Abstract
Cardiovascular diseases (CVD) are now the number one cause of death in low- and middle-income countries (LMIC), such as those in South East Asia (SEA). It is projected that SEA countries will have the greatest total number of deaths due to non-communicable diseases (NCDs) by 2020. In low resource countries, the rising burden of CVDs imposes severe economic consequences that range from impoverishment of families to high health system costs and the weakening of country economies. There are two possible options to be considered for addressing this issue: a “population-based strategy” and/or a “high risk” strategy. The question is, what is the optimal way to reduce the excessive burden of these diseases in the LMICs. We believe that by applying systematic policy and smoking cessation programs with proven effectiveness, there is a chance that the high smoking prevalence, particularly among SEA.
Keywords: Cardiovascular diseases, Prevention, Population-based versus high-risk strategies, Non-laboratory-based method of risk prediction
Cardiovascular diseases (CVD) are now the number one cause of death in low- and middle-income countries (LMIC), such as those in South East Asia (SEA). It is projected that SEA countries will have the greatest total number of deaths due to non-communicable diseases (NCDs) by 2020. In low-resource countries like SEA, the rising burden of CVDs imposes severe economic consequences that range from impoverishment of families to high health system costs and the weakening of country economies.
There are two possible options to be considered for addressing this issue: a “population-based strategy” and/or a “high risk” strategy. Geoffrey Rose has argued that shifting the risk distribution curve by a small amount in a population has a greater beneficial effect in preventing morbidity and mortality than only concentrating on treating patients with high risk.1 Therefore, the question is, whether the optimal way to reduce the excessive burden of these diseases in the LMIC would be the adoption of the “population” versus the “high risk” strategy.
First of all, we have to be aware which of the cardiovascular risk factors contribute most to this dismal state of affairs. The INTERHEART study2 identified 6 risk factors which are positively associated with acute myocardial infarction across 52 countries:
-
♦
Current smoking
-
♦
Raised ApoB/ApoA1 ratio
-
♦
Diabetes
-
♦
Hypertension
-
♦
Abdominal obesity
-
♦
Psychosocial index
Other data indicate that, worldwide, the two most significant cardiovascular and cerebrovascular risk factors with highest ratios of population attributable risk are hypertension and smoking.3
Thinking about a feasible preventive strategy in LMIC, several aspects have to be taken into consideration. Which of the risk factors listed in the INTERHEART study are massively prevalent in LMIC? Doubtlessly, it is hypertension (in both men and women) and smoking (in men). Which of these conditions can be detected in a cost-effective manner? Hypertension and smoking. Finally, which of the above conditions have solid evidence as to the benefit and feasibility of treatment? On top of the list would be hypertension, followed by diabetes and smoking. We have no data about the effectiveness of treating abdominal obesity and even less about manipulating psychosocial index.
The prevalence of hypertension in SEA countries is between 18 and 40% (among men)4–11 while the level of the hypertension awareness and control is generally low or practically nonexistent; for example, in Pakistan only 3–6% of hypertensive are under satisfactory control.11,12 In these countries, the smoking prevalence in males is approximately 48%; among women this is significantly lower (20%).13 Similar to Western countries, in SEA, the incidence and prevalence of obesity (12–18%)14–16 and of diabetes (10–15%) are also increasing.17–19 There is little known about the population level of blood cholesterol in these countries.
It is tempting to think that excess CVD morbidity and mortality in LMIC can be reduced by population-based measures such as changes in nutrition (e.g. low salt, low saturated fat, high intake of vegetables) or behavior (smoking cessation, exercise). Population policies were the main theme of the recent high-level UN non-communicable disease summit.20 As was expected, recommendations regarding nutrition, particularly dietary salt restriction and smoking cessation were given high priority. Generally, the loquacious recommendations emanating from this meeting were met with skepticism as to whether they will have an effect in LMIC.21
In fact, with the exception of anti-smoking policies and programs, these population-based interventions are mostly failures in high-income countries. For example, in countries like USA, Canada, Germany and Austria, there is an increasing prevalence of obesity and diabetes suggesting that recommendations from nutritional advisories and encouragement for physical activity “fall on deaf ears”. In our opinion, it is more than doubtful that measures that failed in economically advanced countries will be successful in LMIC.
This leaves us with the option of the “high risk” strategy. As Geoffrey Rose pointed out in his book, The Strategy of Preventive Medicine, 1992:“The strong attraction of the high-risk preventive strategy is that the intervention is matched to the needs of the individual”.1 To assess the need of the individual we have to assess the level of risk for encountering a major health event in the foreseeable future; hence the various existing methods for calculating the short-term risk or life-long risk.
