Abstract
Objective
To describe treatment options for clinically localized prostate cancer: radical prostatectomy, prostate brachytherapy, external beam radiation, and active surveillance.
Quality of evidence
Prostate-specific antigen (PSA) outcomes presented are from non-randomized, cohort, and other comparisons trials (level II evidence). We describe PSA outcomes from Canadian centres when they are available. One small randomized controlled trial (level I evidence) in localized prostate cancer is available to compare radical prostatectomy with brachytherapy.
Main message
Treatment choice in prostate cancer is based on initial PSA level, clinical stage of disease, and Gleason score, together with baseline urinary function, comorbidities, and patient age. In this article, we describe patients’ eligibility for and the common side effects of all treatment options. Prostate brachytherapy and active surveillance have evolved as new standard treatments of localized prostate cancer. We give a brief overview of the brachytherapy procedure, side effects, and PSA outcomes across Canada, as well as active surveillance guidelines.
Conclusion
Prostate cancer treatment requires a multidisciplinary approach, with input from both urology and radiation oncology. Input from family physicians is often as important in helping guide patients through the treatment decision process.
Résumé
Objectif
Décrire les options de traitement du cancer de la prostate cliniquement localisé: la prostatectomie radicale, la curiethérapie de la prostate, la radiothérapie externe et la surveillance active.
Qualité des données
Les paramètres de l’antigène prostatique spécifique (APS) sont tirés d’études non randomisées, de cohortes et d’autres études comparatives (données probantes de niveau II). Nous décrivons les paramètres de l’APS provenant de centres canadiens lorsqu’ils sont disponibles. Il existe une petite étude randomisée contrôlée (données probantes de niveau I) sur le cancer localisé de la prostate, qui compare la prostatectomie radicale et la curiethérapie.
Message principal
Le choix du traitement du cancer de la prostate se fonde sur le niveau initial de l’APS, le stade clinique de la maladie et le score de Gleason, ainsi que sur la fonction urinaire de base, les maladies concomitantes et l’âge du patient. Dans cet article, nous décrivons les critères d’admissibilité des patients aux différentes options thérapeutiques ainsi que les effets secondaires communs. La curiethérapie de la prostate et la surveillance active sont devenues les nouveaux standards de traitement du cancer localisé de la prostate. Nous donnons un bref aperçu de la procédure de la curiethérapie, de ses effets secondaires et des résultats sur le plan de l’APS au Canada, ainsi que des lignes directrices à suivre pour la surveillance active.
Conclusion
Le traitement du cancer de la prostate exige une approche multidisciplinaire comportant l’intervention à la fois d’un urologue et d’un radio-oncologue. Les contributions d’un médecin de famille sont souvent importantes pour aider à orienter le patient dans le processus décisionnel de l’option thérapeutique.
The purpose of this article is to describe the treatment options available for men with early stage prostate cancer: radical prostatectomy (RP), prostate brachytherapy (PB), external beam radiation therapy (EBRT), and active surveillance (AS). Family physicians are often asked to participate in the discussion with prostate cancer patients regarding available treatment options. Herein, we provide an update for Canadian family physicians on all treatment options for localized prostate cancer, including PB and AS, 2 new standard treatment approaches. We provide eligibility criteria, treatment toxicity, and disease and quality-of-life (QOL) outcomes.
Treatment choice in prostate cancer is based on the well established prognostic factors: initial PSA level, clinical TNM (primary tumour, regional lymph nodes, and distant metastasis) stage, and Gleason score (GS), along with general considerations such as baseline urinary function, comorbidities, and age. Family physicians’ role in counseling patients with localized prostate cancer about treatment options has become increasingly difficult and complex.
Quality of evidence
Two recently published randomized controlled trials (RCTs) of prostate cancer screening1,2 have not only increased controversies surrounding prostate cancer screening, but have also increased demands3 for all radical treatment options. Unfortunately, there are very few randomized studies to compare treatments in localized prostate cancer.
There is only 1 RCT (level I evidence) in localized prostate cancer to compare 2 different treatment options—RP and PB—with each other.4 Level II evidence (non-randomized comparison and cohort studies) is available for all treatments. A large, recently published systematic review compares all treatment options in prostate cancer and provides some insights into the comparative effectiveness of prostate cancer treatments using current modern literature results (848 articles and more than 50 000 patients).5
Variations in outcomes in oncology between institutions are owing to differences in techniques, patient selection, and experience. We believe patients should be informed about expected outcomes of treatment based on the results at the institutions where they will be treated. Therefore, we have focused our outcomes review on disease outcomes from Canadian urology and radiation oncology centres when possible, as this might be more relevant to Canadian family physicians and their patients. In the following section, we describe each treatment option, eligibility criteria, common side effects, treatment outcomes, and QOL.
