Abstract
The objective of this study was to analyze the changes in expression and the possible functions of interleukin-6 (IL-6) in electrical kindling of the basolateral amygdala (BLA) in epileptic rats. Bipolar electrodes were implanted into the BLA of Sprague-Dawley rats, and the rats were then subjected to chronic electrical stimulation through the electrodes to induce kindling. The seizure characteristics and behavioral changes of the rats were observed, and electroencephalograms were recorded during and following kindling. The IL-6 mRNA expression in the hippocampi of the rats was analyzed using semi-quantitative reverse transcription-polymerase chain reaction, and control and topiramate (TPM)-treated groups were compared. The mean time-period required for kindling was 13.50±3.99 days, and the afterdischarge duration (ADD) measured between 21,450 and 119,720 msec. The expression of IL-6 mRNA was significantly upregulated in the kindled rats. TPM was able to depress the seizures and decrease the IL-6 level in the kindled rats. In conclusion, IL-6 mRNA was upregulated in the hippocampi of epileptic rats, and IL-6 may have participated in the process of kindling.
Keywords: interleukin-6, epilepsy, kindling, rat, basolateral amygdale, topiramate
Introduction
A number of studies have suggested that immune factors are involved in the pathogenesis of epilepsy (1–3). During seizures, the cellular immune function becomes abnormal, which leads to changes in the intracorporeal cytokine levels. Among these cytokines, interleukin-6 (IL-6), in particular, has been implicated. IL-6 is generally synthesized by mononuclear phagocytes, vascular endothelial cells and fibroblasts. It is also produced by activated T cells. The main biological function of IL-6 is stimulating the maturation of activated B cells. In the nervous system, neurons and glial cells (including microglia and astrocytes) all secrete IL-6. The hippocampus, neocortex and cerebella exhibit high expression levels of IL-6 and its receptor. Similar to other cytokines, IL-6 binds to the α chain of the IL-6 receptor (IL-6R) on cell surfaces. Subsequently, the α chain and the receptor subunit gp130 (IL-6Rβ chain) form a high-affinity receptor that transmits a signal into cells through the gp130/Jak/STAT route to regulate gene transcription (4). In general, IL-6 has a neuroprotective effect on ischemic lesions, glutamate excitotoxicity and tumor necrosis factor (TNF)-mediated injuries (5–7). Furthermore, IL-6 has been shown to promote neuronal survival. In a study by Thier et al(8), it was revealed that the IL-6 secreted by sensory nerves and the IL-6 receptors jointly acted as neurotrophic factors for the maintenance of neuronal survival. In addition, IL-6 has shown the ability to induce PC12 cells to differentiate into nerve cells (9). IL-6 is able to protect striatal neurons against N-methyl-D-aspartate (NMDA) toxicity (8) and dopaminergic neurons against 1-methyl-4-phenylpyridinium (MPP) toxicity (10). Moreover, IL-6 is able to promote the regeneration of severed peripheral nerve cells (11). März et al(12) demonstrated that IL-6 was able to induce cultured typical stellate cells to transform into fibroblast-like cells, and to induce the expression of neurotrophic factors. Moreover, in different brain regions, IL-6 induced different neurotrophic factors, thus indicating that IL-6 was involved in multiple central nervous system (CNS) functions (11). It has been suggested that IL-6 directly participates in the excitation of nerve cells. Tancredi et al(13) demonstrated that IL-6 inhibited the post-tetanic potentiation (PTP) and the long-term potentiation (LTP) of nerve cells in the hippocampal CA1 region, as well as participating in the plasticity of the hippocampal synapse. Therefore, IL-6 is closely associated with neurological excitability. Qiu et al(14) showed that increased IL-6 levels were capable of notably changing the response to excitatory neurotransmitter during CNS neuronal development, and that IL-6 pretreatment was able to change the level of influx of extracellular calcium ions and the release of stored calcium, thus selectively enhancing the response of intracellular calcium ions to NMDA. In an experiment with mice, IL-6 was shown to be involved in the response to cerebral injury (15). In addition, a number of studies have demonstrated that IL-6 is able to influence the ethological manifestations of animals (16–18). IL-6 has been suggested to be associated with stress (19), as well as certain nervous and mental illnesses, such as infantile autism (20) and migraine (21). Furthermore, IL-6 