Abstract
Objective
To determine the proportion of patients with fragility fractures who can be expected to have low bone mineral density (BMD) at the time of fracture and to assist FPs in deciding whether to refer patients for BMD testing.
Data sources
MEDLINE, EMBASE, and CINAHL were searched from the earliest available dates through September 2009.
Study selection
English-language articles reporting BMD test results of patients with fragility fractures who were managed in an orthopedic environment (eg, fracture clinic, emergency management by orthopedic surgeons, inpatients) were eligible for review. While the orthopedic environment has been identified as an ideal point for case finding, FPs are often responsible for investigation and treatment. Factors that potentially influenced BMD test results (eg, selection of fracture types, exclusion criteria) were identified. Studies with 2 or more selection factors of potential influence were flagged, and rates of low BMD were calculated including and excluding these studies.
Synthesis
The distribution of the proportion of persons with low BMD was summarized across studies using descriptive statistics. We calculated lower boundaries on this distribution, using standard statistical thresholds, to determine a lower threshold of the expected rate of low BMD.
Conclusion
Family physicians evaluating patients with fragility fractures can expect that at least two-thirds of patients with fragility fractures who are older than 50 years of age will have low BMD (T score ≤ −1.0). With this a priori expectation, FPs might more readily conduct a fracture risk assessment and pursue warranted fracture risk reduction strategies following fragility fracture.
Résumé
Objectif
Déterminer la proportion des patients présentant une fracture de fragilité qui risquent d’avoir déjà une densitométrie osseuse (DMO) basse au moment de la fracture et aider le MF à décider quand les diriger vers une ostéodensitométrie.
Source des données
On a consulté MEDLINE, EMBASE et CINAHL à partir des plus anciennes dates disponibles jusqu’à novembre 2009.
Choix des études
Les articles de langue anglaise rapportant les résultats des tests de DMO chez des patients traités en milieu orthopédique pour des fractures de fragilité (p. ex. clinique de fracture, traitement d’urgence par un chirurgien orthopédiste, patients hospitalisés) ont été retenus et révisés. Même si le milieu orthopédique est un endroit idéal pour repérer ce type de cas, les MF sont souvent responsables de l’investigation et du traitement. On a identifié des facteurs qui peuvent influer sur les résultats des tests de DMO (p. ex. le choix des types de fracture, les critères d’exclusion). Pour les études qui avaient au moins 2 facteurs de sélection susceptibles d’avoir une influence, on a calculé les taux de basse DMO en incluant et en excluant ces études.
Synthèse
On a utilisé des statistiques descriptives pour établir, pour l’ensemble des études, la distribution de la proportion des sujets ayant une DMO basse. Les limites inférieures de cette distribution ont été calculées à l’aide de seuils statistiques standards afin de déterminer un seuil inférieur pour le taux de DMO bas attendu.
Conclusion
Le médecin de famille qui évalue des patients de plus de 50 ans qui ont une fracture de fragilité peut s’attendre à ce qu’au moins les deux tiers d’entre eux aient une DMO basse (score de T ≤ −1,0). Compte tenu d’une telle perspective, le MF devrait pouvoir mieux évaluer le risque de nouvelles fractures et instaurer des stratégies reconnues pour en réduire l’incidence.
A person sustaining a low-trauma or fragility fracture, resulting from a fall from standing height or less,1 has a 1.5- to 9.5-fold increased risk of further fracture, including a life-threatening hip fracture.2–4 Bone-sparing medications reduce the risk of subsequent fracture by 30% to 50%5–7; however, uptake of testing or treatment initiation for low bone mass after a fragility fracture is as low as 20%.8–10 This persistent gap between knowledge and action8–10 has spurred numerous efforts to improve rates of investigation, fracture risk assessment, and care to reduce the risk of subsequent fracture, particularly hip fracture.11–14
While patients with fragility fractures are often first seen in an orthopedic environment, responsibility for assessment of future fracture risk, referral for investigation, initiation of treatment, and management of underlying chronic disease often falls to FPs.15 To determine a patient’s risk of future fracture, and future hip fracture, a fracture risk assessment should be conducted using a tool such as FRAX16 or the CAROC system.17 According to the CAROC system, all patients with fragility fractures who are older than 50 years of age have a moderate risk of future fracture. Those who also have low femoral neck bone mineral density (BMD), particularly women, will likely be considered at high risk. A BMD test result is required to complete a fracture risk assessment calculation.16,18,19 In turn, the FP’s decision to order the BMD test required for fracture risk assessment will, in part, be driven by the anticipated yield on the results.
The purpose of this study is to estimate the proportion of patients with fragility fractures who can be expected to have low BMD and to assist FPs in deciding whether to refer patients for BMD testing and conduct a fracture risk assessment. The pivotal role of FPs in directing bone health management could be enhanced by a clear expectation of likely BMD test results in their patients with fragility fractures.