Main message
Radical prostatectomy
For 2 decades, RP has been the most commonly used treatment for healthy men younger than age 70 with localized prostate cancer. Use of PSA-based screening has increased the rate of organ-confined disease at the time of diagnosis, increasing the chances of successful outcomes after surgery. The best candidates for RP are those with organ-confined prostate cancer (low- or intermediate-risk disease, and selected patients with high-risk disease), younger than 70 years of age, who have a more than 10-year life expectancy and no or minimal comorbidities. In patients with pre-existing lower urinary tract symptoms due to benign prostatic hypertrophy, removal of the prostate often improves QOL. The complications of concern are incontinence and erectile dysfunction due to operative damage to the urinary sphincter and erectile nerves. In physician-reported series from single institutions, reported incontinence ranges from 0% to 12% at 6 to 24 months following surgery.6 Patient-reported data suggest that at 24 months, 42% of patients have some leakage, 7% have frequent leakage, and 2% have no urinary control.7 Minimally invasive surgical approaches, such as laparoscopic or robotic RP, offer potentially shorter recovery times but have not been shown to improve cancer control or side effects. Erectile function can be preserved in many men with normal preoperative function who undergo bilateral nerve-sparing RP. Surgical series report potency recovery in as much as 75% to 86% of men8,9; however, potency rates are 30% to 35% in surveys of tumour registry data.10,11 Return of sexual function after surgery is usually gradual and might take 12 to 24 months. Penile rehabilitation is becoming the standard of care after surgery.12 Long-term results of RP will depend on the skill and experience of the surgeon.13,14
Limited long-term results of RP in Canadian centres for patients with low- and intermediate-risk disease indicate 5-year PSA relapse-free survival (PRFS) of 65% to 92%15–18 and 10-year PRFS of 75%.16 A Canadian RCT of 3 versus 8 months of neoadjuvant hormones before prostatectomy was reported in abstract form only: 549 men were stratified by TNM stage, GS, and prebiopsy PSA level. Prostatectomy was completed in 502 men. After 7 years, overall, PSA relapse was 31.5% and not significantly different in the 2 treatment groups.19
Prostate brachytherapy
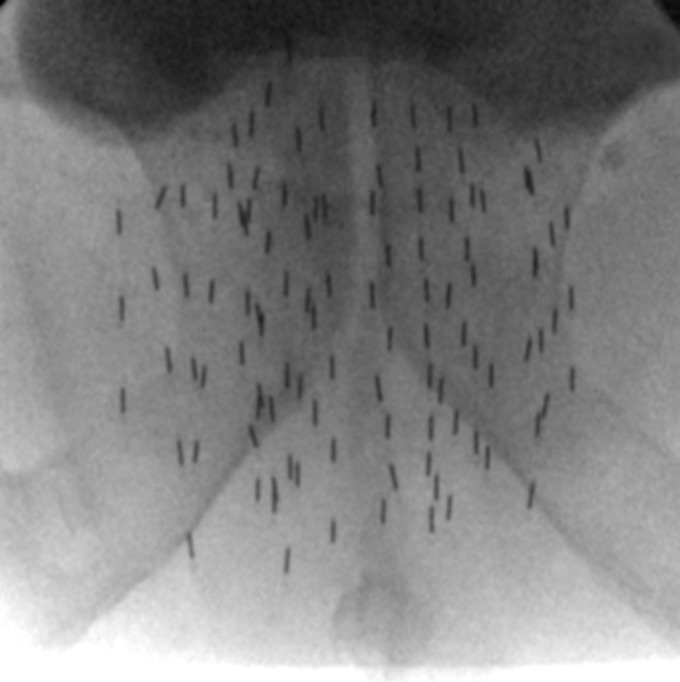
Prostate brachytherapy has acquired worldwide acceptance in the past 5 to 10 years. The term brachytherapy refers to the placement of radioactive sources inside or adjacent to a cancerous tumour. There are 2 forms of PB: low-dose-rate (LDR) brachytherapy where radioactive seeds are permanently implanted into the prostate (Figure 1) and high-dose-rate (HDR) brachytherapy where treatment is administered over about 10 minutes through temporary catheters that contain the radioactive sources. Low-dose-rate brachytherapy is most commonly used as monotherapy for patients with low- or intermediate-risk prostate cancer, or in combination with EBRT for patients with high-risk prostate cancer. High-dose-rate brachytherapy is usually used in combination with EBRT for intermediate- or high-risk disease. Both methods are emerging as the most effective radiation treatments of prostate cancer.5 Prostate brachytherapy is available in several centres in Canada (British Columbia, Alberta, Manitoba, Ontario, Quebec, and New Brunswick).
Figure 1.
Prostate brachytherapy seed implant as seen on fluoroscopy after the procedure
Eligibility for LDR brachytherapy differs among provinces. In all Canadian brachytherapy centres, patients with Canadian consensus criteria (Table 1)20–22 low-risk disease (clinical stage ≤ T2a, PSA level ≤ 10.0 ng/mL, or GS ≤ 6) are eligible for brachytherapy. In British Columbia, Manitoba, Quebec, and Alberta, intermediate-risk patients (clinical stage ≤ T2c, PSA level 10 to 20 ng/mL, or GS ≤ 7) are also eligible for PB. In Ontario, Quebec, and New Brunswick, HDR brachytherapy is used in conjunction with EBRT for high-risk patients. In British Columbia, where the HDR program has just begun, LDR brachytherapy is used in conjunction with EBRT for high-risk patients.
