Abstract
Objective
To evaluate the feasibility of a call-in centre to deliver colorectal cancer (CRC) screening in primary care through self-administered fecal occult blood testing (FOBT).
Design
Four-month intervention study (September 2010 to January 2011) with randomly selected follow-up interviews.
Setting
The family medicine clinics of 3 hospitals in Montreal, Que.
Participants
Letters from doctors invited their patients to contact the call-in centre (N = 761). Eligible patients agreeing to FOBT were sent testing kits that could be returned by mail (N = 100). Randomly selected patients (N = 36) were interviewed to explore the reasons why they did not contact the call-in centre, or why they did or did not adhere to FOBT.
Main outcome measures
Feasibility was assessed by the proportions of patients who contacted the call-in centre, who were eligible for FOBT, and who adhered to FOBT; and by the time between invitation mail-out and contact with the call-in centre, initial telephone contact and receipt of the signed consent form, and FOBT kit mail-out and receipt of the kit by the laboratory. Hierarchical logistic regression evaluated the effect of patient characteristics on feasibility indicators, adjusting for clustering by physician and centre.
Results
Of 761 patients (61.6% female, mean age 61.0 years), 250 (32.9%) contacted the call-in centre, of whom 100 (40.0%) were eligible for and consented to FOBT; 62 (62.0%) of these patients adhered to FOBT. Median (interquartile range) time from invitation mail-out to call-in centre contact was 21 (7 to 29) days, from initial telephone contact to receipt of the signed consent form was 24 (10 to 38) days, and from FOBT kit mail-out to receipt at the laboratory was 23 (18 to 32) days. With the exception of previous cancer diagnosis, patient characteristics were not associated with feasibility indicators. Of the 115 (46.0%) patients determined to be ineligible for FOBT screening, 111 (96.5%) were up to date with or already scheduled for screening.
Conclusion
Feasibility of the call-in centre was demonstrated. Targeting screening-eligible individuals or coupling a call-in service with another evidence-based CRC screening improvement strategy might further improve uptake of fecal testing.
Résumé
Objectif
Évaluer la possibilité d’utiliser un centre d’appel pour le dépistage du cancer colorectal (CCR) en contexte de soins primaires en utilisant un test auto-administré pour la recherche du sang occulte dans les selles (RSOS).
Type d’étude
Étude s’étendant sur 4 mois (de septembre 2010 à janvier 2011) et comprenant des entrevues de suivi auprès de patients choisis au hasard.
Contexte
Les cliniques de médecine familiale de 3 hôpitaux de Montréal, QC.
Participants
Les médecins ont adressé des lettres (N = 761) à leurs patients les invitant à contacter le centre d’appel. Aux patients admissibles (N = 100) qui étaient d’accord, on a envoyé des trousses pour la RSOS, lesquelles pouvaient être retournées par la poste. Des patients choisis au hasard (N = 36) ont été interviewés afin de connaître les raisons pour lesquelles ils n’avaient pas contacté le centre d’appel ou n’avaient pas accepté le test de RSOS.
Principaux paramètres à l’étude
On a évalué la faisabilité du projet à partir de la proportion de patients qui ont contacté le centre d’appel, de patients qui étaient admissibles à la RSOS et de patients qui ont accepté la RSOS; et à partir des délais entre l’envoi de l’invitation et le contact avec le centre d’appel, entre le contact téléphonique initial et la réception du formulaire de consentement signé, et entre l’envoi postal de la trousse de RSOS et sa réception par le laboratoire. L’influence des caractéristiques des patients sur les indicateurs de faisabilité a été évaluée par la régression logistique hiérarchique, après ajustement par segmentation pour les médecins et les centres.
Résultats
Sur 761 patients (dont 61,6 % de femmes âgées en moyenne de 61,0 ans), 250 (32,9 %) ont contacté le centre d’appel, dont 100 (40 %) étaient admissibles et acceptaient le test. Le temps médian (écart interquartile) était de 21 (7 – 29) jours entre l’envoi de l’invitation et le contact avec le centre d’appel, de 24 (10 – 38) jours entre le premier contact téléphonique et la réception de la formule de consentement signée, et de 23 (18 – 32) jours entre l’envoi de la trousse de RSOS et sa réception par le laboratoire. Il n’y avait pas d’association entre les indicateurs de faisabilité et les caractéristiques des patients, sauf pour ceux qui avaient déjà un diagnostic de cancer. Sur les 115 patients (46,0 %) jugés non admissibles au dépistage du CCR, 111 (96,5 %) étaient à jour ou avaient déjà un rendezvous pour le dépistage.