DATA SOURCES
A literature search of interventions to manage patients with fragility fractures was performed using MEDLINE, EMBASE, and CINAHL to identify English-language publications for a systematic review.15 The search period covered the earliest search dates available through September 2009; all articles found were published after 2000. The current review includes a subset of studies that reported BMD test results, as measured by dual-energy x-ray absorptiometry, in patients with fragility fractures who were managed in an orthopedic environment. Fragility fracture was defined as a low-trauma fracture from standing height or less in all studies.1 Full details on the search strategy and key words are available upon request.
Descriptive data (ie, study design, sample size, patient characteristics) were extracted by 2 independent reviewers (J.P. and J.S.). One reviewer (J.P.) extracted BMD test results.
The primary outcome was BMD test results. In all studies, the lowest recorded T score was used to categorize patients according to T score thresholds defined by the World Health Organization: T score greater than −1.0 for normal BMD results; T score of −1.0 or less and greater than −2.5 for mild bone loss; and T score of 2.5 or less for severe bone loss.20 Since mild or severe bone loss lead to consideration of treatment according to guidelines,18 we dichotomized BMD results into “normal” (> −1.0) or “low” (≤ −1.0) BMD results. The distribution of the proportion of persons with low BMD was summarized across studies using descriptive statistics (mean proportion with low BMD, mode, median, standard deviation [SD]). Lower boundaries were calculated using standard statistical thresholds21 to determine the lower threshold of the expected rate of low BMD. A boundary of 2 SDs below the mean described the lower boundary of the distribution at a 95% CI. The fifth percentile provided a nonparametric lower boundary above which 95% of the observations were found in a distribution.
Patient selection and potential bias
The generalizability of this review’s results might be challenged by the degree to which samples in each study were representative of a typical population of patients with fragility fractures. Specific patient groups (eg, elderly patients with hip fractures) are often selected to answer research questions, which might lead the reported BMD test results to be worse or better than one might find in the same physician’s typical practice. We used 2 approaches to estimate if selection factors influenced the rates of low BMD summarized from these studies.
First, we contacted the authors who reported the highest 3 and lowest 3 rates of low BMD to verify reported BMD rates and ascertain whether the reported study sample was representative of their general practices, and in turn of typical patients with fragility fractures who might be seen by FPs. We chose 3 studies at each end of the range because of an observed break in the proportion of patients with low BMD at the higher end of the range (> 90% low BMD), which was mirrored at the low end of the range. These studies were designated as numbers 1, 2, 3, 18, 19, and 20.
Second, we determined if selection factors might have biased the reported rates of low BMD findings. We looked for factors that were likely to result in the lowest rate of low BMD results: wrist fractures only22–26; younger age22,23,26; study sample not restricted to fragility fractures (ie, might have included moderate-trauma fractures but no high-trauma fractures)16,18; and BMD testing received as part of an integrated program (ie, more people with normal BMD might have been tested).16,27–29 We identified which of these factors were present in the selection of subjects, alone or in combination, for each of the studies reviewed. We then reviewed BMD rates including and excluding the studies with 2 or more factors, to determine if the factors affected the observed rate of low BMD.
SYNTHESIS
The literature search identified 2259 articles. A review of titles and abstracts resulted in 422 articles that met our screening criteria. The full articles were reviewed, and 57 articles describing an intervention to improve osteoporosis care in an orthopedic setting were selected for inclusion in the systematic review.15 Twenty of these studies reported BMD test results for at least a subgroup of study participants and were included in the present analysis.
Description of the studies
Descriptive data for the 20 studies are found in Table 1.26–45 Study enrolment ranged from 59 to 5897 participants, with BMD test results available for 4543 patients across all studies (range 29 to 2077). Ten studies were conducted in Europe, 8 in North America, and 2 in Australia or New Zealand. Studies were ordered by increasing proportion of patients with normal BMD: study number 130 had the lowest proportion of patients with normal BMD while study number 2045 had the highest. Studies conducted in Europe had, on average, a slightly higher proportion of patients with low BMD than studies conducted in North America or Australasia (86% vs 81% and 79%, respectively) did.
Table 1.
Proportion of patients from each study with normal test results, osteopenia, and osteoporosis, as well as descriptive data extracted from the studies: N = 20.