Table 1.
Canadian consensus definition of prostate cancer risk stratification
| RISK | DEFINITION |
|---|---|
| Low | ≤ T2a* and iPSA level ≤ 10 ng/mL and GS ≤ 6 |
| Intermediate | ≤ T2c* and iPSA level 10–20 ng/mL or GS ≤ 7 |
| High | ≥ T3a* or iPSA level ≥ 20 ng/mL or GS 8–10 |
GS—Gleason score, iPSA—initial prostate-specific antigen, PSA— prostate-specific antigen.
T1—tumour present, but not detectable clinically or with imaging (T1a—tumour was incidentally found in less than 5% of prostate tissue resected, T1b—tumour was incidentally found in greater than 5% of prostate tissue resected, T1c—tumour was found in a needle biopsy performed owing to an elevated serum PSA level); T2—the tumour can be palpated on examination, but has not spread outside the prostate (T2a—the tumour is in half or less than half of 1 of the prostate gland’s 2 lobes, T2b—the tumour is in more than half of 1 lobe, but not both, T2c—the tumour is in both lobes); T3—the tumour has spread through the prostatic capsule (T3a—the tumour has spread through the capsule on 1 or both sides, T3b—the tumour has invaded 1 or both seminal vesicles); T4—the tumour has invaded other nearby structures.
Low-dose-rate PB is a day-surgery procedure that takes about 1 hour. It is performed under general or spinal anesthesia (occasionally local). Patients are discharged home 2 to 3 hours after the procedure and resume normal activities with minimal recovery time (1 or 2 days). Between 70 and 150 radioactive seeds are implanted into the prostate through the perineum, using 20 to 30 needles, each carrying 2 to 7 seeds.
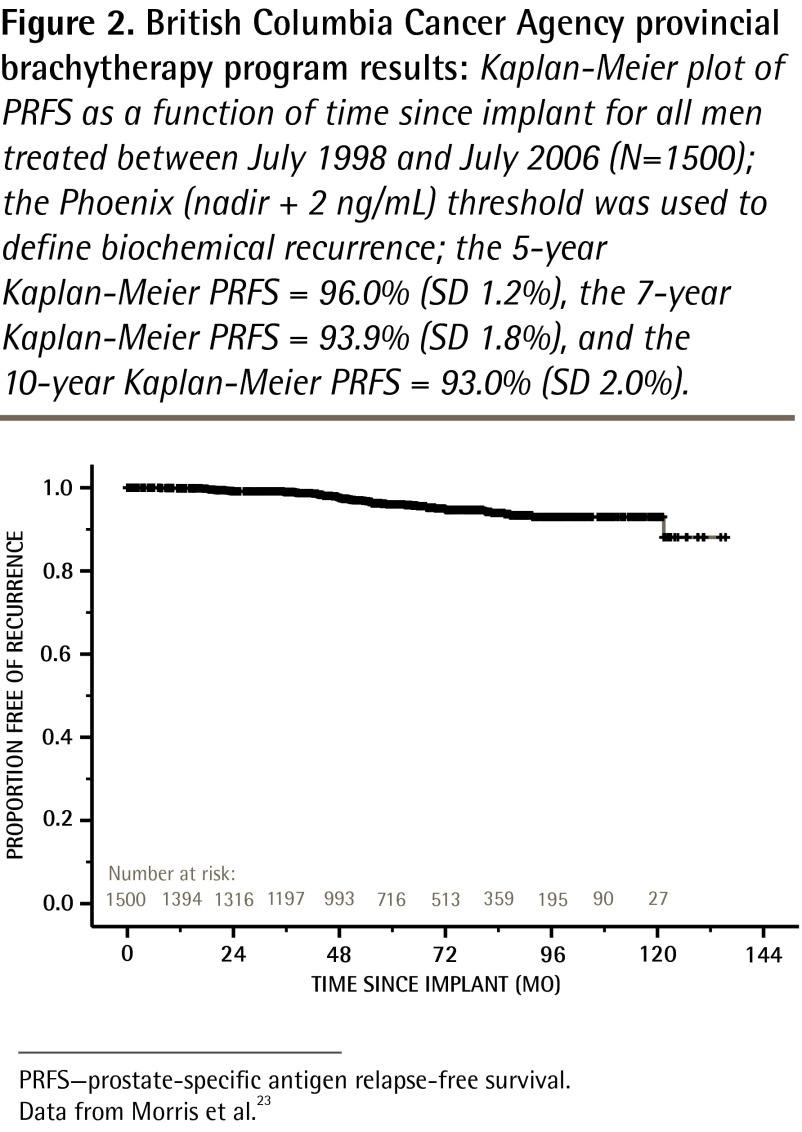
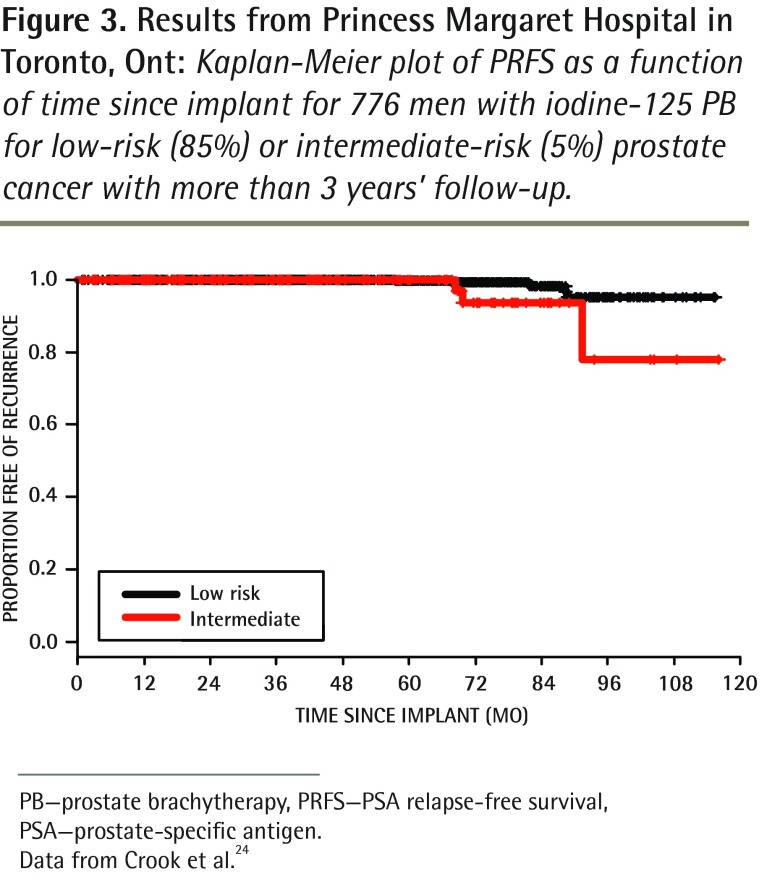