Conclusion
Cette étude confirme la possibilité d’utiliser un centre d’appel. On pourrait peut-être améliorer davantage l’efficacité du test de RSOS en ciblant les personnes admissibles au dépistage ou en couplant l’utilisation d’un centre d’appel à une autre stratégie de dépistage du CCR fondée sur des preuves.
Colorectal cancer (CRC) screening has advanced to the forefront of contemporary preventive health care and is recommended for all individuals aged 50 and older. Many countries and all Canadian provinces have either announced or started implementing organized CRC screening beginning with either fecal occult blood testing (FOBT) or fecal immunochemical testing.1–4 However, although CRC screening is cost-effective,5,6 less than 50% of eligible individuals are screened according to guidelines.7–9 Worldwide, FOBT is the most commonly used and widely available CRC screening method,10 and interest in strategies to increase uptake of FOBT is growing.
Telehealth is a novel approach to health services delivery that improves health care access, provides patient-centred care, and overcomes barriers to traditional health services delivery such as physicians having either inaccurate CRC screening information or insufficient consultation time.11,12 Telehealth can be delivered either as outreach (ie, call-centre staff contact the population) or as call-in (ie, the population contacts call-centre staff). Outreach trials demonstrate increased CRC screening participation by up to 10% without reminders and up to 21% with 2 reminders13–15; however, automated messages failed to improve CRC screening participation.16 Whereas outreach programs expend considerable resources on making the initial contact with potential participants, and might have limited sustainability, call-in centres reduce the burden of the initial contact and can be used to reach individuals in the community.17,18 To date, call-in centres have not been tested for their ability to increase FOBT adherence. Thus, before initiating a large-scale trial, we sought to determine the feasibility of a call-in centre to deliver FOBT screening to primary care patients.
METHODS
Recruitment
An intervention study was conducted between September 2010 and January 2011 in Montreal, Que, in the family medicine clinics of the McGill University Health Centre (Montreal General and Royal Victoria Hospitals), St Mary’s Hospital Centre, and the Sir Mortimer B. Davis Jewish General Hospital Herzl Family Practice Centre. Physician information sessions were held to introduce the study protocol and obtain approval to recruit patients aged 50 to 74 years who were members of the Quebec health insurance plan and who had visited the clinic within the past 3 years. A random sample of 40 patient names per physician was generated by MediVisit, the hospital appointment system database, and physicians were instructed to remove the names of individuals with history of CRC, those who were up to date with screening, and those who were deceased. The abbreviated lists were given to hospital personnel, and the final list of patient names and addresses was generated.
Invitation packages contained a personalized invitation letter signed by the treating physician, consent forms, and a prestamped envelope. The invitation letter provided a telephone number and instructions for contacting the call-in centre, and advised that patients would be screened for eligibility. Patients were informed that the call-in centre hours were Monday, Wednesday, and Friday from 9 am to 5 pm, and Tuesday and Thursday from 2 pm to 9 pm. The call-in centre was staffed by 1 trained research assistant and was based at the research office. Reminder postcards were sent 2 weeks after the initial mail-out to patients who had not contacted the centre. Written communication was in both English and French.
When a patient contacted the call-in centre, the bilingual research assistant used a standardized script to verify patient identity, discuss the importance of CRC screening, determine FOBT eligibility, and explain how to perform FOBT. Some patients mailed in consent forms as the initial contact and were telephoned. Ineligibility for FOBT included the following: history of CRC, colon polyps, inflammatory bowel disease, familial adenomatous polyposis, or hereditary nonpolyposis colon cancer; large-bowel symptoms in the previous month (rectal bleeding not due to hemorrhoids, unintentional weight loss, persistent change in bowel habits, or lower abdominal or rectal pain); and FOBT in the past 2 years, flexible sigmoidoscopy or double-contrast barium enema in the past 5 years, or colonoscopy in the past 10 years. Ineligible patients were encouraged to speak to their physicians about CRC screening. Eligible patients who agreed to FOBT testing were asked to complete a 5-minute survey about health and demographic characteristics, and to sign and mail consent forms to the research office. Patients were informed that we would mail FOBT kits only to those who returned signed consent forms, and that they were to mail completed FOBT kits to the laboratory for analysis. Patients had 2 months from mail-out of the FOBT kit to complete the test and send it to the laboratory. However, because of the 3-week turnaround, we extended this by 1 month. The laboratory sent FOBT results to treating physicians and the research team. Primary care physicians were responsible for contacting patients to follow up on positive FOBT results, and the study gastroenterologist agreed to provide colonoscopy to such patients within 2 months of receiving family physicians’ requests.