| STUDY NO. | AUTHOR, YEAR | COUNTRY | SETTING | NO. OF BMD TEST RESULTS |
BMD TEST RESULTS
|
2 OR MORE SELECTION FACTORS OF POTENTIAL INFLUENCE* | ||
|---|---|---|---|---|---|---|---|---|
| NORMAL, % | OSTEOPENIA, % | OSTEOPOROSIS, % | ||||||
| 1 | Schmid et al,30 2004 | Switzerland | Outpatient fracture clinic | 29 | 0 | 28 | 72 | |
| 2 | Sidwell et al,31 2004 | New Zealand | Orthogeriatric rehabilitation ward | 158 | 4 | 17 | 79 | |
| 3 | Becker et al,29 2006 | United States | Orthopedic and rehabilitation inpatient wards | 61 | 7 | 23 | 70 | |
| 4 | Hegeman et al,32 2005 | The Netherlands | Outpatient fracture clinic | 100 | 13 | 20 | 67 | |
| 5 | Astrand et al,33 2006 | Sweden | Emergency department and orthopedic inpatient ward | 239 | 13 | 45 | 42 | X |
| 6 | Mulherin et al,34 2003 | United Kingdom | Emergency department | 91 | 14 | 34 | 52 | |
| 7 | Chevalley et al,35 2002 | Switzerland | Orthopedic inpatient ward and outpatient clinic | 242 | 14 | 45 | 41 | |
| 8 | Hegeman et al,36 2004 | The Netherlands | Departments of surgery and traumatology | 94 | 15 | 34 | 51 | |
| 9 | Levasseur et al,37 2007 | France | Orthopedic inpatient ward | 32 | 16 | 50 | 34 | |
| 10 | Rozental et al,28 2008 | United States | Orthopedic outpatient clinic | 32 | 16 | 50 | 34 | X |
| 11 | Gallacher,38 2005 | United Kingdom | Orthopedic and trauma departments | NR | 17 | 46 | 37 | |
| 12 | Harrington et al,39 2005 | United States | Orthopedic inpatient ward and outpatient clinic | 154 | 18 | 50 | 32 | |
| 13 | McLellan et al,26 2003 | United Kingdom | Emergency department, orthopedic inpatient ward, and fracture clinic | 2077 | 18 | 42 | 40 | |
| 14 | van Helden et al,27 2008 | The Netherlands | Emergency department and orthopedic inpatient ward | 568 | 21 | 44 | 35 | X |
| 15 | Majumdar et al,40 2007 | Canada | Orthopedic surgery department | 120 | 21 | 34 | 45 | |
| 16 | Harrington and Lease,41 2007 | United States | Orthopedic inpatient ward and outpatient clinic | 232 | 2 | 57 | 21 | |
| 17 | Kuo et al,42 2007 | Australia | Outpatient fracture clinic | 135 | 24 | 46 | 30 | X |
| 18 | Majumdar et al,43 2008 | Canada | Emergency and fracture clinics | 95 | 28 | 52 | 20 | X |
| 19 | Cuddihy et al,44 2004 | United States | Orthopedic inpatient ward and outpatient clinic | 42 | 29 | 50 | 21 | X |
| 20 | Majumdar et al,45 2004 | Canada | Emergency department | 42 | 31 | 17 | 52 | |
BMD—bone mineral density, NR—not reported.
Selection factors of potential bias included study of wrist fractures only, younger age, study sample not restricted to fragility fractures (ie, might have included moderate-trauma fractures but no high-trauma fractures), and BMD testing received as part of an integrated program (ie, more people with normal BMD might have been tested).