In the field of cardiovascular and cerebrovascular medicine, a classic method for CVD risk assessment is the use of the Framingham risk engine. There are a number of other risk assessment systems e.g. SCORE, Q-risk, Sheffield tables, etc. These tools have a reasonably good predictive power but each of them requires the input of blood cholesterol value. Considering the need for mass screenings in LMIC, taking a venous blood sample from each screened individual can be a deterrent financially and psychologically.
Currently, we have at disposal a non-laboratory risk assessment method developed by Gaziano et al22 specifically considered for use in LMICs. This simplified, non-invasive and cost-effective approach provides a CVD risk assessment model, which has been shown to be as accurate as a laboratory-based model. The validity of the Gaziano et al model was assessed using C-statistics in a cohort of 6186 people without a history of cardiovascular disease or cancer who were participating in the NHANES I Epidemiologic Follow-up Study (NHEFS).23 Gaziano et al22 documented that the predictive value of this non-laboratory risk engine is identical to those for whom blood cholesterol was determined.
One of the numerous advantages of this non-laboratory-based method of risk prediction is that the risk assessment can be completed in one clinic visit with minimum equipment needed: A tape measure, scale, and an automated blood-pressure machine. This non-laboratory risk assessment procedure uses information on risk factors such as age, measured systolic BP (using an automated BP measuring device), calculated BMI (based on measurements of height and weight) and self-reported information on diabetes and smoking status. Applying this information on the assessment charts, the combination of these risk factors is converted into a score that reflects one of three 5-year CVD risk prediction categories for the individual: low (<5%, 5–10%), moderate (11%–20%) and high (21–30%, >30%). Separate prediction charts exist for men and women (see also Figs. 1 and 2).
Fig. 1.
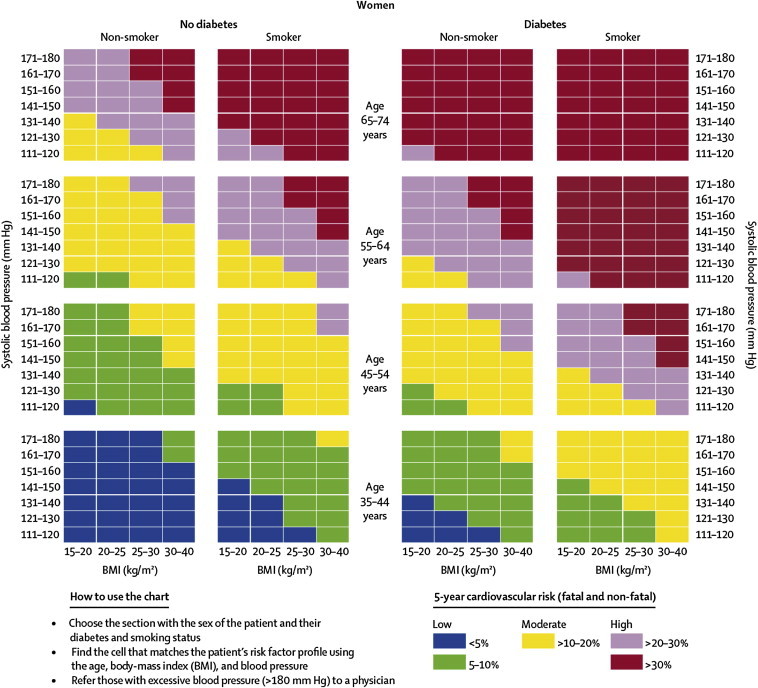
Risk prediction chart for cardiovascular disease using non-laboratory-based measures (Women).
Source: Gaziano TA, Young CR, Fitzmaurice G, Atwood S, Gaziano JM. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: the NHANES I Follow-up Study cohort. Lancet 2008;371:923–31. Reproduced with permission of Elsevier.
Fig. 2.