Structured interviews
To explore the reasons why some patients did not contact the call-in centre, why some did not adhere to FOBT, and why some did adhere to FOBT, 1 secretary from each family practice unit conducted telephone interviews with 4 randomly selected patients in each of the 3 categories using standardized scripts and questionnaires (total of 12 interviews per secretary). The secretaries recorded patient responses directly on the questionnaires and 2 members of the research team categorized the responses.
Statistical analysis
Feasibility of the call-in centre was determined by the following indicators: proportion of invited patients who contacted the call-in centre, proportion of patients who were eligible for FOBT from among those who contacted the call-in centre, and proportion of patients who adhered to FOBT from among those who were eligible and consented; and elapsed time between the invitation mail-out and call-in centre contact, elapsed time between call-in centre contact and receipt of consent forms, and elapsed time between FOBT kit mail-out to receipt of the completed test by the laboratory.
Associations between patient characteristics and participation rates were estimated using Bayesian hierarchical logistic regression modeling with 3 levels: patients (the first level), who were nested within physicians (the second level), who were in turn nested within institutions (the third level). Such models account for clustered data by allowing physician-specific intercepts to vary around an institution mean, while the institution-specific intercepts vary around an overall mean. Three distinct models were fitted for the probability of 1) participants making contact among all invitees, as a function of patient age (< 65 or ≥ 65 years) and sex; 2) participants being eligible among those who made contact, as a function of age and sex; and 3) participants completing FOBT among those who were eligible and who consented, as a function of age, sex, family history of cancer, diabetes, hypertension, and country of birth (Canada or outside Canada). Noninformative prior distributions were used throughout so that the data informed the final estimates. We estimated the model parameters via the Gibbs sampler using WinBUGS.
Ethics approval
Ethics approval for the study was obtained from the McGill University Faculty of Medicine Institutional Review Board and the local research ethics boards before study initiation.
RESULTS
Recruitment
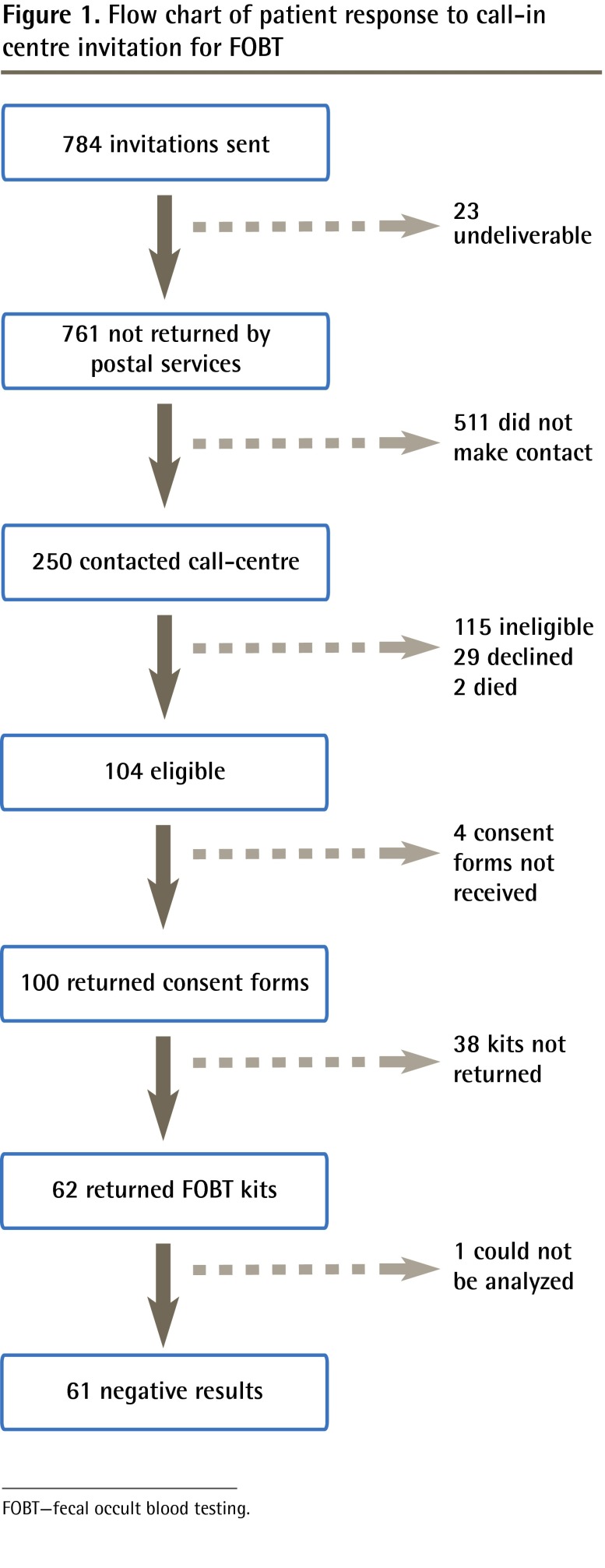
We approached 55 physicians for approval to recruit their patients; 42 (76.4%) agreed and signed invitation letters, 34 (61.8%) of whom actually referred patients. The remaining 8 (14.5%) did not refer patients. Each physician contributed between 5 and 40 patients (mean [standard deviation (SD)] of 23 [10.6] patients). A total of 784 invitation packages were mailed out, of which 23 (2.9%) were undeliverable and returned to the research office by postal services. Among the 761 patients whose invitation letters were not returned, the mean (SD) age was 61.0 (7.3) years, and 469 (61.6%) were female. Two-weeks after the invitation mail-out, reminder postcards were sent to 633 (83.2%) nonrespondents.