To our knowledge, more than 12 000 patients have been implanted so far in all Canadian centres. As is the case for surgery, long-term results depend on quality-assurance standards. A 5-year PRFS rate of 95.6% has been published for the first consecutive 1006 low- and intermediate-risk patients treated in British Columbia (Figure 2, updated 10-year results).23 In Ontario the reported actuarial 7-year PRFS rate was 95.2% for 776 men treated at Princess Margaret Hospital in Toronto for low-risk (85%) or intermediate-risk (5%) prostate cancer with a median follow-up of 54 months (Figure 3).24 Data from Quebec showed a 5-year PRFS rate of 90.5% for both low- and intermediate-risk groups in 1723 patients from Hôtel-Dieu de Québec of the Centre hospitalier universitaire de Québec.25,26
Figure 2.
British Columbia Cancer Agency provincial brachytherapy program results: Kaplan-Meier plot of PRFS as a function of time since implant for all men treated between July 1998 and July 2006 (N=1500); the Phoenix (nadir + 2 ng/mL) threshold was used to define biochemical recurrence; the 5-year Kaplan-Meier PRFS = 96.0% (SD 1.2%), the 7-year Kaplan-Meier PRFS = 93.9% (SD 1.8%), and the 10-year Kaplan-Meier PRFS = 93.0% (SD 2.0%).
PRFS—prostate-specific antigen relapse-free survival.
Data from Morris et al.23
Figure 3.
Results from Princess Margaret Hospital in Toronto, Ont: Kaplan-Meier plot of PRFS as a function of time since implant for 776 men with iodine-125 PB for low-risk (85%) or intermediate-risk (5%) prostate cancer with more than 3 years’ follow-up.
PB—prostate brachytherapy, PRFS—PSA relapse-free survival, PSA—prostate-specific antigen.
