Abstract
The challenge in understanding cognitive impairment in schizophrenia is that people with this illness have deficits in an array of domains. Here we briefly review evidence regarding the pattern of deficits within three domains: context processing, working memory and episodic memory. We suggest that there may be a common mechanism driving deficits in these domains -- an impairment in the ability to actively represent goal information in working memory to guide behavior, a function we refer to as proactive control. We suggest that such deficits in proactive control reflect impairments in dorsolateral prefrontal cortex, its interactions with other brain regions such as parietal cortex, thalamus, and striatum, and the influence of neurotransmitter systems such as dopamine, GABA and glutamate.
Centrality of Cognition in Schizophrenia
When we think of the core symptoms of illness such as schizophrenia, we think of people who hear voices, see visions and have false beliefs about reality (i.e., delusions). However, clinicians have long recognized that abnormalities in cognitive function are a key component of schizophrenia, one of the most debilitating psychiatric disorders. As such, the last three decades have witnessed a relative explosion of research on cognition in schizophrenia, much of it couched within the framework of understanding the cognitive neuroscience of schizophrenia. This emphasis on cognition in schizophrenia is in part due to the growing body of research suggesting that cognitive function in schizophrenia is one of the most critical determinants of quality of life and function in schizophrenia, potentially more so than the severity of other symptoms of schizophrenia such as hallucinations or delusions [1].
The challenge we face in understanding the nature of cognitive impairments in schizophrenia is that at least on the surface, individuals with this illness appear to have deficits in a diverse array of domains, such as working memory, language function, executive function, episodic memory, processing speed, attention, inhibition and sensory processing [2, 3]. While it is certainly possible that this seemingly diverse array of impairments reflects dissociable deficits, each with its own psychological and neural pathophysiology, this explanation does little to help us conceptualize the nature of cognitive dysfunction in this illness, or in other psychotic disorders (see Text Box 1). Instead, a number of researchers have argued that a common mechanism contributing to cognitive impairments across a range of domains in schizophrenia is an inability to actively represent goal information in working memory needed to guide behavior, and that this deficit reflects impairments in the function of the dorsolateral prefrontal cortex prefrontal cortex (DLPFC), its interactions with other brain regions such as the parietal cortex, the thalamus, and the striatum, and the influence of neurotransmitter systems such as dopamine, GABA and glutamate [4-6]. In the sections below, we provide a discussion of the evidence for such impairment in schizophrenia, and how it manifests in domains typically referred to as executive control, working memory and episodic memory.
Text Box 1. Cognition Across Psychotic Disorders.
Schizophrenia is often referred to as a “non-affective psychosis.” There are other illnesses, such as schizoaffective disorder, major depression with psychosis, and bipolar disorder that are often referred to as “affective psychoses” because they involve difficulties with mood (depression or mania) in addition to symptoms such as hallucination or delusions. A key question is whether the nature and/or severity of cognitive impairment found in affective psychoses is similar or different to that found in schizophrenia. If qualitatively different, this would argue for a fundamentally different role for cognition in affective psychoses. However, if the pattern or profile of cognitive impairment is similar, such a result would be consistent with the hypothesis that there are common dimensions of psychopathology across the affective and nonaffective psychoses [78]. Both empirical and meta-analytic studies have fairly consistently shown that the degree of cognitive impairment in schizophrenia is worse than the degree of cognitive impairment in bipolar disorder and psychotic major depression [e.g., 79, 80, 81]. The literature on the comparison of schizoaffective disorder to schizophrenia is mixed, with some studies finding very similar magnitudes of cognitive impairments in these two disorders [82, 83], but others finding evidence of worse impairment in schizophrenia [84].