is closely associated with neurological excitability; thus it has been proposed that IL-6 may be involved in epilepsy. In 1999, Jankowsky and Patterson (2) observed increased levels of oncostatin M (OSM), leukemia inhibitory factor (LIF), cardiotrophin-1 (CT-1), ciliary neurotrophic factor (cNTF) and other cytokines in the hippocampi of pilocarpine-induced epileptic rats, and proposed that cytokines were possibly intercellular informational molecules in the mechanism underlying the formation of epilepsy. D’Arcangle et al(22) later demonstrated that IL-6 was able to reduce synapsin I phosphorylation, and transgenic mice with the synapsin I deletion have been shown to be susceptible to epileptiform seizures during growth (23). Therefore, IL-6 may be involved in epileptic formation. Steffensen et al(24) observed that although IL-6 had a certain protective effect on nerve cell damage under general conditions, the continuously high expression of IL-6 in transgenic mice increased neuron deficiency, gliosis and hippocampal seizure discharge, inhibited the θ rhythm and participated in epileptogenesis. Results from a number of studies (25–27) have indicated that convulsions caused by viral infections may be associated with IL-6. In a previous study, IL-6 was shown to aggravate thermal shock in young mice (28). Moreover, in a study by Lehtimäki et al(29), northern blotting and in situ hybridization were used to assess the effects of kainic acid on the levels of cytokines and their corresponding receptors in the CNS of rats, and it was revealed that IL-6 and IL-6R expression levels had increased. IL-6 has also been suggested to be involved in the control of epilepsy, as well as neuronal damage and protection. Kalueff et al(30) revealed that IL-6 was capable of aggravating chemical-induced rat convulsions. Furthermore, in a study by Peltola et al(31), it was observed in the clinic that, following an epileptic attack, IL-6 levels in the cerebrospinal fluid of patients appeared to increase. It was therefore proposed that IL-1, IL-6 and TNF were regulators of colloid cells following CNS damage. In addition, Lehtimäki et al observed that a one-time epileptic attack was capable of increasing the IL-6 concentration in the blood of patients (32). A Japanese study showed that among pediatric patients with influenza and neurological complications, the IL-6 concentration in the blood of the convulsion group was higher than that of the delirium group and the control group, respectively (33). Therefore, the interrelation between IL-6 and epilepsy requires further study. As clinical research is limited in the actual condition of patients, it is only possible to detect the expression of IL-6 in cerebrospinal fluid or blood. Furthermore, the expression of IL-6 in living brain tissue is unclear. However, the majority of the previous animal experiments have used a chemical-induced epileptic rat model, with which it was difficult to avoid the impact of the chemicals themselves on the toxicity and stimulatory effect of IL-6. Therefore, the present study used the electrical kindling rat model of epilepsy to explore the possible roles of IL-6 and antiepileptic drugs in the incidence and treatment of epilepsy. By observing the changes in the IL-6 mRNA expression level in the hippocampi of electrically kindled epileptic rats, and the effect of an antiepileptic drug on the rats, this methodology was able to avoid the direct toxic action of chemical drugs and reduce the interference of the chemicals, thus making any obtained conclusions more reliable.
Topiramate (TPM) is a second-generation antiepileptic drug that affects a variety of cellular mechanisms (34), such as the inhibition of voltage-dependent sodium channels and the negative modulation of L-type calcium channels. TPM has been suggested to exhibit neuroprotective and antiepileptic effects (35), and was therefore used in this study.
Materials and methods
Animals and grouping
A total of 24 specific pathogen-free (SPF) male Sprague-Dawley (SD) rats, with body weights ranging from 180 to 280 g, were obtained from the Experimental Animal Center of Soochow University (License no. SYXK-Su-2002-0037; Suzhou, China). The rats were randomly divided into four groups: blank control, surgical control, epilepsy and TPM, with six rats in each group. The rats were bred in separate cages away from intense light, under a 24-h circadian cycle, and were allowed to freely eat and drink. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (8th edition, 2011). The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Soochow University.