Data from Crook et al.24
Recovery time after the procedure is short; patients are able to resume usual daily activity within days. Most men experience moderate irritative and obstructive urinary symptoms lasting several months; more than 90% have minimal or no urinary symptoms in the long term.27–29 Approximately 5% to 10% of patients will experience urinary retention and require Foley catheters; the urinary retention usually resolves within a few days. Mild self-limiting rectal irritation affects 20% to 30% of patients. Rectal bleeding occurs in 2% to 7% of patients.30 Serious rectal injury requiring major surgical intervention such as colostomy is very rare (fewer than 1 of every 500 to 1000 patients). Erectile dysfunction rates after brachytherapy are favourable.10,31,32 As with surgery, younger patients and those with better pretreatment function are likely to have better outcomes.33,34 Oral cyclic guanosine monophosphate–specific phosphodiesterase inhibitors (sildenafil, vardenafil, and tadalafil) are helpful. A recent study from British Columbia of more than 1400 patients showed preserved erectile function rates 8 years after brachytherapy of 60% to 80% (in those aged younger than 60 years), 55% to 60% (in those aged 60 to 69 years), and 20% to 30% (in those older than 70 years).34 The Toronto experience in 1111 men showed that 82.8% of men retained satisfactory erectile function beyond 5 years.24,29,33 A recent JAMA publication reported erectile function outcomes after radical prostate cancer treatments in 1913 patients treated in 9 US university-affiliated hospitals.11 Reported rates of erectile function at 2 years after prostatectomy, brachytherapy, and EBRT were overall 35%, 58%, and 37%, respectively. Pretreatment sexual health-related QOL score, age, race or ethnicity, body mass index, and treatment were all independent predictors of functional erections 2 years after treatment. Overall, multivariable logistic regression models predicting erectile function estimated 2-year function probabilities from as low as 10% or less, to as high as 70% or greater depending on the individual’s pretreatment patient characteristics and the treatment received.11
External beam radiation therapy
External beam radiation therapy is a well established treatment for localized prostate cancer. In combination with androgen deprivation therapy (ADT), it is a standard approach for intermediate- and high-risk prostate cancer. Multiple RCTs (level I evidence) demonstrate a clinical benefit, including improvement in disease-free and overall survival, with the addition of ADT.35–37 Higher radiation doses, which can be delivered safely with technologic advances, are critical to achieving optimal tumour control.38 External beam radiation therapy is suitable for patients in all risk groups and, unlike PB, there are very few contraindications for EBRT, which can be offered to men for whom general or spinal anesthetic is not suitable, or those with various serious comorbidities, a large prostate size (greater than 70 g), or high-risk disease. In general, EBRT alone, or in combination with ADT in patients with low- and intermediate-risk prostate cancer, provides PRFS of 70% to 90% with 5-year follow-up and 50% to 70% with 10-year follow-up.5 While there are very few studies comparing the side effects of EBRT and PB, EBRT is generally associated with substantially fewer urinary side effects, but greater rectal toxicity.39 External beam radiation therapy is commonly delivered over a period of 7 to 8 weeks of daily treatments. Combination of EBRT and brachytherapy is considered to be more effective than EBRT alone in some intermediate-risk patients and in high-risk patients.5
A matched-pair analysis by Pickles et al from the BC Cancer Agency (level II evidence), showed that men treated with PB had superior PRFS outcomes when compared with conventional-dose EBRT. Seven-year PRFS rates were 95% for PB and 75% for EBRT.39 Unlike brachytherapy, patients treated with EBRT continue to experience more PSA failures with longer follow-up.5
Active surveillance
Active surveillance is a novel approach in which patients with minimal disease are closely followed, with treatment initiated only if the disease progresses. About 20% to 40% of prostate cancer detected by screening might not require any treatment, remaining asymptomatic until patients die of other causes.40 However, men under AS require careful follow-up so that curative treatment can be offered in a timely fashion should tumour progression occur. Patients suitable for AS include those with clinically localized prostate cancer, PSA levels less than 10 ng/mL, 2 or fewer positive biopsy cores, and GS of 6 or less. Rebiopsy within 6 to 12 months of the initial biopsy is mandatory in order to exclude more extensive or aggressive disease that might have been missed. Repeated periodic biopsy is also a part of regular follow-up.
The recently published Toronto experience with AS showed that out of 453 patients under AS, only 30% required and received treatment within 6.8 years of follow-up41; however, their outcome was remarkably unsuccessful, with a PRFS rate of less than 50% at 3 years. The risk of losing the opportunity for cure with this approach has not been fully quantified. Healthy young men can be more assured of a successful outcome if they are treated sooner rather than later. The ongoing anxiety of harbouring untreated cancer might be unacceptable to some patients. Active surveillance will become more attractive when accurate prediction of the biologic behaviour of individual cancers becomes possible using modern tissue and serum biomarkers. International studies are actively accruing patients using this approach and results will be available in several years.
Watchful waiting is distinct from AS in that it is reserved for patients who are elderly or who have considerable comorbidities. Other treatment options such as high-intensity focused ultrasound and cryotherapy are not considered standard approaches for early stage prostate cancer.42
Comparison between RP and PB (level I evidence)
One small Italian trial randomized 200 patients to receive RP or PB.4 With a median follow up of 5 years, PRFS was the same in both groups (91%). At 6 months and 1 year follow-up, brachytherapy patients had more irritative urinary symptoms but better sexual function. After 5 years, RP patients had a higher incidence of urinary stricture (6.5% vs 2%) and urinary incontinence (18% vs 0%). Sexual function recovery was greater with brachytherapy in the first year after treatment (60% vs 40%); however, it was virtually identical (65% vs 68%) after 5 years between the 2 arms. There was no difference in overall QOL measures between the 2 groups of patients.