Despite the evidence of a larger magnitude of cognitive impairment in schizophrenia as compared to affective psychoses, the literature is very consistent in demonstrating that the profile of cognitive impairment is similar across schizophrenia and affective psychoses. In other words, the relative severity of impairments across different cognitive domains tends to be very similar in bipolar disorder, psychotic major depression and schizoaffective disorders as compared to schizophrenia [e.g., 79, 83, 85]. Thus, the literature on cognitive dysfunction in psychosis suggests that all of the psychoses (affective or non-affective) are associated with some level of cognitive impairment, that it may be equally severe in schizophrenia and schizoaffective disorder, but that it may be less severe for individuals with psychotic bipolar disorder and psychotic major depression. However, importantly from the perspective of understanding etiology, the profile or pattern of cognitive impairment across affective psychoses is very similar to that seen in schizophrenia. This finding is consistent with the idea that there are common mechanisms that lead to cognitive dysfunction across psychotic disorders and with a growing emphasis on identifying core neural system that contribute to impairments that cut across traditional diagnostic boundaries [86].
Context, Proactive Control and Goal Representation in Schizophrenia
In previous work based in part upon computational modeling, Cohen and colleagues put forth the hypothesis that intact function of dopamine in DLPFC was responsible for the processing of context, and that a disturbance in this mechanism was responsible for a range of cognitive deficits in schizophrenia [e.g., 7, 8, 9]. In this framework context refers to prior task-relevant information, including task instructions, the results of processing prior stimuli, and goals that are represented and maintained in working memory in a form that can bias the selection of an appropriate behavioral response. One critical insight that emerged from this work was that a single deficit in one aspect of executive control could contribute to deficits in cognitive domains often treated as independent. More specifically, deficits in working memory, attention, inhibition, and language processing in schizophrenia can arguably all be understood in terms of a deficit in context-processing, as each of these domains requires the active representation of such context information for effective function [see for full discussion., 8, 9]. Numerous prior studies have provided support for these hypotheses concerning context-processing deficits in schizophrenia (for a review, see [10]), as well as evidence for impairments in individuals at risk for schizophrenia [e.g., 11, 12, 13] suggesting that such deficits may be associated with liability to schizophrenia as well as manifest illness.
In more recent years, the key role of context processing in cognition and in schizophrenia has been reconceptualized somewhat more broadly as the function of proactive cognitive control [5, 14-16]. This conceptualization builds upon earlier ideas of context processing to argue for flexible mechanisms of cognitive control that allow humans to deal with the diversity of challenges that we face in everyday life. In this theory, termed dual mechanisms of control [5, 14, 15], a distinction is made between proactive and reactive modes of cognitive control. The proactive control mode can be thought of as a form of “early selection,” in which goal-relevant information is actively maintained in a sustained or anticipatory manner, before the occurrence of cognitively demanding events. This allows for optimal biasing of attention, perception, and action systems in a goal-driven manner. By goal information, we mean information about what one needs to accomplish in this particular task situation or the intended outcome of a series of actions or mental operations. In real life settings, such goals may include the main points you wish to communicate in a conversation, or the need to organize a shopping trip so that you can make sure to get everything you need. In contrast, in the reactive mode, attentional control is recruited as a “late correction;” mechanism that is mobilized only when needed, such as after a high-interference event is detected (e.g., you encounter unexpected distracting stimuli and need to retrieve the topic of your conversation). Thus, proactive control relies on the anticipation and prevention of interference before it occurs, whereas reactive control relies on the detection and resolution of interference after its onset.