Patient age ranged from 46 to 102 years. Three studies had a higher mean age of participants29,31,37; 3 studies had a lower mean age of participants.33,42,43 The proportion of women ranged from 53% to 100%. Two studies included only women.34,36 All fracture types were represented across the studies; 6 studies included only wrist fractures,28,34,36,43–45 and 1 study included only hip fractures.40
Low BMD rates
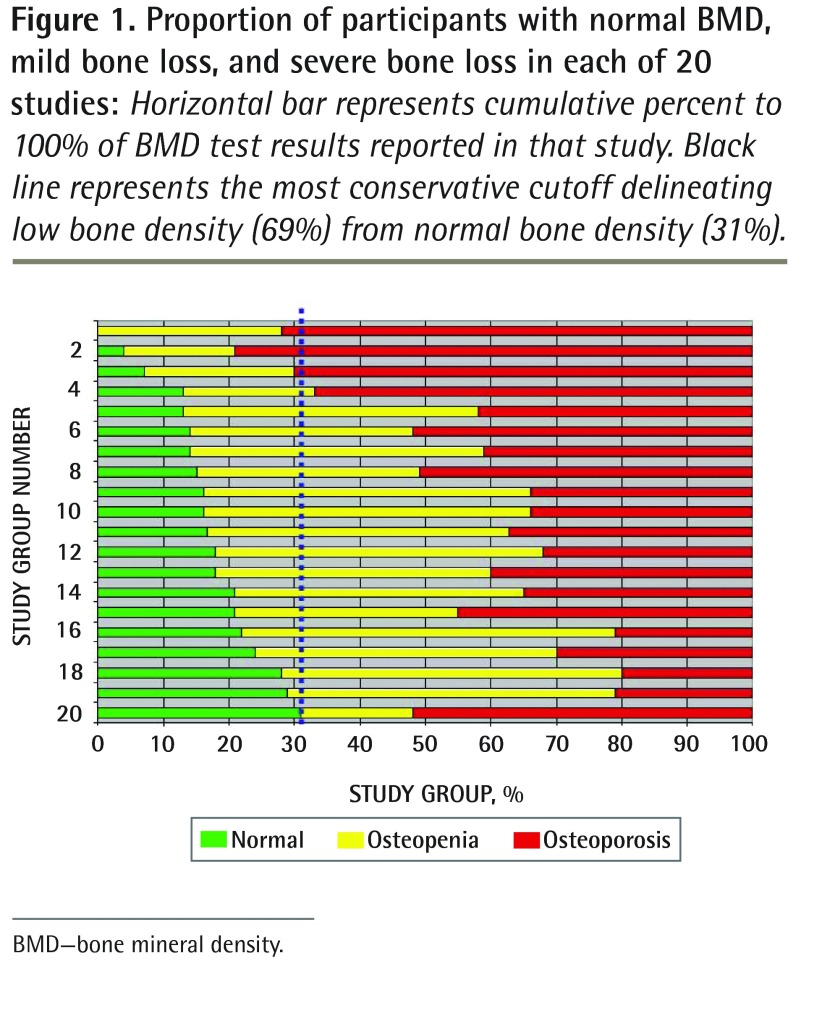
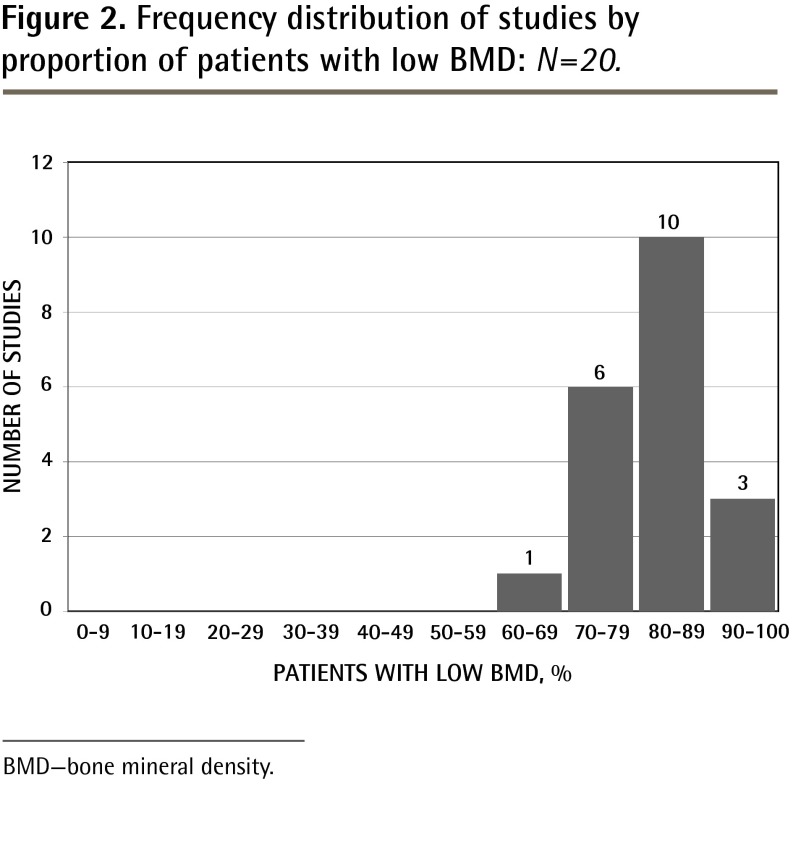
The proportion of patients with low BMD ranged from 69%45 to 100%30 across studies (Table 1,26–45Figure 1). The average proportion (low BMD rate) was 83% (SD 7.7%); the median was 83.5%. Most studies reported a low BMD rate between 70% and 89% (Figure 2).
Figure 1.
Proportion of participants with normal BMD, mild bone loss, and severe bone loss in each of 20 studies: Horizontal bar represents cumulative percent to 100% of BMD test results reported in that study. Black line represents the most conservative cutoff delineating low bone density (69%) from normal bone density (31%).
BMD—bone mineral density.
Figure 2.
Frequency distribution of studies by proportion of patients with low BMD: N=20.
BMD—bone mineral density.
Using the lower boundaries of the distribution to estimate the lower end of what one might expect to see for low BMD findings, the boundary of mean minus 2 SDs was 67.6%, and the nonparametric lower boundary of the fifth percentile of the distribution was 69%.
Selection factors of potential influence
Contact with the authors of the 6 studies at the margins confirmed our interpretation of reported low BMD rates in 5 studies.29–31,44,45 An Australian study providing education, BMD assessment, reporting of results, and treatment recommendations directly to patients with fragility fractures confirmed a slightly higher proportion of people with low BMD owing to the study design.42 Demographic and enrolment information confirmed the representativeness of the samples to a typical general orthopedic practice.
Six studies included only wrist fractures28,34,36,43–45; 3 studies had a lower mean age of participants (< 65 years)33,42,43; 1 study included some patients with moderate-trauma fractures27; and 17 studies reported that BMD testing was conducted as part of a designed program.26–29,31–42,44 These selection factors would have biased BMD test results toward a smaller proportion of the sample presenting with “low BMD.” Six studies27,28,33,42–44 had 2 or more selection factors (Table 1).26–45 Exclusion of these studies led to a lower boundary (mean minus 2 SDs) of 69%, and of 70% at the fifth percentile, indicating a consistent estimation of low BMD findings. The new mean was 85% (range 69% to 100%; SD 7.5%), compared with 83% (SD 7.7%) when the 6 studies were included. The 6 studies with 2 or more selection factors are compared with the remaining 14 studies in Figure 3.