Quality of life
The largest QOL study prospectively measured outcomes reported at multiple centres before and after RP, PB, and EBRT. The study included 1201 patients and 625 spouses.43 Each prostate cancer treatment was associated with a distinct pattern of change in QOL in the urinary, sexual, bowel, and hormonal domains. These changes influenced satisfaction with treatment outcomes among patients and their partners. Brachytherapy resulted in fewer effects on bowel and sexual function than surgery did; however, urinary irritation was more common than with surgery. In general, PB is associated with worse functional and symptom scores in the first year, but better scores and functional outcomes in subsequent years.44 Prospective QOL measures in 190 patients with a minimum 5 years of follow-up, who had undergone either RP or brachytherapy (patients’ choice),29 showed better urinary function, sexual function, higher overall QOL, and overall satisfaction with brachytherapy versus RP.
Role of family physicians
No one treatment of localized prostate cancer has proven to be clearly superior. For many patients, making the treatment decision can be challenging. Men are often required to process a large amount of previously unfamiliar information and assess the risks and benefits of various treatments while limiting the possibility of regretting the decision at a later date.45 Information given by urologists and radiation oncologists can be sometimes contradictory, confusing, and overwhelming. More so, such specialists are likely to recommend the treatment that they commonly provide. Patients are known to often rely on spouses, family members, friends, anecdotes, media, and the Internet to help make decisions.46
Previous studies have indicated that patients have poor knowledge and unrealistic expectations of treatment, and physician judgments concerning patient preferences are often inaccurate.46 A Queen’s University study of a cognitive-based decision aid for patients with early stage prostate cancer demonstrated wide variation in treatment attributes and issues important to patients during the decision-making process. The study also describes a high likelihood that the importance of various attributes and concerns is likely to change during the decision-making process.45 Many studies, including a recent RCT,47 have showed that the use of decision aids in early stage prostate cancer can increase overall knowledge, reduce patients’ anxiety, and increase their involvement in the decision-making process. As decision aids are not routinely used in clinical practice, various models for their dissemination and knowledge translation are being developed.46
Family physicians can provide further support to patients and their partners, when appropriate. They might further explore various treatment attributes and patient preferences regarding different treatment options, and at the same time, limit the cognitive burden. Focused discussion on the most important issues for each individual patient is likely to increase patient and partner involvement in the decision-making process. The Queen’s University Decision Aid in Early Stage Prostate Cancer computer program download might be useful.48
Conclusion
Decisions regarding the appropriate choice of treatment in men with localized prostate cancer require input from urology and radiation oncology. Discussion of the various options can be complex, and reaching a decision on how to proceed is sometimes difficult. Family physicians are often helpful in providing further information, and helping guide patients through the decision-making process.
Acknowledgments
We thank the Canadian Association of Radiation Oncology and the Canadian Brachytherapy Group for providing the information about Canadian brachytherapy programs. We also thank Dr Deb Feldman-Stewart and Dr Michael Brundage from the Division of Cancer Care and Epidemiology of the Queen’s University Cancer Research Institute for providing access to the Queen’s University Decision Aid in Early Stage Prostate Cancer computer program download.
EDITOR’S KEY POINTS
No one treatment of localized prostate cancer has proven to be clearly superior. For many patients, making the treatment decision can be challenging. Family physicians are often helpful in providing information and guiding patients through the decision-making process.
This article describes the treatment options available for men with early stage prostate cancer: radical prostatectomy, prostate brachytherapy, external beam radiation therapy, and active surveillance.
The use of decision aids in early stage prostate cancer can increase overall patient knowledge, reduce patients’ anxiety, and increase their involvement in the decision-making process, and physicians might find them useful when supporting patients who are facing decisions about early stage prostate cancer treatment.
POINTS DE REPÈRE DU RÉDACTEUR
Aucun traitement du cancer localisé de la prostate ne s’est à lui seul révélé clairement supérieur. Pour de nombreux patients, le choix du traitement peut être difficile. Les médecins de famille apportent souvent une aide précieuse dans la communication de renseignements et l’orientation des patients dans le processus décisionnel.
Cet article décrit les options de traitement à la disposition des hommes atteints d’un cancer de la prostate à un stade précoce: la prostatectomie radicale, la curiethérapie de la prostate, la radiothérapie de la prostate et la surveillance active.