This theory postulates that proactive control depends on actively representing information in lateral prefrontal cortex, and that the updating and maintenance of such information depends on precise inputs from neurotransmitter systems such as dopamine into prefrontal cortex. As outlined in detail in [17], this theory does not argue that proactive and reactive control are mutually exclusive, and individuals are likely using some balance between the two modes to successfully meet most ongoing cognitive demands. However, Braver [17] has argued that the two control modes can be distinguished based on their temporal characteristics (e.g., when they are engaged in the course of cognitive processing), the requirement to actively maintain control representations over time for proactive control, and the need for temporally precise interactions with the dopamine system. Further, Braver has suggested that there may be biases to favor one processing mode over the other, which may be dependent on task demands (e.g., high conflict situations may push toward a proactive control mode) and individual differences factors such as working memory capacity, fluid intelligence, and even personality traits such as reward sensitivity. Further, proactive control may be particularly vulnerable to disruption, given that it is resource demanding, and dependent upon temporally precise dopamine-prefrontal interactions. Thus, populations characterized by disordered prefrontal and dopamine function (such as schizophrenia and/or older adults) may rely more heavily on reactive control, as it may be more robust in the face of such dysfunction [5].
In support of this theory, there is ample evidence for an association between impairments in DLPFC activity and deficits of proactive control in schizophrenia [18, 19], for both medicated [20] and unmedicated patients [7, 21], as well as those at risk for the development of schizophrenia [22, 23]. For example, an elegant meta-analysis of imaging studies of executive control and proactive control conducted by Minzenberg and colleagues [18] provided robust evidence for reduced activity in DLPFC in schizophrenia (see Figure 1). Further, there is growing evidence for a critical role for impaired connectivity between the DLPFC and other cognitive control related brain regions [24-27], positive impacts of dopamine enhancement on cognitive control in psychosis [28, 29], and intriguing evidence for GABAergically mediated [30] impairment in neural oscillations that may support representations in DLPFC [31, 32].
Figure 1. Brain Regions Showing Altered Activity During Executive Function in Schizophrenia.
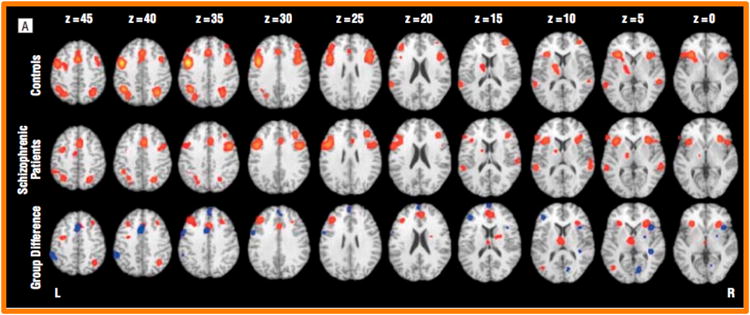
Brain regions with significant activation across executive function task types. In the bottom row, clusters in which controls showed more activation than schizophrenic patients are in red and clusters in which schizophrenic patients showed more activation than controls are in blue. Reproduced, with permission, from [18].
Working Memory in Schizophrenia
Although there is a huge amount of work on executive function and proactive cognitive control in schizophrenia, there is perhaps even more data on working memory deficits in this illness [3, 33]. However, simply saying that individuals with schizophrenia have deficits on working memory tasks is not particularly informative, as working memory is a complex construct with different subprocesses. To understand what specific aspects of working memory might be impaired in schizophrenia and how this might relate to the proactive control impairments described above, it is helpful to refer to current models of working memory function, such as Baddeley's model [34]. This model proposes four major components of working memory: 1) the visuo-spatial sketch pad, a short-term storage buffer for visual information; 2) the phonological loop, a short-term storage buffer for verbal information; 3) a central executive that supports the manipulation and transformation of information held within the storage buffers; and 4) an episodic buffer, in which complex, multi-modal events are integrated and stored online [34]. The central executive has been associated with the function of DLPFC in numerous studies, while the storage buffers have been associated with both inferior frontal and posterior parietal function [35].