Figure 3.
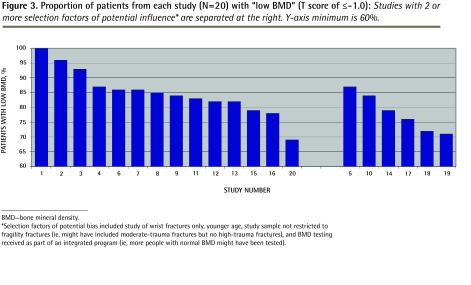
Proportion of patients from each study (N=20) with “low BMD” (T score of ≤ −1.0): Studies with 2 or more selection factors of potential influence* are separated at the right. Y-axis minimum is 60%.
BMD—bone mineral density.
*Selection factors of potential bias included study of wrist fractures only, younger age, study sample not restricted to fragility fractures (ie, might have included moderate-trauma fractures but no high-trauma fractures), and BMD testing received as part of an integrated program (ie, more people with normal BMD might have been tested).
Notwithstanding, the summary rate of all 20 studies clearly demonstrated that low (ie, below normal) BMD can be expected in at least two-thirds of patients with fragility fractures. This will lead to a classification of moderate or high risk of future fracture. The rate of very low BMD (ie, osteoporosis) ranged from 20% to 79% and exceeded 41% in more than half the studies (Table 1).26–45
DISCUSSION
Clinical fracture risk assessment requires a BMD test result, which is often unavailable when patients older than 50 years of age are first seen by their FPs following fragility fracture. Our review suggests that FPs can expect that this test will yield informative results, with at least two-thirds of these patients having low BMD. This expected high yield might aid the decision making about whether to conduct a BMD test for completion of a fracture risk assessment—a pivotal first step in reducing the risk of future fracture.
Effective therapies supported by clinical practice guidelines17 are available to mitigate fracture risk. However, previous work has identified the gap between existing rates of investigation and treatment of bone loss in patients with fragility fractures and the rates recommended by clinical guidelines.8–10,46 This gap has spurred the introduction of care programs to improve testing and treatment uptake,24,26,43,47 and many programs include BMD testing as part of the bone loss screening program.26,48 Rozental et al28 found that BMD tests, combined with accurate reporting of results, improved the likelihood of guideline-based care. We believe that having an accurate expectation for the outcome of testing and the likelihood of low BMD can also set accurate expectations about the importance of care to reduce risk of future fracture and potentially influence the uptake of clinical practice guidelines.
Strengths and limitations
Our study has several strengths. We began with a robust review of the literature on interventions to improve fragility fracture management in orthopedic settings, where patients are often referred back to FPs for bone health management. We had strict screening and selection factors with multiple reviewers, and thus we are confident that we have captured most of the relevant studies. We contacted the authors of studies whose rates were at either end of the range to confirm their data. We used the lower end of our distribution of low BMD rates, rather than the mean or median, to provide a conservative but convincing estimate of the likely rate FPs will find in their practices.
Our study also has limitations. We analyzed BMD results at a group level, but most studies did not segregate results by sex or age, which are key components of fracture risk assessment tools and would influence the proportion of people that would be found to have low BMD.
Conclusion
We found that 69% to 100% of patients with fragility fractures who underwent BMD testing across 20 intervention studies had low BMD. Family physicians working with patients older than 50 years of age with fragility fractures can have an a priori expectation that more than two-thirds of such patients will have low BMD. Using current algorithms for calculation of 10-year fracture risk, the co-occurrence of a prevalent fragility fracture, age older than 50 years, and a BMD test showing low bone mass will result in these patients being at moderate or high risk of subsequent fracture, with many in the high-risk category.16–18 Most patients will have an indication for pharmacotherapy, shown to reduce risk of future fracture; the remainder will require surveillance for optimization of calcium and vitamin D intake, modification of lifestyle risks of fracture, and fall prevention. With this information in mind, we hope FPs will feel confident to proceed with BMD testing, congruent with evidence-based guidelines for investigation and treatment.13,17,49,50
Acknowledgments
We thank Victoria Elliot-Gibson for background research and assistance with article selection, Rebeka Sujic and Iman Ahsan for their help with article selection, and Sarah Dyer for coordination support. We also thank Dagmar Gross for assistance with preparation of the manuscript. We thank Osteoporosis Canada and the Ontario Osteoporosis Strategy for their interest in and support for this project. This study was supported by a grant from the Ontario Ministry of Health and Long-Term Care as part of the Ontario Osteoporosis Strategy. The views expressed in this article are those of the researchers and do not necessarily reflect the opinions of the Ontario Ministry of Health and Long-Term Care.