Call-in centre contact
Of the 761 patients, 250 (32.9%) contacted the call-in centre, 206 (82.4%) by telephone and 44 (17.6%) by mailing in the signed consent form (Figure 1).
Figure 1.
Flow chart of patient response to call-in centre invitation for FOBT
FOBT—fecal occult blood testing.
Eligibility for FOBT
Of the 250 patients who contacted the call-in centre, 100 (40.0%) were eligible for and consented to FOBT. Those eligible for FOBT were, on average, 59.5 years of age; 67.0% were female; 57.0% were born in Canada; 8.0% had history of cancer other than CRC; and 83.0% had close family members or friends diagnosed with some form of cancer (Table 1). Of the 115 patients determined to be ineligible for FOBT screening, 111 (96.5%) were up to date with or already scheduled for screening (Table 2), and 4 (3.5%) were ineligible for other reasons (ie, age, medical coverage, language [did not read English or French], polyp history). A remaining 29 (11.6%) patients declined to participate. On 2 occasions family members contacted the call-in centre to indicate that the patient was deceased.
Table 1.
Characteristics of patients who were eligible for and consented to FOBT screening: N = 100.
| CHARACTERISTIC | VALUE |
|---|---|
| Mean (SD) age, y | 59.5 (7.1) |
| Female, %* | 67.0 |
| Born in Canada, %* | 57.0 |
| Had diabetes, %* | 6.0 |
| Had hypertension, %* | 29.0 |
| Current or past diagnosis of cancer, %* | 8.0 |
| Family member or friend diagnosed with cancer, %* | 83.0 |
FOBT—fecal occult blood testing.
Numbers and percentages are the same, as there were 100 eligible patients.
Table 2.
Screening histories of patients ineligible for FOBT screening: N = 111.
| SCREENING HISTORY | N (%)* |
|---|---|
| FOBT in the past year | 24 (21.6) |
| DCBE in the past 5 years | 3 (2.7) |
| Flexible sigmoidoscopy in the past 5 years | 1 (0.9) |
| Colonoscopy in past 10 years | 83 (74.8) |
| Untested but have a test scheduled | 4 (3.6) |
DCBE—double-contrast barium enema, FOBT—fecal occult blood testing.
Does not total to 100%, as categories are not mutually exclusive.
Completed FOBT
Of the 100 patients who returned consent forms and were sent FOBT kits, 62 (62.0%) returned FOBT kits to the laboratory, 1 (1.6%) of which could not be analyzed. All analyzed FOBT results were negative.
Elapsed time
Median (interquartile range) time from the invitation mail-out to call-in centre contact was 21 (7 to 29) days, from initial telephone contact to receipt of the signed consent form was 24 (10 to 38) days, and from FOBT kit wail-out to receipt by the laboratory was 23 (18 to 32) days.
Hierarchical modeling results
We found little evidence of associations among patient characteristics and the 3 feasibility indicators (Table 3), but wide credible intervals did not rule out effects for most variables. The one exception was that among participants eligible for FOBT, those with previous cancer diagnoses (other than CRC) were less likely to complete FOBT (odds ratio 0.14, 95% Bayesian credible interval 0.01 to 0.96) compared with those without previous cancer diagnoses.
Table 3.