There is relatively little consistent evidence for either behavioral or neurobiological impairments in working memory in schizophrenia that can be unambiguously attributed to deficits in either the verbal or visual-spatial buffer systems [36]. In contrast, there is robust evidence that individuals with schizophrenia have difficulty with processes attributed to the central executive component of working memory [36]. One argument used to support the presence of central executive dysfunction in schizophrenia is the fact that patients with this illness have deficits on working memory tasks with all different material types, with relatively little evidence for selective deficits with one material type over another [3, 33]. Another argument is that individuals with schizophrenia consistently show deficits on tasks designed to measure a range of functions ascribed to the central executive, including manipulation [37, 38], interference control and/or dual-task coordination [e.g., 39], and information updating and temporal indexing [e.g., 40]. However, not all studies have shown manipulation impairments in schizophrenia [41] and manipulation is not always differentially impaired compared to maintenance of information in working memory [e.g., 42, 43]
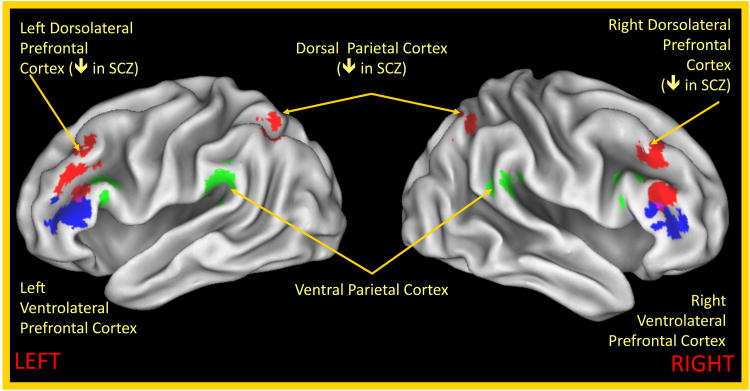
Further, there is very robust evidence for altered DLPFC function among individuals with schizophrenia and their relatives during performance of working memory tasks that require central executive type functions, though the exact nature of the DLPFC impairment can vary as a function of both sample and task characteristics [44, 45]. For example, work comparing working memory performance during a task requiring updating and maintenance of information to control conditions with similar verbal and spatial processing requirements (see Figure 2) suggested reduced activity in bilateral DLPFC and dorsal parietal cortex in schizophrenia [46]. However, this study found relatively intact activity of ventrolateral prefrontal and parietal regions typically associated with the function of domain specific storage buffers (e.g., verbal rehearsal, visual spatial scratchpad) [46]. There is also even evidence for impaired DLPFC activity during encoding or maintenance of information during working memory tasks that do not necessarily place heavy demands on central executive function (e.g., spatial delayed response tasks) [47, 48], suggesting the possibility that central executive or proactive control mechanisms could be contributing to impaired performance in task contexts that might not normally be associated with strong proactive control demands in healthy individuals. Importantly, as with proactive control, there is evidence suggesting a key role for impaired connectivity between DLPFC and other working memory related regions (e.g., parietal cortex, thalamus and striatum) in understanding working memory in schizophrenia [46, 49, 50], as well as evidence for altered gamma and theta oscillatory activity in prefrontal regions [e.g., 51, 52].
Figure 2. Regions Showing Altered Activity During Working Memory in Schizophrenia.
Regions in red showed reduced activity in individuals with schizophrenia compared to controls in a comparison of working memory performance to a control task in [46]. Regions in blue and green on the left hemisphere showed greater activity for verbal and than non-verbal working memory in both healthy controls and individuals with schizophrenia. Regions in blue and green on the right hemisphere are homologous regions to those on the left hemisphere.
The discussion above primarily focuses on the common role of DLPFC in proactive control and working memory impairments in schizophrenia. One can also consider the ways in which the cognitive mechanisms involved in proactive control may influence the magnitude and timing of brain activation in working memory tasks. A failure to use proactive control would suggest that patients may show reduced activity during encoding and/or maintenance in lateral prefrontal regions if they fail to effectively encode or maintain information over the delay in preparation for responding. If so, when a response is needed, they may need to try to retrieve the memoranda, potentially resulting in increased activation in brain regions associated with memory retrieval or response selection. A number of studies that examined the timecourse of activity during working memory trials have shown evidence for reduced activity during encoding and maintenance periods in DLPFC, as well as other working memory related brain regions [5, 47, 48, 53]. Further, studies that have specifically examined retrieval related activity have found evidence for increased activation among individuals with schizophrenia in either the same or different regions that showed reduced encoding/maintenance related activation [5, 54].