EDITOR’S KEY POINTS
This study determined the proportion of patients with fragility fractures who would be expected to have low bone mineral density (BMD) at the time of fracture. This information can set an expectation of the rate of low BMD in patients with fragility fractures to assist fracture risk assessment and facilitate clinical decision making.
The proportion of patients with low BMD in a literature review of 20 studies reporting results for 4543 patients ranged from 69% to 100% across the studies. The average proportion or rate of low BMD (ie, T score < −1.0) was 83%. Most studies reported a low BMD rate between 70% and 89%.
Family physicians working with patients older than 50 years of age with fragility fractures can have an a priori expectation that more than two-thirds of these patients will have low bone mass. Using current algorithms for calculation of 10-year fracture risk, all patients who have a prevalent fragility fracture, are older than 50 years of age, and have BMD test results that reveal low bone mass will be at moderate or high risk of refracture; these patients should be evaluated by their physicians and be provided with appropriate guideline-based treatment, which might include pharmacotherapy.
POINTS DE REPÈRE DU RÉDACTEUR
Dans cette étude, on a déterminé la proportion des patients avec une fracture de fragilité pour lesquels on s’attendrait à ce qu’il aient déjà une densitométrie osseuse (DMO) basse avant la fracture. Cette information permettra de prévoir les taux de DMO basse chez ces patients et d’évaluer le risque de nouvelles fractures, facilitant ainsi la prise de décisions cliniques.
Dans une revue de la littérature portant sur les résultats de 20 études et comprenant 4543 patients, la proportion de ceux avec une DMO basse variait entre 69 et 100 %. En moyenne, la proportion des taux de DMO basse (c.-à-d. ayant un score de T ≤ −1,0) était de 83 %. Dans la plupart des études, ce taux variait entre 70 et 89 %.
Le médecin de famille qui travaille avec des patients de plus de 50 ans avec une fracture pathologique peut a priori s’attendre à ce que plus des deux tiers de ces patients aient une faible densité osseuse. Les algorithmes actuels pour calculer le risque de fracture sur 10 ans permettent de prévoir que tous les patients de plus de 50 ans qui ont une fracture pathologique et un test de DMO révélant une masse osseuse basse présentent un risque modéré à élevé de nouvelle fracture; ces patients devraient être évalués par leur médecin et recevoir les traitements recommandés par les directives de pratique, ce qui pourrait comprendre une médication.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors contributed to the concept and design of the study; literature review; analysis and interpretation of the studies; and preparing the manuscript for submission.
Competing interests
The Osteoporosis Screening Program at the study site is outside of the Ontario Osteoporosis Strategy and is supported through unrestricted research grants from Merck Frosst Canada and Co. and the Alliance for Better Bone Health. Dr Bogoch serves as a consultant for Warner Chilcott, has received unrestricted research support from the Alliance for Better Bone Health and from Amgen Canada, and has received honoraria from Warner Chilcott and Merck Frosst Canada Ltd.
References
- 1.World Health Organization. Guidelines for preclinical evaluation and clinical trials in osteoporosis. Geneva, Switz: World Health Organization; 1998. Available from: whqlibdoc.who.int/publications/1998/9241545224_eng.pdf. Accessed 2013 Nov 14. [Google Scholar]
- 2.Haentjens P, Autier P, Collins J, Velkeniers B, Vanderschueren D, Boonen S. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women. A meta-analysis. J Bone Joint Surg Am. 2003;85-A(10):1936–43. doi: 10.2106/00004623-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721–39. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 4.Schrøder HM, Petersen KK, Erlandsen M. Occurrence and incidence of the second hip fracture. Clin Orthop Relat Res. 1993;(289):166–9. [PubMed] [Google Scholar]
- 5.Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C. Meta-analyses of therapies for postmenopausal osteoporosis. IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002;23(4):570–8. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- 6.Levis S, Quandt SA, Thompson D, Scott J, Schneider DL, Ross PD, et al. Alendronate reduces the risk of multiple symptomatic fractures: results from the fracture intervention trial. J Am Geriatr Soc. 2002;50(3):409–15. doi: 10.1046/j.1532-5415.2002.50102.x. [DOI] [PubMed] [Google Scholar]