Regression model results for the effect of patient characteristics on feasibility of a call-in centre for FOBT
| MODEL | CHARACTERISTIC | AOR* | 95% CRI |
|---|---|---|---|
| Probability of participants contacting the call-in centre among all invitees (N = 761) | Age (≥ 65 y) | 1.14 | 0.82–1.57 |
| Sex (male) | 0.89 | 0.65–1.21 | |
| Probability of participants being eligible for FOBT among those who made contact (N = 250) | Age (≥ 65 y) | 0.57 | 0.31–1.05 |
| Sex (male) | 0.77 | 0.42–1.39 | |
| Probability of participants returning FOBT kits among those who were eligible and who consented (N = 100) | Age (≥ 65 y) | 3.60 | 0.94–17.60 |
| Sex (male) | 0.81 | 0.25–2.45 | |
| Family history of cancer | 1.77 | 0.43–7.68 | |
| Diabetes | 2.35 | 0.22–39.20 | |
| Hypertension | 1.50 | 0.39–6.63 | |
| Cancer diagnosis† | 0.14 | 0.01–0.96 | |
| Born in Canada | 0.82 | 0.26–2.41 |
AOR—adjusted odds ratios, CrI—Bayesian credible interval, FOBT—fecal occult blood testing.
Adjusted for all other variables in each model.
Other than colorectal cancer.
Interview findings
Among patients who did not contact the call-in centre, most expressed that receiving mailed letters from their doctors was a good way to let them know about a test that looked for potential disease. Time was the main reason given for not contacting the call-in centre; patients used words such as “busy,” “no time,” or “not a good time.” Lack of interest and previous CRC screening were also reasons for not participating. Among patients who were not adherent to FOBT, 7 reported “being busy,” that they “forgot,” they “lost [the] envelope,” or that it was “not a good time,” while 5 indicated the dietary and medication restrictions were too difficult to follow. Some patients indicated they might have adhered to FOBT had a reminder call been provided. Patients who did adhere to FOBT explained that they had “done it before,” that they had “health concerns or family history,” or that it was “easy to do.” Most were told by their family doctors to undergo CRC screening and expressed interest in knowing their FOBT results as well as the findings of the study.
DISCUSSION
The feasibility of delivering FOBT screening to primary care patients using a call-in centre model was demonstrated, as one-third of invitees contacted the call-in centre. Of those who made contact, 40.0% were eligible for and consented to FOBT, while most of those who were not eligible for FOBT were up to date with screening. Furthermore, 62.0% of those who consented also adhered to FOBT. Only 1 (1.6%) of the 62 completed FOBT kits could not be analyzed, a low rejection rate that could be attributed to the revisions our team made to the written FOBT kit instructions. Results for all of the 61 completed, analyzable FOBT kits were negative, a finding that is within 2 SDs of the expected number based on a positivity rate of 3%.19 Understanding the 3-week turnaround times between mail-out and call-in centre contact, call-in centre contact and receipt of consent forms, and FOBT kit mail-out and receipt at the laboratory might be helpful to the planning of future studies. These findings have implications for future fecal test–based screening improvement interventions. First, based on our review of novel FOBT interventions that showed that single interventions improved FOBT rates by up to 17%,20–23 while multimodal interventions increased FOBT rates by as much as 40%,24–27 a call-in centre could be included as part of a multicomponent approach to increasing fecal test–based screening. Second, having access to a registry of completed colonoscopy and FOBT results might help physicians determine when their patients become eligible for screening28; despite most physicians having reviewed their patient medical files, many were unable to determine that their patients were up to date with screening.
Our findings were consistent with those of previous telehealth intervention research that showed improved cancer screening rates.13–15 Dietrich et al showed an increase in the proportion of patients who underwent CRC screening, from 39% to 63%, with an average of 4 reminder calls.14 In a predominantly black population, telephone outreach delivered CRC screening to 27.0% of intervention participants compared with only 6.1% of controls.13
Using hierarchical regression, little evidence was found for associations among patient characteristics and feasibility indicators. The exception was having a previous cancer diagnosis, which was associated with decreased FOBT adherence, possibly owing to the psychological burden of detecting another cancer. This finding might have been affected by unmeasured confounding. Moreover, the wide credible intervals around some of the odds ratios for other patient characteristics suggest a lack of power to detect differences.