This brief summary does not cover the body of work suggesting robust impairments in working memory capacity and processing speed in schizophrenia (see Text Box 2). However, recent findings suggesting that the prefrontal cortex may provide top-down support to enhance working memory capacity raises the intriguing possibility that proactive control mechanisms could even influence working memory capacity in schizophrenia [55].
Text Box 2. Working Memory Capacity and Processing Speed in Schizophrenia.
Numerous studies have documented reduced working memory capacity in schizophrenia, including studies using change detection measures [e.g., 87, 88-91] that are thought to be a relatively “pure” measure of storage capacity [92]. Such capacity deficits cannot be solely accounted for by impaired control of attention [88] or by impaired precision of visual representations [90]. Recent work has shown that when the contributions of rehearsal, grouping and chunking are reduced, working memory capacity is in the range of 3-5 items [92]. Several different lines of research point to the parietal cortex as a key node in the working memory network that drives these capacity limitations [93-96]. Work on the coding or signal properties of the parietal cortex that are responsible for this limitation suggests several potential mechanisms. One proposal is that items are maintained in WM as unique patterns of coordinated firing across many neurons [97]. In such models, the number of high frequency oscillations (i.e., gamma range) that can be embedded in a lower frequency oscillation (e.g., theta) determines the number of distinct items that can be maintained in memory [97]. Alternatively, another hypothesis has been that lateral inhibition mechanisms involved in neuronal representation limit the number of items that can be simultaneously maintained. Importantly, there is also data to suggest that DLPFC mechanisms can help increase capacity by virtue of enhanced excitatory input that competes with lateral inhibition mechanisms [55]. As such, it is plausible that one mechanism contributing to impaired working memory capacity in schizophrenia is a reduction in DLPFC input that can normally serve to boost working memory capacity, a hypotheses that remains to be tested.
There is also robust evidence that individuals with schizophrenia show slowed processing speed. In fact, meta-analytic work suggested that impairments in processing speed as indexed by performance on Digit Symbol Coding type tasks reflect one of the largest effects sizes for cognitive impairment in schizophrenia [98]. One possibility is that such slowed processing speed is related to the integrity of white matter tracks supporting the speed of neuronal communication [99]. However, it should be noted that in many ways, the Digit Symbol Coding Task is a type of working memory task, as efficient performance can be aided by the ability to rapidly bind representations in working memory and use them to guide performance. As such, further research is needed to determine the specific mechanisms that lead to impaired performance on such tasks.
Episodic Memory in Schizophrenia
When one discusses episodic memory functions a typical focus is on encoding, binding, relational, and retrieval mechanisms that are frequently associated with the function of medial temporal regions, including the hippocampus [56, 57]. In schizophrenia, there is meta-analytic evidence for greater impairments in relational than item memory [58]. More recently, clinical researchers have begun to use tasks derived form the animal literature on hippocampal function, such as the transitive interference test, which measures the ability to learn the relationships among hierarchically arranged stimulus pairs. Individuals with schizophrenia are impaired on critical conditions of this task requiring relational processing, but not on conditions that require simpler associative reinforcement mappings [59, 60]. Other work has used eye-movement measures of relational memory, shown to be impaired in patients with hippocampal lesions [61, 62], to identify relational memory impairments in schizophrenia [63, 64]. Still other work has provided evidence for greater deficits in recollection than familiarity in schizophrenia, which have also been interpreted as reflecting relational memory impairments [65, 66].
