muscular dystrophy. Similarly, in dystrophin-deficient skeletal muscles from mdx mice both soluble and particulate nNOS was greatly reduced compared with C57 control mice. nNOS mRNA was also reduced in mdx muscle in contrast to mRNA levels for a dystrophin binding protein, alpha 1-syntrophin. nNOS levels increased dramatically from 2 to 52 weeks of age in C57 skeletal muscle, which may indicate a physiological role for NO in aging-related processes. Biochemical purification readily dissociates nNOS from the dystrophin-glycoprotein complex. Thus, nNOS is not an integral component of the dystrophin-glycoprotein complex and is not simply another dystrophin-associated protein since the expression of both nNOS mRNA and protein is affected by dystrophin expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman J. E., Chao D. S., Gee S. H., McGee A. W., Craven S. E., Santillano D. R., Wu Z., Huang F., Xia H., Peters M. F. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996 Mar 8;84(5):757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Brenman J. E., Chao D. S., Xia H., Aldape K., Bredt D. S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995 Sep 8;82(5):743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Bulfield G., Siller W. G., Wight P. A., Moore K. J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995 Mar 10;80(5):675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Cho K. O., Hunt C. A., Kennedy M. B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992 Nov;9(5):929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coulton G. R., Morgan J. E., Partridge T. A., Sloper J. C. The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol. 1988 Jan-Feb;14(1):53–70. doi: 10.1111/j.1365-2990.1988.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Jaros E. Ultrastructure of the skeletal muscle in the X chromosome-linked dystrophic (mdx) mouse. Comparison with Duchenne muscular dystrophy. Acta Neuropathol. 1988;77(1):69–81. doi: 10.1007/BF00688245. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Kahl S. D., Campbell K. P. Purification of dystrophin from skeletal muscle. J Biol Chem. 1991 May 15;266(14):9161–9165. [PubMed] [Google Scholar]
- Ervasti J. M., Ohlendieck K., Kahl S. D., Gaver M. G., Campbell K. P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990 May 24;345(6273):315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hutter O. F., Burton F. L., Bovell D. L. Mechanical properties of normal and mdx mouse sarcolemma: bearing on function of dystrophin. J Muscle Res Cell Motil. 1991 Dec;12(6):585–589. doi: 10.1007/BF01738447. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O., Ervasti J. M., Leveille C. J., Slaughter C. A., Sernett S. W., Campbell K. P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992 Feb 20;355(6362):696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Khurana T. S., Watkins S. C., Chafey P., Chelly J., Tomé F. M., Fardeau M., Kaplan J. C., Kunkel L. M. Immunolocalization and developmental expression of dystrophin related protein in skeletal muscle. Neuromuscul Disord. 1991;1(3):185–194. doi: 10.1016/0960-8966(91)90023-l. [DOI] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Law D. J., Allen D. L., Tidball J. G. Talin, vinculin and DRP (utrophin) concentrations are increased at mdx myotendinous junctions following onset of necrosis. J Cell Sci. 1994 Jun;107(Pt 6):1477–1483. doi: 10.1242/jcs.107.6.1477. [DOI] [PubMed] [Google Scholar]
- Love D. R., Hill D. F., Dickson G., Spurr N. K., Byth B. C., Marsden R. F., Walsh F. S., Edwards Y. H., Davies K. E. An autosomal transcript in skeletal muscle with homology to dystrophin. Nature. 1989 May 4;339(6219):55–58. doi: 10.1038/339055a0. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Campbell K. P. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994 Jan;17(1):2–15. doi: 10.1002/mus.880170103. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Ervasti J. M., Ohlendieck K., Kahl S. D., Campbell K. P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992 Dec 10;360(6404):588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- McMillan K., Bredt D. S., Hirsch D. J., Snyder S. H., Clark J. E., Masters B. S. Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11141–11145. doi: 10.1073/pnas.89.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan K., Masters B. S. Prokaryotic expression of the heme- and flavin-binding domains of rat neuronal nitric oxide synthase as distinct polypeptides: identification of the heme-binding proximal thiolate ligand as cysteine-415. Biochemistry. 1995 Mar 21;34(11):3686–3693. doi: 10.1021/bi00011a025. [DOI] [PubMed] [Google Scholar]
- Miles A. M., Bohle D. S., Glassbrenner P. A., Hansert B., Wink D. A., Grisham M. B. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J Biol Chem. 1996 Jan 5;271(1):40–47. doi: 10.1074/jbc.271.1.40. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Nonaka I., Hirai S., Ozawa E. Reciprocal expression of dystrophin and utrophin in muscles of Duchenne muscular dystrophy patients, female DMD-carriers and control subjects. J Neurol Sci. 1993 Oct;119(1):43–52. doi: 10.1016/0022-510x(93)90190-a. [DOI] [PubMed] [Google Scholar]
- Nguyen T. M., Ellis J. M., Love D. R., Davies K. E., Gatter K. C., Dickson G., Morris G. E. Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: presence at neuromuscular junctions, in the sarcolemma of dystrophic skeletal muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J Cell Biol. 1991 Dec;115(6):1695–1700. doi: 10.1083/jcb.115.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T., Yokoyama T., Fujisawa H., Kurashima Y., Esumi H. Structural diversity of neuronal nitric oxide synthase mRNA in the nervous system. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1014–1022. doi: 10.1006/bbrc.1993.1726. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Campbell K. P. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J Cell Biol. 1991 Dec;115(6):1685–1694. doi: 10.1083/jcb.115.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Matsumura K., Kahl S. D., Leveille C. J., Campbell K. P. Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron. 1991 Sep;7(3):499–508. doi: 10.1016/0896-6273(91)90301-f. [DOI] [PubMed] [Google Scholar]
- Pasternak C., Wong S., Elson E. L. Mechanical function of dystrophin in muscle cells. J Cell Biol. 1995 Feb;128(3):355–361. doi: 10.1083/jcb.128.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting C. P., Phillips C. DHR domains in syntrophins, neuronal NO synthases and other intracellular proteins. Trends Biochem Sci. 1995 Mar;20(3):102–103. doi: 10.1016/s0968-0004(00)88973-2. [DOI] [PubMed] [Google Scholar]
- Senter L., Ceoldo S., Petrusa M. M., Salviati G. Phosphorylation of dystrophin:effects on actin binding. Biochem Biophys Res Commun. 1995 Jan 5;206(1):57–63. doi: 10.1006/bbrc.1995.1009. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989 Jun 30;244(4912):1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Tidball J. G., Law D. J. Dystrophin is required for normal thin filament-membrane associations at myotendinous junctions. Am J Pathol. 1991 Jan;138(1):17–21. [PMC free article] [PubMed] [Google Scholar]
- Tinsley J. M., Blake D. J., Roche A., Fairbrother U., Riss J., Byth B. C., Knight A. E., Kendrick-Jones J., Suthers G. K., Love D. R. Primary structure of dystrophin-related protein. Nature. 1992 Dec 10;360(6404):591–593. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- Yang B., Ibraghimov-Beskrovnaya O., Moomaw C. R., Slaughter C. A., Campbell K. P. Heterogeneity of the 59-kDa dystrophin-associated protein revealed by cDNA cloning and expression. J Biol Chem. 1994 Feb 25;269(8):6040–6044. [PubMed] [Google Scholar]