Preparation of electrically kindled animal models
All rats, with the exception of those in the blank control group, were anaesthetized with 4% (mass concentration) chloral hydrate and fixed onto the brain stereotaxic apparatus. The incisor was set to 2.4 mm below the ear rod. Based on the spectra, the left basolateral amygdaloid nucleus of the rats was determined (2.0 mm caudal to the bregma, 5.0 mm lateral to the midline and 8.0 mm ventral to the dura). A bipolar electrode with a diameter of 0.20 mm was inserted and fixed onto the surface of the skull. The rats were left to recuperate for 1 week following the surgery, and were then stimulated with 2-sec-long 50-Hz, 400-μA continuous single-phase square waves with a width of 1 msec, once daily. For the blank control and surgical control groups, no stimulation was applied. Following Racines’s grading (36), the seizure intensities were observed and recorded. If the rats presented a grade 5 reaction five consecutive times, the kindling was deemed successful and stimulation was immediately stopped.
Synchronous recording of a video electroencephalogram
A Cadwell video electroencephalogram analysis system (Cadwell Laboratories, Inc., Kennewick, WA, USA) was used to synchronously record the background electroencephalogram of the rats in the free state and the afterdischarge duration (ADD) during electrical stimulation. Afterdischarge (AD) refers to a period of epileptiform activity observed with at least five confirmable high-amplitude, multiple-spiked waves that appear following electrical stimulation. ADD is the duration from the end of stimulation to the end of the AD.
Drug experiment and specimen acquisition
Following successful kindling, 2 ml/day normal saline was intragastrically administered to the rats in the blank control, surgical control and epilepsy groups, whereas 85 mg/kg/day TPM was intragastrically administered to the rats in the TPM group. For all groups, the intragastric administration was conducted for eight days. In the epilepsy and TPM groups, electrical stimulation was conducted once prior to the intragastric administration, according to the parameters required for kindling. The characteristics of the seizures and the corresponding electroencephalograms were recorded simultaneously. At the end of the intragastric administration, the electrical stimulation was repeated, and the resulting electroencephalograms were recorded. Moreover, the characteristics of the seizure and the changes in the electroencephalograms between the epilepsy and TPM groups were compared. Twenty-four hours subsequent to the second electrical stimulation, the rats were anaesthetized and the thoracic cavity was opened. Following the puncturing of the aortic arch, the brain of each rat was extracted and washed with phosphate-buffered saline (PBS) and the right cerebral hippocampi were homogenized. The homogenates were stored at −70°C for future use.
Reverse transcription-polymerase chain reaction (RT-PCR)
The homogenized hippocampal tissues were treated with TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) for cell lysis, and then the RNA was extracted with chloroform, precipitated with isopropyl alcohol, eluted with ethyl alcohol and dissolved in diethyl pyrocarbonate (DEPC) solution. Following this, an ultraviolet spectrophotometer (752 Ultraviolet Spectrophotometer Model; Shanghai Precision Scientific Instrument Corp., Shanghai, China) was used to measure the absorbance at 260 nm (A260), and the RNA concentration was calculated. Subsequently, reverse transcription was performed and the cDNA product was stored at 4°C. The obtained cDNA was amplified using PCR with the following primers: β-actin (expected product size of 265 bp) forward primer (P1), 5′-AGGCCGGCTTCGCGGGCGAC-3′, and reverse primer (P2), 5′-CTCGGGAGCCACACGCAGCTC-3′; and IL-6 (expected product size of 442 bp) forward primer (P3), 5′-GAGAAAAGAGTTGTGCAATGGC-3′, and reverse primer (P4), 5′-ACTAGGTTTGCCGAGTAGACC-3′. The PCR conditions were as follows: initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 sec, annealing at 56°C for 30 sec and extension at 72°C for 1 min, followed by a final extension step at 72°C for 7 min. Subsequent to electrophoresis, the PCR amplification products were analyzed using a gel imaging analysis system. A BioCaptMW analysis system (BioCaptMW version 99.05 s system; Vilber Courmat, Marine-La-Vallee, France) was used to detect the gray value of each band, and β-actin was used as the internal reference to semiquantitatively determine the IL-6 mRNA expression levels.