Le recours à des aides à la décision à un stade précoce du cancer de la prostate peut accroître les connaissances générales du patient, réduire son anxiété et augmenter sa participation au processus décisionnel; les médecins peuvent les trouver utiles dans l’assistance aux patients aux prises avec une décision au sujet du traitement à choisir pour le cancer de la prostate à un stade précoce.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors contributed to the literature review and analysis, and to preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Woloshin S, Schwartz LM. Numbers needed to decide. J Natl Cancer Inst. 2009;101(17):1163–5. doi: 10.1093/jnci/djp263. [DOI] [PubMed] [Google Scholar]
- 4.Giberti C, Chiono L, Gallo F, Schenone M, Gastaldi E. Radical retropubic prostatectomy versus brachytherapy for low-risk prostatic cancer: a prospective study. World J Urol. 2009;27(5):607–12. doi: 10.1007/s00345-009-0418-9. [DOI] [PubMed] [Google Scholar]
- 5.Grimm P, Billiet I, Bostwick DG, Dicker AP, Frank SJ, Immerzeel J, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the prostate cancer results study group. BJU Int. 2012;109(Suppl 1):22–9. doi: 10.1111/j.1464-410X.2011.10827.x. [DOI] [PubMed] [Google Scholar]
- 6.Eastham JA, Kattan MW, Rogers E, Goad JR, Ohori M, Boone TB, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156(5):1707–13. [PubMed] [Google Scholar]
- 7.Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the prostate cancer outcomes study. JAMA. 2000;283(3):354–60. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 8.Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172(6 Pt 1):2227–31. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 9.Walsh PC, Marschke P, Ricker D, Burnett AL. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology. 2000;55(1):58–61. doi: 10.1016/s0090-4295(99)00397-0. [DOI] [PubMed] [Google Scholar]
- 10.Mettlin CJ, Murphy GP, Sylvester J, McKee RF, Morrow M, Winchester DP. Results of hospital cancer registry surveys by the American College of Surgeons: outcomes of prostate cancer treatment by radical prostatectomy. Cancer. 1997;80(9):1875–81. [PubMed] [Google Scholar]
- 11.Alemozaffar M, Regan MM, Cooperberg MR, Wei JT, Michalski JM, Sandler HM, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306(11):1205–14. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulhall JP, Bella AJ, Briganti A, McCullough A, Brock G. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med. 2010;7(4 Pt 2):1687–98. doi: 10.1111/j.1743-6109.2010.01804.x. [DOI] [PubMed] [Google Scholar]
- 13.Klein EA, Bianco FJ, Serio AM, Eastham JA, Kattan MW, Pontes JE, et al. Surgeon experience is strongly associated with biochemical recurrence after radical prostatectomy for all preoperative risk categories. J Urol. 2008;179(6):2212–7. doi: 10.1016/j.juro.2008.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianco FJ, Jr, Riedel ER, Begg CB, Kattan MW, Scardino PT. Variations among high volume surgeons in the rate of complications after radical prostatectomy: further evidence that technique matters. J Urol. 2005;173(6):2099–103. doi: 10.1097/01.ju.0000158163.21079.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkhateeb SS, Alibhai SM, Finelli A, Fleshner NE, Jewett MA, Zlotta AR, et al. Does nerve-sparing radical prostatectomy increase the risk of positive surgical margins and biochemical progression? Urol Ann. 2010;2(2):58–62. doi: 10.4103/0974-7796.65107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodde M, Harel F, Lacombe L, Fradet Y. Substratification of high-risk localised prostate cancer treated by radical prostatectomy. World J Urol. 2008;26(3):225–9. doi: 10.1007/s00345-008-0252-5. [DOI] [PubMed] [Google Scholar]
- 17.Klotz LH, Goldenberg SL, Jewett MA, Fradet Y, Nam R, Barkin J, et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. World J Urol. 2003;170(3):791–4. doi: 10.1097/01.ju.0000081404.98273.fd. [DOI] [PubMed] [Google Scholar]
- 18.Shariat SF, Walz J, Roehrborn CG, Zlotta AR, Perrotte P, Suardi N, et al. External validation of a biomarker-based preoperative nomogram predicts biochemical recurrence after radical prostatectomy. J Clin Oncol. 2008;26(9):1526–31. doi: 10.1200/JCO.2007.12.4669. [DOI] [PubMed] [Google Scholar]
- 19.Gleave ME, Goldenberg SL, Chin JL, Saad F, Klotz J, Jewett M, et al. CUOGP-95 randomized comparative study of 3 vs 8 months of neoadjuvant hormone therapy prior to prostatectomy 7 year PSA relapse free survival rate. Urology. 2009;181(Suppl 4):755. [Google Scholar]
- 20.Lukka H, Warde P, Pickles T, Morton G, Brundage M, Souhami L, et al. Controversies in prostate cancer radiotherapy: consensus development. Can J Urol. 2001;8(4):1314–22. [PubMed] [Google Scholar]
- 21.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, editors. AJCC cancer staging manual. 6th ed. New York, NY: Springer; 2002. [Google Scholar]