Semistructured interviews were conducted to gain insight into reasons for participation and nonparticipation. Lack of time and forgetfulness were the main reasons patients did not contact the call-in centre and did not adhere to FOBT. Others have shown that repeated reminder calls boost FOBT rates by 18.3%.21 Facilitators to FOBT adherence included family history of CRC, pervious FOBT screening, ease of doing the test, physician recommendation, and health concerns. As one of the main barriers to FOBT compliance is collection of stool samples, those with, as opposed to those without, previous FOBT experience would be more likely to participate.29 Most patients who adhered to FOBT had received recommendations from their family doctors to undergo CRC screening, mirroring the findings of others that there is an association between receipt of physician CRC screening referral and increased use of screening.30–33
Strengths and limitations
Consideration must be given to the study limitations and strengths. First, generalizability might be limited, as our results might not be applicable to individuals without primary care physicians, universal health insurance coverage, mailing addresses, or telephones, or to those who are not fluent in English or French. Second, selection bias was possible because the decision about who to invite to participate in the study was left to some degree to the physicians’ discretion. Physicians might have selected patients who were likely to adhere to or benefit from screening. Third, given that many patients who contacted the centre were up to date with screening, and that many of those who participated had family members or friends who had had cancer, health-conscious individuals might have been more likely to participate. We could not assess the influence of patient characteristics on call-in contact and FOBT eligibility because detailed information was available only for those patients eligible for FOBT. Nevertheless, our telehealth intervention systematically delivered CRC screening to active clinic patients. Our use of quantitative methods and structured interviews helped us to estimate the expected participation rates in a future study and to understand reasons for nonparticipation. Finally, with more than 4.4 million Canadians lacking family physicians,34 a call-in centre could be used to reach individuals in this population.
Conclusion
A call-in centre service appears to be feasible for delivering FOBT screening to primary care patients. Given that nearly half of patients contacting the call-in centre were up to date with CRC screening, having access to a registry of past colonoscopy and FOBT examination results might help physicians make better CRC screening decisions at the point of care. Moreover, in that multimodal interventions have been shown to improve FOBT screening uptake more than single-intervention approaches, combining a call-in centre with another evidence-based improvement strategy might further increase participation in CRC screening.
EDITOR’S KEY POINTS
Although colorectal cancer (CRC) screening has been shown to be cost-effective, less than 50% of eligible individuals are screened according to guidelines. Telehealth is a novel approach to health services delivery that improves health care access, provides patient-centred care, and overcomes barriers to traditional health services delivery. This study aimed to evaluate the feasibility of a call-in centre to deliver CRC screening in primary care.
A third of the patients invited to contact the call-in centre did so. Of those, 40.0% were eligible for and consented to screening; 62.0% of these individuals completed screening (8.1% of the total sample). Almost all of the patients who contacted the call-in centre and were ineligible for screening were up to date with or already scheduled for screening. In follow-up telephone interviews, lack of time and forgetting were the most common reasons given for not contacting the call-in centre or for not completing screening.
This study demonstrated that a call-in centre model is feasible for delivering CRC screening. However, given that many of those who contacted the centre were up to date with screening, and that many who completed screening had family members or friends who had had cancer, it is possible more health-conscious individuals are more likely to participate in such programs.
POINTS DE REPÈRE DU RÉDACTEUR
Même s’il est établi que le dépistage du cancer colorectal (CCR) est efficace par rapport à son coût, moins de 50 % des sujets admissibles sont évalués conformément aux directives de pratique. Télésanté est une nouvelle façon de dispenser des services de santé qui facilite l’accès aux soins, dispense des soins centrés sur le patient et permet de contourner les obstacles qui gênent la dispensation des services de santé traditionnels. Cette étude avait pour but de déterminer si l’on pouvait utiliser un centre d’appel pour faciliter le dépistage du CCR en contexte de soins primaires.
Un tiers des patients invités ont contacté le centre d’appel. Parmi eux, 40 % étaient admissibles au dépistage et y ont consenti; 62,0 % ont complété le dépistage (8,1 % de l’échantillon total). Presque tous ceux qui ont contacté le centre d’appel et qui n‘étaient pas admissibles étaient à jour ou avaient un rendez-vous pour un dépistage. Lors des entrevues téléphoniques de suivi, les raisons les plus souvent invoquées pour n’avoir pas contacté le centre d’appel ou pour n’avoir pas complété le dépistage étaient les contraintes de temps et l’oubli.
Cette étude a démontré qu’il était possible d’utiliser un centre d’appel pour promouvoir le dépistage du CCR. Toutefois, étant donné que plusieurs de ceux qui ont contacté le centre étaient déjà à jour dans leur dépistage et que plusieurs de ceux qui ont complété le dépistage avaient des amis ou des membres de leurs familles qui avaient eu un cancer, il est possible que les personnes les plus préoccupées de leur santé soient plus susceptibles de participer à de tels programmes.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
Drs Sewitch, Joseph, and Barkun contributed to the concept and design of the study. Drs Grad, Yaffe, Pavilanis, and Roper contributed to acquisition of data. Drs Sewitch, Grad, and Joseph and Ms Jiang contributed to the analysis and interpretation of data. This article was drafted and revised critically for important intellectual content by all authors. All authors gave final approval of the version to be published.