Statistical analysis
The SPSS statistical software package (SPSS, Inc., Chicago, IL, USA) was used for all statistical analysis. The experimental data are expressed as the mean ± standard deviation. The means of the measurement data were compared among the multiple groups using one-way analysis of variance (ANOVA), once the homogeneity of the variance had been confirmed. The Student-Newman-Keuls (SNK) method was used for comparisons between groups. A Fisher’s test was used for the count data. P<0.05 was considered to indicate a statistically significant difference.
Results
Kindling and electroencephalogram records
With regard to the rat kindling process, the shortest period required to achieve kindling was 7 days, while the longest period was 19 days. The mean number of days was 13.50±3.99 days. In the epilepsy group, all the rats presented with grade 5 seizures. Following TPM treatment (the TPM group), the intensity, amplitude and duration of the seizures were decreased, or the seizures of the rats were completely controlled. Among the TPM group, one rat exhibited grade 1 seizures, three rats exhibited grade 3 seizures and two rats exhibited grade 4 seizures. None of the rats exhibited grade 5 seizures in the TPM group. The two groups were significantly different with regard to seizure grade (P<0.05). At all recorded points, the ADD values of the electrically kindled rats were between 21,450 and 119,720 msec (Fig. 1). Moreover, the mean ADD values of the epilepsy and TPM groups were 78,205.67±32,567.93 and 23,880.83±20,184.50 msec, respectively. The ADD of the TPM group was significantly shorter than that of the epilepsy group (P<0.05).
Figure 1.
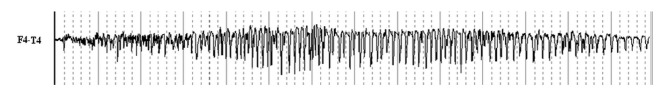
Discharge of epileptic seizures in the rat.
RT-PCR detection of IL-6
The variance analyses of the semiquantitative RT-PCR results for IL-6 mRNA in the hippocampi of the rats in the four treatment groups were significantly different (F=5.959, P<0.05). As shown in Fig. 2, compared with the levels in the two control groups (1.57±0.69 for the blank control group and 1.80±0.54 for the surgical control group), the IL-6 mRNA levels (mean 2.83±0.40) in the hippocampi of rats in the epilepsy group were significantly increased (P<0.05). In addition, the TPM group (2.18±0.56) had a different recovery rate; however, the difference in IL-6 expression was not significant between the TPM and epilepsy groups (P>0.05; Fig. 2).
Figure 2.
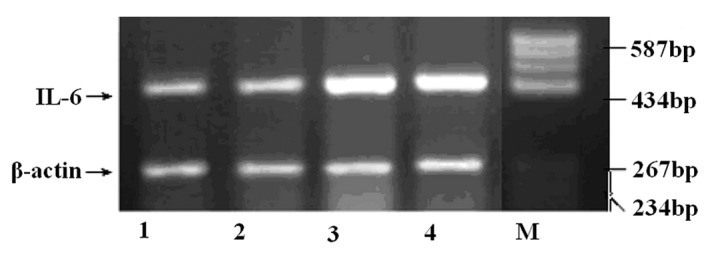
Electrophoresis of the reverse transcription polymerase chain reaction products of interleukin-6 (IL-6). Lane 1, blank control; lane 2, surgical control; lane 3, epilepsy; lane 4, topiramate (TPM); lane M: marker.
Discussion
As a model for temporal lobe epilepsy, the kindling model is widely used in studies of epilepsy, since the spontaneous limbic system epilepsy caused by electrical kindling is similar to temporal lobe epilepsy. In the CNS, a number of regions are able to act as electrical stimulation sites for kindling rats. Among these regions, the basolateral amygdaloid nucleus has the most extensive conduction bundle link, and may be the focus of the epileptiform manifestations in the limbic system (36). In the present study, a solid electrode was inserted into the rats, and electroencephalograms were recorded when the rats were in the free state. This surgical method was convenient, and the detection results and the actual values were reliable. Furthermore, compared with the chemical-induced epilepsy model, the surgical method avoided the interference of drug toxicity on the experimental data and improved the reliability of the results.