- 22.Union for International Cancer Control. TNM classification of malignant tumours. 6th ed. Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- 23.Morris WJ, Keyes M, Palma D, Spadinger I, McKenzie MR, Agranovich A, et al. Population-based study of biochemical and survival outcomes after permanent 125I brachytherapy for low- and intermediate-risk prostate cancer. Urology. 2009;73(4):860–7. doi: 10.1016/j.urology.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 24.Crook J, Borg J, Evans A, Toi A, Saibishkumar EP, Fung S, et al. 10-year experience with I-125 prostate brachytherapy at the Princess Margaret Hospital: results for 1,100 patients. Int J Radiat Oncol Biol Phys. 2011;80(5):1323–9. doi: 10.1016/j.ijrobp.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 25.Zebentout O, Apardian R, Beaulieu L, Harel F, Martin AG, Vigneault E. Clinical outcome of intermediate risk prostate cancer treated with iodine 125 monotherapy: the Hôtel-dieu of Quebec experience. Cancer Radiother. 2010;14(3):183–8. doi: 10.1016/j.canrad.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Martin AG, Roy J, Beaulieu L, Pouliot J, Harel F, Vigneault E. Permanent prostate implant using high activity seeds and inverse planning with fast simulated annealing algorithm: a 12-year Canadian experience. Int J Radiat Oncol Biol Phys. 2007;67(2):334–41. doi: 10.1016/j.ijrobp.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 27.Keyes M, Miller S, Moravan V, Pickles T, McKenzie M, Pai H, et al. Predictive factors for acute and late urinary toxicity after permanent prostate brachytherapy: long-term outcome in 712 consecutive patients. Int J Radiat Oncol Biol Phys. 2009;73(4):1023–32. doi: 10.1016/j.ijrobp.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Crook J, Fleshner N, Roberts C, Pond G. Long-term urinary sequelae following 125iodine prostate brachytherapy. J Urol. 2008;179(1):141–6. doi: 10.1016/j.juro.2007.08.136. [DOI] [PubMed] [Google Scholar]
- 29.Crook JM, Gomez-Iturriaga A, Wallace K, Ma C, Fung S, Alibhai S, et al. Comparison of health-related quality of life 5 years after SPIRIT: surgical prostatectomy versus interstitial radiation intervention trial. J Clin Oncol. 2011;29(4):362–8. doi: 10.1200/JCO.2010.31.7305. [DOI] [PubMed] [Google Scholar]
- 30.Keyes M, Spadinger I, Liu M, Pickles T, Pai H, Hayden A, et al. Rectal toxicity and rectal dosimetry in low-dose-rate iodine-125 permanent prostate implants: a long-term study in 1006 patients. Brachytherapy. 2012;11(3):199–208. doi: 10.1016/j.brachy.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Cesaretti JA, Kao J, Stone NN, Stock RG. Effect of low dose-rate prostate brachytherapy on the sexual health of men with optimal sexual function before treatment: analysis at > or = 7 years of follow-up. BJU Int. 2007;100(2):362–7. doi: 10.1111/j.1464-410X.2007.07016.x. [DOI] [PubMed] [Google Scholar]
- 32.Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys. 2002;54(4):1063–8. doi: 10.1016/s0360-3016(02)03030-4. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Iturriaga Pina A, Crook J, Borg J, Lockwood G, Fleshner N. Median 5 year follow-up of 125iodine brachytherapy as monotherapy in men aged < or = 55 years with favorable prostate cancer. Urology. 2010;75(6):1412–6. doi: 10.1016/j.urology.2009.04.101. [DOI] [PubMed] [Google Scholar]
- 34.Hayden AJ, Keyes M, Moravan V, McKenzie M, Pickles T. Erectile function following I125 permanent prostate brachytherapy: 5 and 8 years results in 1411 men. Radiother Oncol. 2010;96(Suppl 2):S3. [Google Scholar]
- 35.Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11(11):1066–73. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 36.Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 37.Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61(5):1285–90. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 38.Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Pickles T, Keyes M, Morris WJ. Brachytherapy or conformal external radiotherapy for prostate cancer: a single-institution matched-pair analysis. Int J Radiat Oncol Biol Phys. 2010;76(1):43–9. doi: 10.1016/j.ijrobp.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 40.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374–83. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 42.BCCA Prostate Brachytherapy Program–treatment policy and brachytherapy eligibility. Vancouver, BC: BC Cancer Agency; 2012. Available from: www.bccancer.bc.ca/HPI/CancerManagementGuidelines/Genitourinary/Prostate/Brachytherapy.htm. Accessed 2013 Oct 28. [Google Scholar]
- 43.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 44.Wyler SF, Engeler DS, Seelentag W, Ries G, Schmid HP. Health-related quality of life after radical prostatectomy and low-dose-rate brachytherapy for localized prostate cancer. Urol Int. 2009;82(1):17–23. doi: 10.1159/000176019. [DOI] [PubMed] [Google Scholar]
- 45.Feldman-Stewart D, Brundage MD, Van Manen L, Svenson O. Patient-focussed decision-making in early-stage prostate cancer: insights from a cognitively based decision aid. Health Expect. 2004;7(2):126–41. doi: 10.1111/j.1369-7625.2004.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin GA, Aaronson DS, Knight SJ, Carroll PR, Dudley RA. Patient decision aids for prostate cancer treatment: a systematic review of the literature. CA Cancer J Clin. 2009;59(6):379–90. doi: 10.3322/caac.20039. [DOI] [PubMed] [Google Scholar]
- 47.Feldman-Stewart D, Tong C, Siemens R, Alibhai S, Pickles T, Robinson JT, et al. The impact of explicit values clarification exercises in a patient decision aid emerges after the decision is actually made: evidence from a randomized controlled trial. Med Decis Making. 2012;32(4):616–26. doi: 10.1177/0272989X11434601. [DOI] [PubMed] [Google Scholar]
- 48.Decision aid computer program for early-stage prostate cancer patients. Kingston, ON: Queen’s University; www.queensu.ca/cce/resources/patienteducation/decisionaid.html. Accessed 2012 Aug 14. [Google Scholar]