Many researchers have taken this body of work to suggest that episodic memory impairments in schizophrenia reflect medial temporal lobe deficits, with a specific focus on the hippocampus [e.g., 67]. However, it has become increasingly clear that prefrontal structures also make important contributions to episodic memory. Damage to the prefrontal cortex can lead to episodic memory deficits, although episodic memory is typically not the only cognitive function impaired in these individuals [e.g., 68]. Such findings have contributed to the hypothesis that prefrontal cortex damage alters episodic memory by impairing strategic contributions to memory formation and retrieval [e.g., 68]. For example, studies have shown activation of ventral prefrontal regions such as Brodmann's areas 45 an 47 when participants are asked to processing verbal information use semantic elaboration strategies [e.g., 69] that promote subsequent memory. In addition, one of the most compelling findings supporting a key role for such prefrontal structures in episodic memory are results showing that the increased activation during encoding in frontal regions such as BA 45 and 47 is very strongly predictive of subsequent memory performance [e.g., 69]. Further, there is recent work suggesting the DLPFC may contribute specifically to successful relational memory formation and retrieval [e.g., 70, 71].
Such findings in healthy individuals raise the possibility that at least some episodic memory impairments among individuals with schizophrenia also reflect deficits in prefrontally mediated cognitive functions that contribute to successful memory encoding and retrieval, including strategic and proactive control mechanisms that may facilitate memory formation. Consistent with this hypothesis, a number of studies suggest that individuals with schizophrenia are impaired in their ability to generate effective mnemonic strategies (for review, see [36]). However, when provided with strategies that promote successful episodic encoding, individuals with schizophrenia are typically able to benefit as much as controls from these strategies [e.g., 72, 73, 74], and can even show intact item recognition when provided with support for effective encoding [75]. Further, a meta-analysis of brain activity alterations during episodic memory performance in schizophrenia showed consistent evidence for reduced activation in both ventrolateral and DLPFC, but did not find consistent evidence for altered hippocampal activity [76]. Recent work on relational memory encoding and retrieval has shown evidence for impaired DLPFC function associated with impaired relational memory function in schizophrenia [77]. Although these findings by no means exclude medial temporal lobe contributions to impaired episodic memory in schizophrenia, they do suggest a need to also take into account a role for prefrontally mediated cognitive functions. These findings also do not directly point to a specific contribution of proactive control in episodic memory impairments and primarily focus on the evidence for DLPFC involvement in episodic memory impairments in schizophrenia. Thus, further work is needed to determine whether the ability to detect the need for strategies or the ability to use effective strategies specifically engages proactive control mechanisms. Nonetheless, the findings on strategic changes in schizophrenia and the role of DLPFC in episodic memory suggests that examining the role of proactive control deficits may be a fruitful avenue for future research on episodic memory in schizophrenia.
Conclusion
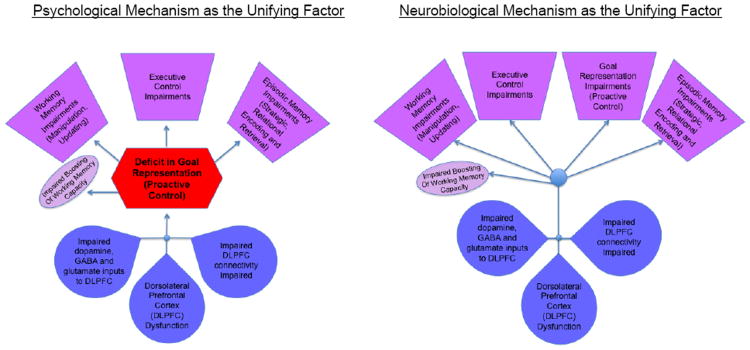
As this brief review highlights, individuals with schizophrenia show significant deficits in a number of different cognitive domains, including executive function, working memory and episodic memory. We have suggested that there is a common mechanism contributing to these deficits – an impairment in proactive control that can influence performance in a wide variety of cognitive domains. Further, we have suggested that at the neural level, a common denominator to such deficits may be impaired function of DLPFC, its connectivity with other brain regions, and the neurotransmitter systems that support precise updating and maintenance of goal representations that enable proactive control. We should note however, that there are a number of open questions (see Text Box 3) and at least two ways to conceptualize this hypothesis. One is that the unifying factor is really the psychological mechanism of goal representation/proactive control, as illustrated in Figure 3. In other words, this hypothesis would suggest that the influence of DLPFC function on the range of cognitive domains we have highlighted in this review is mediated through proactive control deficits. However, alternatively, as shown in Figure 3, it may be that that there are multiple cognitive functions that are impacted by DLPFC dysfunction directly, but that these effects are not mediated through proactive control deficits. Further work will be needed to tease apart these options.