The mechanisms that regulate IL-6 expression in the hippocampus have yet to be elucidated. IL-1, interferon (IFN) and TNF, as well as certain neurotransmitters, are capable of promoting IL-6 secretion (37–39), and it was observed that following LTP induction with an embedded electrode, the expression levels of certain cytokines were notably increased. In particular, IL-6 levels were more significantly increased than those of the other cytokines (39). In a study on the changes in IL-6 expression following cerebral ischemia in rats, Ali et al(40) demonstrated that NMDA and ionomycin (a calcium ion carrier) upregulated IL-6 mRNA expression, which indicated that the NMDA receptor-mediated calcium ion influx induced the neurons to generate IL-6. Therefore, LTP and calcium ion influx induce neurons to generate IL-6. The present study demonstrated that the level of IL-6 expression in the hippocampi of electrically kindled rats was increased. One potential mechanism for this is that the electrical kindling increased the levels of excitatory neurotransmitters and decreased the levels of γ-aminobutyric acid (GABA) in the neurons, thereby increasing the influx of neuronal calcium ions, and, consequently, neuronal IL-6 expression. Furthermore, the LTP induced by electrical stimulation may also have been involved in promoting IL-6 secretion.
The involvement of IL-6 with nervous system diseases has been an area of increasing focus, and IL-6 is generally recognized to exhibit neuroprotective effects (41). However, compared with wild-type mice, the hippocampal seizure discharge rates of transgenic mice with continuously high IL-6 expression have been shown to be increased, whereas the θ rhythm of the transgenic mice was inhibited; this continuously high IL-6 expression has been indicated to be involved in seizure formation (24). In the present study, the IL-6 expression in the hippocampi of the electrically kindled rats was increased. The preliminary increase in IL-6 expression may have been a protective mechanism intended to preserve the neurons. However, the continuously high expression may have been involved in the kindling process by increasing gliosis and hippocampal seizure discharge, among other mechanisms (25). Antiepileptic drugs decrease IL-6 expression by varying degrees, and may therefore be useful for maintaining the appropriate IL-6 levels. TPM reduces the frequency and intensity of seizures in epileptic rats, in addition to reducing the cerebral injury caused by epilepsy or anoxia (42). In the present study, although TPM was able to induce the recovery of the IL-6 levels, the difference between the IL-6 levels in the TPM and epilepsy groups was not significant. This phenomenon may have been due to the relatively small sample size utilized, as well as insufficient control over the experimental conditions in the study. Thus, this result remains to be verified in future studies with larger sample sizes. Further studies are also required to investigate the exact mechanisms underlying the action of IL-6 in epilepsy.
Acknowledgements
The authors would like to thank Professor Yin Qi-Zhang and Mrs. Gong Shan for their technical assistance in the establishment of the model. This study was supported by grants from the Project of Science and Technology Commission of Shanghai Baoshan in China (no. 09-E-3) and from the ‘Medical-Engineering (Science) Cross-Research Fund’ of Shanghai Jiaotong University (no. YG2010MS16).