Competing interests
None declared
References
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics . Canadian Cancer Statistics 2011. Toronto, ON: Canadian Cancer Society; 2011. [Google Scholar]
- 2.Telford JJ. Canadian guidelines for colorectal cancer screening. Can J Gastroenterol. 2011;25(9):479–81. doi: 10.1155/2011/285926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Cancer Screening Network . Inventory of colorectal cancer screening activities in ICSN countries, May 2008. Bethesda, MD: National Cancer Institute; 2008. Available from: http://appliedresearch.cancer.gov/icsn/colorectal/screening.html. Accessed 2013 Nov 15. [Google Scholar]
- 4.Choi KS, Jun JK, Lee HY, Hahm MI, Oh JH, Park EC. Increasing uptake of colorectal cancer screening in Korea: a population-based study. BMC Public Health. 2010;10:265. doi: 10.1186/1471-2458-10-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heitman SJ, Manns BJ, Hilsden RJ, Fong A, Dean S, Romagnuolo J. Cost-effectiveness of computerized tomographic colonography versus colonoscopy for colorectal cancer screening. CMAJ. 2005;173(8):877–81. doi: 10.1503/cmaj.050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Colorectal cancer screening: health impact and cost effectiveness. Am J Prev Med. 2006;31(1):80–9. doi: 10.1016/j.amepre.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Sewitch MJ, Fournier C, Ciampi A, Dyachenko A. Colorectal cancer screening in Canada: results of a national survey. Chronic Dis Can. 2008;29(1):9–21. [PubMed] [Google Scholar]
- 8.Colorectal Cancer Association of Canada [website] Majority of Canadians surveyed not checking for colon cancer. Toronto, ON: Colorectal Cancer Association of Canada; 2010. Available from: www.colorectal-cancer.ca/en/news-and-resources/majority-cancer. Accessed 2013 Nov 15. [Google Scholar]
- 9.Zarychanski R, Chen Y, Bernstein CN, Hebert PC. Frequency of colorectal cancer screening and the impact of family physicians on screening behaviour. CMAJ. 2007;177(6):593–7. doi: 10.1503/cmaj.070558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson VS, Patnick J, Davies AK, Nadel MR, Smith RA, Atkin WS, et al. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122(6):1357–67. doi: 10.1002/ijc.23273. [DOI] [PubMed] [Google Scholar]
- 11.Sewitch MJ, Burtin P, Dawes M, Yaffe M, Snell L, Roper M, et al. Colorectal cancer screening: physicians’ knowledge of risk assessment and guidelines, practice, and description of barriers and facilitators. Can J Gastroenterol. 2006;20(11):713–8. doi: 10.1155/2006/609746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klabunde CN, Lanier D, Breslau ES, Zapka JG, Fletcher RH, Ransohoff DF, et al. Improving colorectal cancer screening in primary care practice: innovative strategies and future directions. J Gen Intern Med. 2007;22(8):1195–205. doi: 10.1007/s11606-007-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basch CE, Wolf RL, Brouse CH, Shmukler C, Neugut A, DeCarlo LT, et al. Telephone outreach to increase colorectal cancer screening in an urban minority population. Am J Public Health. 2006;96(12):2246–53. doi: 10.2105/AJPH.2005.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich AJ, Tobin JN, Cassells A, Robinson CM, Greene MA, Sox CH, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Ann Intern Med. 2006;144(8):563–71. doi: 10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgibbon ML, Ferreira MR, Dolan NC, Davis TC, Rademaker AW, Wolf MS, et al. Process evaluation in an intervention designed to improve rates of colorectal cancer screening in a VA medical center. Health Promot Pract. 2007;8(3):273–81. doi: 10.1177/1524839907302210. [DOI] [PubMed] [Google Scholar]
- 16.Simon SR, Zhang F, Soumerai SB, Ensroth A, Bernstein L, Fletcher RH, et al. Failure of automated telephone outreach with speech recognition to improve colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2010;170(3):264–70. doi: 10.1001/archinternmed.2009.522. [DOI] [PubMed] [Google Scholar]
- 17.Bellavance M, Beland MJ, van Doesburg NH, Paquet M, Ducharme FM, Cloutier A. Implanting telehealth network for paediatric cardiology: learning from the Quebec experience. Cardiol Young. 2004;14(6):608–14. doi: 10.1017/S1047951104006055. [DOI] [PubMed] [Google Scholar]