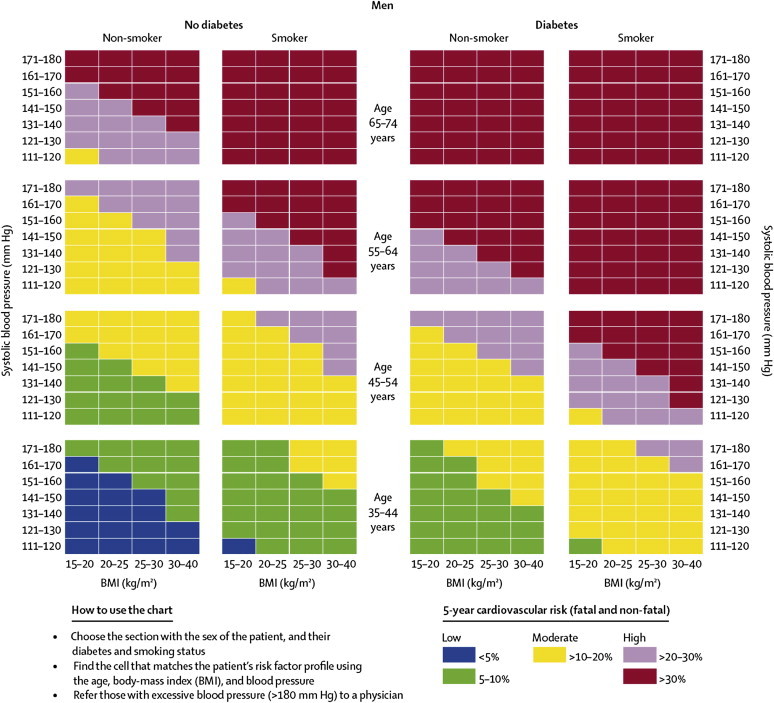
Risk prediction chart for cardiovascular disease using non-laboratory-based measures (Men).
Source: Gaziano TA, Young CR, Fitzmaurice G, Atwood S, Gaziano JM. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: the NHANES I Follow-up Study cohort. Lancet 2008;371:923–31. Reproduced with permission of Elsevier.
According to Gaziano et al, “a risk prediction value can be ascertained and a treatment decision can be made within the same 5- to 10-min visit, without the cost or the time needed to wait for laboratory results”.
The need to have in LMIC an inexpensive cardiovascular and cerebrovascular risk assessment model has been recognized by the WHO and International Society of Hypertension. These organizations have also produced a non-laboratory risk assessment model (World Health Organization (2007). Prevention of cardiovascular disease. Pocket guidelines for assessment and management of cardiovascular risk, Geneva), which is similar to the one developed by Gaziano et al. The disadvantage of the WHO/ISSH model is that it has not been yet validated the same way as the Gaziano model.
On the other hand, the disadvantage of both models is in the fact that so far these have not been adjusted to special needs of populations in LMIC. However, for the time being, even an imperfect tool has a great potential for playing a useful role in CVD prevention in these populations. The local investigator should also be encouraged to create cohorts which would enable to prepare a more accurate risk engine for the local population.
Considering and amalgamating the above information, we propose a step-wise approach for CVD prevention in LMIC. As a starting point, hypertension (a highly prevalent risk factor for which effective and cost-effective treatment options are currently available) should be addressed first. If a feasible strategy should be tested, we have to take into account the fact that in most LMICs the available health services are not ready to provide a high quality help to all hypertensive. We concur with MacMahon et al24 that the first step to be considered for the prevention and control of CVDs would be: “whether care can be given to those at the highest risk of fatal or catastrophic events… because most of those for whom blood pressure lowering is recommended receive no treatment whatsoever”. As such, it would be a great success if it was possible to demonstrate the possibility of achieving good control of hypertension at least among those hypertensive individuals who are at the highest risk of CVD events.
This requires a tool enabling a rapid and low cost triage of the hypertensive population to low, medium and high-risk individuals, such as the Gaziano assessment engine. This cost/effective triage, quickly identifies the highest risk individuals and these can be taken care with top priority according to a standard treatment protocol.
This approach has many advantages:
-
1.
It reduces the number of patients who have to be treated immediately to manageable levels;
-
2.
The therapy starts with antihypertensive drugs of proven effectiveness end efficacy;
-
3.
Cheap, generic antihypertensive drugs are available and affordable
This “high risk” approach is not interfering with potential “population strategies.” We believe that by applying systematic policy and smoking cessation programs with proven effectiveness, there is a chance that the high smoking prevalence, particularly among SEA males can be reduced. On the other hand, it remains to be seen whether dietary habits, which have developed over thousands of years and are also primarily determined by economic possibilities of toiling masses in these LMIC areas, could be changed effectively.
Conflicts of interest
All authors have none to declare.