References
- 1.Jankowsky JL, Patterson PH. The role of cytokines and growth factors in seizures and their sequelae. Prog Neurobiol. 2001;63:125–149. doi: 10.1016/s0301-0082(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 2.Jankowsky JL, Patterson TA. Differential regulation of cytokine expression following pilocarpine-induced seizure. Exp Neurol. 1999;159:333–346. doi: 10.1006/exnr.1999.7137. [DOI] [PubMed] [Google Scholar]
- 3.Maroso M, Balosso S, Ravizza T, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Fuller GM. Phosphorylation and internalization of gp130 occur after IL-6 activation of Jak2 kinase in hepatocytes. Mol Biol Cell. 1994;5:819–828. doi: 10.1091/mbc.5.7.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funk JA, Gohlke J, Kraft AD, McPherson CA, Collins JB, Jean HG. Voluntary exercise protects hippocampal neurons from trimethyltin injury: possible role of interleukin-6 to modulate tumor necrosis factor receptor-mediated neurotoxicity. Brain Behav Immun. 2011;25:1063–1077. doi: 10.1016/j.bbi.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Qiu YH, Li B, Ma SH, Peng YP. Neuroprotection of interleukin-6 against NMDA-induced apoptosis and its signal-transduction mechanisms. Neurotox Res. 2011;19:484–495. doi: 10.1007/s12640-010-9215-x. [DOI] [PubMed] [Google Scholar]
- 8.Thier M, März P, Otten U, Weis J, Rose-John S. Interleukin-6 (IL-6) and its soluble receptor support survival of sensory neurons. J Neurosci Res. 1999;55:411–422. doi: 10.1002/(SICI)1097-4547(19990215)55:4<411::AID-JNR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Sterneck E, Kaplan DR, Johnson PF. Interleukin-6 induces expression of peripherin and cooperates with Trk receptor signaling to promote neuronal differentiation in PC12 cells. J Neurochem. 1996;67:1365–1374. doi: 10.1046/j.1471-4159.1996.67041365.x. [DOI] [PubMed] [Google Scholar]
- 10.Akaneya Y, Takahashi M, Hatanaka H. Interleukin-1 beta enhances survival and interleukin-6 protects against MPP+ neurotoxicity in cultures of fetal rat dopaminergic neurons. Exp Neurol. 1995;136:44–52. doi: 10.1006/exnr.1995.1082. [DOI] [PubMed] [Google Scholar]
- 11.Toulmond S, Vige X, Fage D, Benavides J. Local infusion of interleukin-6 attenuates the neurotoxic effects of NMDA on rat striatal cholinergic neurons. Neurosci Lett. 1992;144:49–52. doi: 10.1016/0304-3940(92)90713-h. [DOI] [PubMed] [Google Scholar]
- 12.März P, Heese K, Dimitriades-Schmutz B, Rose-John S, Otten U. Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia. 1999;26:191–200. doi: 10.1002/(sici)1098-1136(199905)26:3<191::aid-glia1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Tancredi V, D’Antuono M, Cafè C, et al. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- 14.Qiu Z, Parsons KL, Gruol DL. Interleukin-6 selectively enhances the intracellular calcium response to NMDA in developing CNS neurons. J Neurosci. 1995;15:6688–6699. doi: 10.1523/JNEUROSCI.15-10-06688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quarta S, Vogl C, Constantin CE, Üçeyler N, Sommer C, Kress M. Genetic evidence for an essential role of neuronally expressed IL-6 signal transducer gp130 in the induction and maintenance of experimentally induced mechanical hypersensitivity in vivo and in vitro. Mol Pain. 2011;7:73. doi: 10.1186/1744-8069-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee RH, Mills EA, Schwartz N, et al. Neurodevelopmental effects of chronic exposure to elevated levels of pro-inflammatory cytokines in a developing visual system. Neural Dev. 2010;5:2. doi: 10.1186/1749-8104-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton MD, Sparkman NL, Johnson RW. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. J Neuroinflammation. 2011;8:54. doi: 10.1186/1742-2094-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, Rooij SE. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res. 2011;14:615–622. doi: 10.1089/rej.2011.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankord R, Zhang R, Flak JN, Solomon MB, Albertz J, Herman JP. Stress activation of IL-6 neurons in the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2010;299:R343–R351. doi: 10.1152/ajpregu.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei H, Zou H, Sheikh AM, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Burstein R, Levy D. Local action of the proinflammatory cytokines IL-1β and IL-6 on intracranial meningeal nociceptors. Cephalalgia. 