- 7.Watts NB, Josse RG, Hamdy RC, Hughes RA, Manhart MD, Barton I, et al. Risedronate prevents new vertebral fractures in postmenopausal women at high risk. J Clin Endocrinol Metab. 2003;88(2):542–9. doi: 10.1210/jc.2002-020400. [DOI] [PubMed] [Google Scholar]
- 8.Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int. 2004;15(10):767–78. doi: 10.1007/s00198-004-1675-5. [DOI] [PubMed] [Google Scholar]
- 9.Giangregorio L, Papaioannou A, Cranney A, Zytaruk N, Adachi JD. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum. 2006;35(5):293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Papaioannou A, Giangregorio L, Kvern B, Boulos P, Ioannidis G, Adachi JD. The osteoporosis care gap in Canada. BMC Musculoskelet Disord. 2004;5:11. doi: 10.1186/1471-2474-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royal Australian College of General Practitioners. Clinical guideline for the prevention and treatment of osteoporosis in postmenopausal women and older men. South Melbourne, Aust: Royal Australian College of General Practitioners; 2010. [Google Scholar]
- 12.Brown JP, Josse RG, Scientific Advisory Council of the Osteoporosis Society of Canada 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002;167(10 Suppl):S1–34. Errata in: CMAJ 2003;168(5):544, CMAJ 2003;168(4):400, CMAJ 2003;168(6):676. [PMC free article] [PubMed] [Google Scholar]
- 13.National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. [Google Scholar]
- 14.Cooper C, Mitchell P, Kanis JA. Breaking the fragility fracture cycle. Osteoporos Int. 2011;22(7):2049–50. doi: 10.1007/s00198-011-1643-9. [DOI] [PubMed] [Google Scholar]
- 15.Sale JE, Beaton D, Posen J, Elliot-Gibson V, Bogoch E. Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int. 2011;22(7):2067–82. doi: 10.1007/s00198-011-1544-y. [DOI] [PubMed] [Google Scholar]
- 16.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182(17):1864–73. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siminoski K, Leslie WD, Frame H, Hodsman A, Josse RG, Khan A, et al. Recommendations for bone mineral density reporting in Canada. Can Assoc Radiol J. 2005;56(3):178–88. [PubMed] [Google Scholar]
- 19.Leslie WD, Lix LM, Manitoba Bone Density Program Simplified 10-year absolute fracture risk assessment: a comparison of men and women. J Clin Densitom. 2010;13(2):141–6. doi: 10.1016/j.jocd.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(6):368–81. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 21.Freedman D, Pisani R, Purves R. Statistics. 3rd ed. New York, NY: WW Norton & Company Inc; 1998. [Google Scholar]
- 22.Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34(1):195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Bogoch ER, Elliot-Gibson V, Beaton DE, Jamal SA, Josse RG, Murray TM. Effective initiation of osteoporosis diagnosis and treatment for patients with a fragility fracture in an orthopaedic environment. J Bone Joint Surg Am. 2006;88(1):25–34. doi: 10.2106/JBJS.E.00198. [DOI] [PubMed] [Google Scholar]
- 24.Bogoch ER, Elliot-Gibson V, Escott BG, Beaton DE. The osteoporosis needs of patients with wrist fracture. J Orthop Trauma. 2008;22(8 Suppl):S73–8. doi: 10.1097/BOT.0b013e31815e9ff7. [DOI] [PubMed] [Google Scholar]
- 25.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286(22):2815–22. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 26.McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int. 2003;14(12):1028–34. doi: 10.1007/s00198-003-1507-z. [DOI] [PubMed] [Google Scholar]
- 27.Van Helden S, van Geel AC, Geusens PP, Kessels A, Nieuwenhuijzen Kruseman AC, Brink PR. Bone and fall-related fracture risks in women and men with a recent clinical fracture. J Bone Joint Surg Am. 2008;90(2):241–8. doi: 10.2106/JBJS.G.00150. [DOI] [PubMed] [Google Scholar]
- 28.Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953–61. doi: 10.2106/JBJS.G.01121. [DOI] [PubMed] [Google Scholar]
- 29.Becker C, Crow S, Toman J, Lipton C, McMahon DJ, Macaulay W, et al. Characteristics of elderly patients admitted to an urban tertiary care hospital with osteoporotic fractures: correlations with risk factors, fracture type, gender and ethnicity. Osteoporos Int. 2006;17(3):410–6. doi: 10.1007/s00198-005-0001-1. [DOI] [PubMed] [Google Scholar]
- 30.Schmid L, Henzen C, Schlumpf U, Babst R. Improving secondary prevention in fragility fracture patients: the impact of a simple clinical information procedure. J Appl Res. 2004;4(4):570–5. [Google Scholar]
- 31.Sidwell AI, Wilkinson TJ, Hanger HC. Secondary prevention of fractures in older people: evaluation of a protocol for the investigation and treatment of osteoporosis. Intern Med J. 2004;34(3):129–32. doi: 10.1111/j.1444-0903.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 32.Hegeman JH, Willemsen G, van Nieuwpoort J, Kreeftenberg HG, van der Veer E, Slaets JP, et al. Effective case-finding of osteoporosis in a fracture and osteoporosis clinic in Groningen: an analysis of the first 100 patients [article in Dutch] Aktuelle Traumatol. 2005;35(1):34–9. [Google Scholar]