References
- 1.Rose Geoffrey. University Press; Oxford: 1992. The Strategy of Preventive Medicine. [Google Scholar]
- 2.Yusuf S., Hawken S., Ôunpuu S. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 3.Ezzati M., Lopez A.D., Rodgers A. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 4.Singh R.B., Fedacko J., Pella D. Prevalence and risk factors for prehypertension and hypertension in five Indian cities. Acta Cardiol. 2011 Feb;66(1):29–37. doi: 10.1080/ac.66.1.2064964. [DOI] [PubMed] [Google Scholar]
- 5.Jonas J.B., Nangia V., Matin A. Prevalence, awareness, control and associations of arterial hypertension in a rural central India population: the Central India Eye and Medical Study. Am J Hypertens. 2010;23:347–350. doi: 10.1038/ajh.2009.276. [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj R., Kandori A., Marwah R. Prevalence, awareness and control of hypertension in rural communities of Himachal Pradesh. J Assoc Physicians India. 2010 Jul;58:423–424. 429. [PubMed] [Google Scholar]
- 7.By Y., Mr N.G., Ag U. Prevalence, awareness, treatment, and control of hypertension in rural areas of davanagere. Indian J Community Med. 2010 Jan;35(1):138–141. doi: 10.4103/0970-0218.62578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Midha T., Idris M.Z., Saran R.K., Srivastav A.K., Singh S.K. Prevalence and determinants of hypertension in the urban and rural population of a north Indian district. East Afr J Public Health. 2009 Dec;6(3):268–273. [PubMed] [Google Scholar]
- 9.Mohan V., Deepa M., Farooq S. Prevalence, awareness and control of hypertension in Chennai – The Chennai Urban Rural Epidemiology Study (CURES – 52) J Assoc Physicians India. 2007;55:326–332. [PubMed] [Google Scholar]
- 10.Jafar T.H., Levey A.S., Jafary F.H. Ethnic subgroup differences in hypertension in Pakistan. J Hypertens. 2003;21:905–912. doi: 10.1097/00004872-200305000-00014. [DOI] [PubMed] [Google Scholar]
- 11.The Collaborative Group Pakistan Hypertension League & Pakistan Cardiac Society . 2009. National Guidelines For Detection, Prevention, Control & Management of Hypertension. [Google Scholar]
- 12.Saleheen D., Hashmi S.K., Zaidi M. Evaluation of therapeutic control in a Pakistani population with hypertension. J Eval Clin Pract. 2010 Dec;16(6):1081–1084. doi: 10.1111/j.1365-2753.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- 13.Global Adult Tobacco Survey (GATS): India. World Health Organization; 2009. 2010. [Google Scholar]
- 14.Geetha L., Deepa M., Anjana R.M., Mohan V. Prevalence and clinical profile of metabolic obesity and phenotypic obesity in Asian Indians. J Diabetes Sci Technol. 2011 Mar 1;5(2):439–446. doi: 10.1177/193229681100500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta D.K., Shah P., Misra A. Secular trends in prevalence of overweight and obesity from 2006 to 2009 in urban Asian Indian adolescents aged 14–17 years. PLoS One. 2011 Feb 23;6(2) doi: 10.1371/journal.pone.0017221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khadilkar V.V., Khadilkar A.V., Cole T.J., Chiplonkar S.A., Pandit D. Overweight and obesity prevalence and body mass index trends in Indian children. Int J Pediatr Obes. 2011 Jun 6 doi: 10.3109/17477166.2010.541463. (2–2):e216–24. [DOI] [PubMed] [Google Scholar]
- 17.Vaz N.C., Ferreira A.M., Kulkarni M.S., Vaz F.S. Prevalence of diabetes mellitus in a rural population of Goa, India. Natl Med J India. 2011 Jan–Feb;24(1):16–18. [PubMed] [Google Scholar]
- 18.Ravikumar P., Bhansali A., Ravikiran M. Prevalence and risk factors of diabetes in a community-based study in North India: the Chandigarh Urban Diabetes Study (CUDS) Diabetes Metab. 2011 Jun;37(3):216–221. doi: 10.1016/j.diabet.2010.10.004. Epub 2010 Dec 30. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran A., Snehalatha C. Current scenario of diabetes in India. J Diabetes. 2009 Mar;1(1):18–28. doi: 10.1111/j.1753-0407.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 20.The Lancet Oncology Editorial Two days in New York: reflections on the UN NCD summit. Lancet Oncol. 2011;12:981. doi: 10.1016/S1470-2045(11)70272-8. [DOI] [PubMed] [Google Scholar]
- 21.Horton R. Offline: despicable effects and disgraceful actions. Lancet. 2011;378:1208. [Google Scholar]
- 22.Gaziano T.A., Young C.R., Fitzmaurice G. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: the NHANES I Follow-up Study Cohort. Lancet. 2008;371:923–931. doi: 10.1016/S0140-6736(08)60418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandya A., Weinstein M.C., Gaziano T.A. A comparative assessment of non-laboratory-based versus commonly used laboratory-based cardiovascular disease risk scores in the NHANES III Population. PLoS One. 2011;6:e20416. doi: 10.1371/journal.pone.0020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacMahon S., Alderman M.H., Lindholm L.H. Blood-pressure-related disease is a global health priority. Lancet. 2008;371:1480–1482. doi: 10.1016/S0140-6736(08)60632-7. [DOI] [PubMed] [Google Scholar]