2012;32:66–72. doi: 10.1177/0333102411430848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Arcangelo G, Tancredi V, Onofri F, D’Antuono M, Giovedì S, Benfenati F. Interleukin-6 inhibits neurotransmitter release and the spread of excitation in the rat cerebral cortex. Eur J Neurosci. 2000;12:1241–52. doi: 10.1046/j.1460-9568.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 23.Terada S, Tsujimoto T, Takei Y, et al. Inhibitory synaptic transmission in mice lacking synapsin I. Neurosci Res. 1997;28(Suppl 1):S57. doi: 10.1083/jcb.145.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffensen SC, Campbell IL, Henriksen SJ. Site-specific hippocampal pathophysiology due to cerebral overexpression of interleukin-6 in transgenic mice. Brain Res. 1994;652:149–153. doi: 10.1016/0006-8993(94)90329-8. [DOI] [PubMed] [Google Scholar]
- 25.Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011;85:6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Once initiated, viral encephalitis-induced seizures are consistent no matter the treatment or lack of interleukin-6. J Neurovirol. 2011;17:496–499. doi: 10.1007/s13365-011-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuda M, Morimoto T, Suzuki Y, Shinonaga C, Ishida Y. Interleukin-6 attenuates hyperthermia- induced seizures in developing rats. Brain Dev. 2007;29:644–648. doi: 10.1016/j.braindev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Lehtimäki KA, Peltola J, Koskikallio E, Keränen T, Honkaniemi J. Expression of cytokines and cytokine receptors in the rat brain after kainic acid-induced seizures. Brain Res Mol Brain Res. 2003;110:253–260. doi: 10.1016/s0169-328x(02)00654-x. [DOI] [PubMed] [Google Scholar]
- 30.Kalueff AV, Lehtimaki KA, Ylinen A, Honkaniemi J, Peltola J. Intranasal administration of human IL-6 increases the severity of chemically induced seizures in rats. Neurosci Lett. 2004;365:106–110. doi: 10.1016/j.neulet.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 31.Peltola J, Hurme M, Miettinen A, Keränen T. Elevated levels of interleukin-6 may occur in cerebrospinal fluid from patients with recent epileptic seizures. Epilepsy Res. 1998;31:129–133. doi: 10.1016/s0920-1211(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 32.Lehtimäki KA, Keränen T, Palmio J, et al. Increased plasma levels of cytokines after seizures in localization-related epilepsy. Acta Neurol Scand. 2007;116:226–230. doi: 10.1111/j.1600-0404.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 33.Fukumoto Y, Okumura A, Hayakawa F, et al. Serum levels of cytokines and EEG findings in children with influenza associated with mild neurological complications. Brain Dev. 2007;29:425–430. doi: 10.1016/j.braindev.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Menachem E, Sander JW, Stefan H, Schwalen S, Schauble B. Topiramate monotherapy in the treatment of newly or recently diagnosed epilepsy. Clin Ther. 2008;30:1180–1195. doi: 10.1016/s0149-2918(08)80045-8. [DOI] [PubMed] [Google Scholar]
- 35.Edmonds HL, Jr, Jiang YD, Zhang PY, Shank R. Topiramate as a neuroprotectant in a rat model of global ischemia-induced neurodegeneration. Life Sci. 2001;69:2265–2277. doi: 10.1016/s0024-3205(01)01306-6. [DOI] [PubMed] [Google Scholar]
- 36.Castillo CG, Mendoza S, Freed WJ, Giordano M. Intranigral transplants of immortalized GABAergic cells decrease the expression of kainic acid-induced seizures in the rat. Behav Brain Res. 2006;171:109–115. doi: 10.1016/j.bbr.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Gadient RA, Otten UH. Interleukin-6 (IL-6) - a molecule with both beneficial and destuctive potentials. Prog Neurobiol. 1997;52:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 38.Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 39.Jankowsky JL, Derrick BE, Patterson PH. Cytokine responses to LTP induction in the rat hippocampus: a comparison of in vitro and in vivo techniques. Learn Mem. 2000;7:400–412. doi: 10.1101/lm.32600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali C, Nicole O, Docagne F, et al. Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J Cereb Blood Flow Metab. 2000;20:956–966. doi: 10.1097/00004647-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Calkavur S, Akisu M, Olukman O, et al. Genetic factors that influence short-term neurodevelopmental outcome in term hypoxic-ischaemic encephalopathic neonates. J Int Med Res. 2011;39:1744–1756. doi: 10.1177/147323001103900517. [DOI] [PubMed] [Google Scholar]
- 42.Pitkänen A. Efficacy of current antiepileptics to prevent neurodegeneration in epilepsy models. Epilepsy Res. 2002;50:141–160. doi: 10.1016/s0920-1211(02)00076-1. [DOI] [PubMed] [Google Scholar]