- 33.Astrand J, Thorngren KG, Tägil MM. One fracture is enough! Experience with a prospective and consecutive osteoporosis screening program with 239 fracture patients. Acta Orthop. 2006;77(1):3–8. doi: 10.1080/17453670610045623. [DOI] [PubMed] [Google Scholar]
- 34.Mulherin D, Williams S, Smith JA, Edwards J, Sheeran TP, Price T. Identification of risk factors for future fracture in patients following distal forearm fracture. Osteoporos Int. 2003;14(9):757–60. doi: 10.1007/s00198-003-1441-0. [DOI] [PubMed] [Google Scholar]
- 35.Chevalley T, Hoffmeyer P, Bonjour JP, Rizzoli R. An osteoporosis clinical pathway for the medical management of patients with low-trauma fracture. Osteoporos Int. 2002;13(6):450–5. doi: 10.1007/s001980200053. [DOI] [PubMed] [Google Scholar]
- 36.Hegeman JH, Oskam J, van der Palen J, Ten Duis HJ, Vierhout PA. The distal radial fracture in elderly women and the bone mineral density of the lumbar spine and hip. J Hand Surg Br. 2004;29(5):473–6. doi: 10.1016/j.jhsb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Levasseur R, Sabatier JP, Guilcher C, Guaydier-Souquières G, Costentin-Pignol V, Jean-Jacques PY, et al. Medical management of patients over 50 years admitted to orthopedic surgery for low-energy fracture. Joint Bone Spine. 2007;74(2):160–5. doi: 10.1016/j.jbspin.2006.03.003. Epub 2006 Jul 21. [DOI] [PubMed] [Google Scholar]
- 38.Gallacher SJ. Setting up an osteoporosis fracture liaison service: background and potential outcomes. Best Pract Res Clin Rheumatol. 2005;19(6):1081–94. doi: 10.1016/j.berh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Harrington JT, Barash HL, Day S, Lease J. Redesigning the care of fragility fracture patients to improve osteoporosis management: a health care improvement project. Arthritis Rheum. 2005;53(2):198–204. doi: 10.1002/art.21072. [DOI] [PubMed] [Google Scholar]
- 40.Majumdar SR, Beaupre LA, Harley CH, Hanley DA, Lier DA, Juby AG, et al. Use of a case manager to improve osteoporosis treatment after hip fracture: results of a randomized controlled trial. Arch Intern Med. 2007;167(19):2110–5. doi: 10.1001/archinte.167.19.2110. [DOI] [PubMed] [Google Scholar]
- 41.Harrington JT, Lease J. Osteoporosis disease management for fragility fracture patients: new understandings based on three years’ experience with an osteoporosis care service. Arthritis Rheum. 2007;57(8):1502–6. doi: 10.1002/art.23093. [DOI] [PubMed] [Google Scholar]
- 42.Kuo I, Ong C, Simmons L, Bliuc D, Eisman J, Center J. Successful direct intervention for osteoporosis in patients with minimal trauma fractures. Osteoporos Int. 2007;18(12):1633–9. doi: 10.1007/s00198-007-0418-9. [DOI] [PubMed] [Google Scholar]
- 43.Majumdar SR, Johnson JA, McAlister FA, Bellerose D, Russell AS, Hanley DA, et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ. 2008;178(5):569–75. doi: 10.1503/cmaj.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuddihy MT, Amadio PC, Gabriel SE, Pankratz VS, Kurland RL, Melton LJ., 3rd A prospective clinical practice intervention to improve osteoporosis management following distal forearm fracture. Osteoporos Int. 2004;15(9):695–700. doi: 10.1007/s00198-004-1597-2. [DOI] [PubMed] [Google Scholar]
- 45.Majumdar SR, Rowe BH, Folk D, Johnson JA, Holroyd BH, Morrish DW, et al. A controlled trial to increase detection and treatment of osteoporosis in older patients with a wrist fracture. Ann Intern Med. 2004;141(5):366–73. doi: 10.7326/0003-4819-141-5-200409070-00011. [DOI] [PubMed] [Google Scholar]
- 46.Foley KA, Foster SA, Meadows ES, Baser O, Long SR. Assessment of the clinical management of fragility fractures and implications for the new HEDIS osteoporosis measure. Med Care. 2007;45(9):902–6. doi: 10.1097/MLR.0b013e3180536764. [DOI] [PubMed] [Google Scholar]
- 47.Kanis JA, Johansson H, Oden A, McCloskey EV. Assessment of fracture risk. Eur J Radiol. 2009;71(3):392–7. doi: 10.1016/j.ejrad.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 48.Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am. 2008;90(Suppl 4):188–94. doi: 10.2106/JBJS.H.00628. [DOI] [PubMed] [Google Scholar]
- 49.Marsh D, Akesson K, Beaton DE, Bogoch ER, Boonen S, Brandi ML, et al. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int. 2011;22(7):2051–65. doi: 10.1007/s00198-011-1642-x. [DOI] [PubMed] [Google Scholar]
- 50.Boden SD, Einhorn TA, Morgan TS, Tosi LL, Weinstein JN. An AOA critical issue. The future of the orthopaedic surgeon-proceduralist or keeper of the musculoskeletal system? J Bone Joint Surg Am. 2005;87(12):2812–21. doi: 10.2106/JBJS.E.00791. [DOI] [PubMed] [Google Scholar]