- 18.Bunik M, Glazner JE, Chandramouli V, Emsermann CB, Hegarty T, Kempe A. Pediatric telephone call centers: how do they affect health care use and costs? Pediatrics. 2007;119(2):e305–13. doi: 10.1542/peds.2006-1511. [DOI] [PubMed] [Google Scholar]
- 19.Hol L, van Leerdam ME, van Ballegooijen M, van Vuuren AJ, van Dekken H, Reijerink JC, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut. 2010;59(1):62–8. doi: 10.1136/gut.2009.177089. [DOI] [PubMed] [Google Scholar]
- 20.Stokamer CL, Tenner CT, Chaudhuri J, Vazquez E, Bini EJ. Randomized controlled trial of the impact of intensive patient education on compliance with fecal occult blood testing. J Gen Intern Med. 2005;20(3):278–82. doi: 10.1111/j.1525-1497.2005.40023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosen DM, Feldstein AC, Perrin N, Rosales AG, Smith DH, Liles EG, et al. Automated telephone calls improved completion of fecal occult blood testing. Med Care. 2010;48(7):604–10. doi: 10.1097/MLR.0b013e3181dbdce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JK, Reis V, Liu S, Conn L, Groessl EJ, Ganiats TG, et al. Improving fecal occult blood testing compliance using a mailed educational reminder. J Gen Intern Med. 2009;24(11):1192–7. doi: 10.1007/s11606-009-1087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus AC, Mason M, Wolfe P, Rimer BK, Lipkus I, Strecher V, et al. The efficacy of tailored print materials in promoting colorectal cancer screening: results from a randomized trial involving callers to the National Cancer Institute’s Cancer Information Service. J Health Commun. 2005;10(Suppl 1):83–104. doi: 10.1080/10810730500257754. [DOI] [PubMed] [Google Scholar]
- 24.Potter MB, Phengrasamy L, Hudes ES, McPhee SJ, Walsh JM. Offering annual fecal occult blood tests at annual flu shot clinics increases colorectal cancer screening rates. Ann Fam Med. 2009;7(1):17–23. doi: 10.1370/afm.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh JM, Salazar R, Nguyen TT, Kaplan C, Nguyen LK, Hwang J, et al. Healthy colon, healthy life: a novel colorectal cancer screening intervention. Am J Prev Med. 2010;39(1):1–14. doi: 10.1016/j.amepre.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu SP, Taylor V, Yasui Y, Chun A, Yip MP, Acorda E, et al. Promoting culturally appropriate colorectal cancer screening through a health educator: a randomized controlled trial. Cancer. 2006;107(5):959–66. doi: 10.1002/cncr.22091. [DOI] [PubMed] [Google Scholar]
- 27.Potter MB, Walsh JM, Yu TM, Gildengorin G, Green LW, McPhee SJ. The effectiveness of the FLU-FOBT program in primary care: a randomized trial. Am J Prev Med. 2011;41(1):9–16. doi: 10.1016/j.amepre.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Sewitch MJ, Barkun AN. Fighting colorectal cancer with information technology. CMAJ. 2011;183(9):1053–4. doi: 10.1503/cmaj.111-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahlquist DA. Occult blood screening. Obstacles to effectiveness. Cancer. 1992;70(5 Suppl):1259–65. doi: 10.1002/1097-0142(19920901)70:3+<1259::aid-cncr2820701511>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Zapka JG, Puleo E, Vickers-Lahti M, Luckmann R. Healthcare system factors and colorectal cancer screening. Am J Prev Med. 2002;23(1):28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 31.Madlensky L, Esplen MJ, Gallinger S, McLaughlin JR, Goel V. Relatives of colorectal cancer patients: factors associated with screening behavior. Am J Prev Med. 2003;25(3):187–94. doi: 10.1016/s0749-3797(03)00202-2. [DOI] [PubMed] [Google Scholar]
- 32.Brenes GA, Paskett ED. Predictors of stage of adoption for colorectal cancer screening. Prev Med. 2000;31(4):410–6. doi: 10.1006/pmed.2000.0729. [DOI] [PubMed] [Google Scholar]
- 33.Manne S, Markowitz A, Winawer S, Guillem J, Meropol NJ, Haller D, et al. Understanding intention to undergo colonoscopy among intermediate-risk siblings of colorectal cancer patients: a test of a mediational model. Prev Med. 2003;36(1):71–84. doi: 10.1006/pmed.2002.1122. [DOI] [PubMed] [Google Scholar]
- 34.Statistics Canada. Health Fact Sheets. Ottawa, ON: Statistics Canada; 2011. Access to a regular medical doctor, 2010. [Google Scholar]