Text Box 3. Key Questions For Future Research On Proactive Control in Schizophrenia.
Do impairments in the ability to strategically manipulate information for effective encoding into episodic memory reflect the same psychological or neural mechanisms as proactive control impairments?
Schizophrenia is not the only population characterized by impairments in prefrontal function and dopamine, as such deficits are also found in healthy aging, other psychiatric disorders (e.g., bipolar) and substance abusing individuals. To what extent are such deficits similar or different across these populations, and do proactive control/DLPFC deficits reflect a final common pathway that can account for findings of similar cognitive and functional impairments across such populations?
There are other examples of impairments in “predictive” behavior in schizophrenia, such as impaired smooth pursuit performance. Do these types of impairments also reflect altered “proactive” control deficits, or do they reflect different cognitive and neural mechanisms?
Is the unifying factor among a least a subset of cognitive impairments in schizophrenia the cognitive mechanisms involved in proactive control or DLPFC dysfunction?
Does the hypothesis regarding proactive control deficits in schizophrenia point to specific pathways for treatment?
Figure 3. Potential Common Mechanisms of Cognitive Dysfunction in Schizophrenia.
Figure illustrating two potential pathways linking deficits in goal maintenance/proactive control, DLPFC function, and other cognitive impairments in schizophrenia. The figure on the left illustrates a pathway in which the influence of DLPFC dysfunction on deficits in cognitive domains such as executive control, working memory and episodic memory in schizophrenia is mediated by an impairment in proactive control, which leads to impairments in these other domains. The figure on the right illustrates a pathway by which DLPFC function directly influences cognitive function in many domains in schizophrenia (including proactive control), but in which deficits in other cognitive domains are not mediated through proactive control impairments.
We do not mean to imply that all aspects of cognitive impairment in schizophrenia can be fully explained by these mechanisms. Schizophrenia is a complex disorder and it is clear that it would be an oversimplification to suggest that a single mechanism could explain the diversity of impairments found in this illness. Nonetheless, we think it important to raise the possibility that there is a common core mechanism that can help explain at least of subset of impairments, and which may serve as an excellent target for therapeutic interventions that could serve to broadly enhance cognitive function and outcome in this illness. The question of how to use psychological and/or pharmacological approaches to remediate cognitive impairments found in schizophrenia is a highly active area of current research and the focus of the article by Minzenberg and Carter in this same issue [100].
Acknowledgments
Deanna Barch receives funding from the NIH, the McDonnell Center for Higher Brain Function, and NARSAD. Alan Ceaser has been funded by the National Science Foundation.
Glossary
- Context
Information that can be used to modify the interpretation of an event or the response of an individual to stimuli in their environment. Context is information that must be “actively” held in mind so that it can be used mediate task appropriate behavior. Context information can be a specific prior stimulus, the result of processing a sequence of prior stimuli, or more abstract information such as task instructions. Language processing provides a useful example of context information, as in the following sentence: “In order to keep pigs, you need a pen.” In this sentence, the first clause serves as context that biases you towards the appropriate meaning of the word “pen” (a fenced enclosure) for this sentence, rather than the more common meaning of the word “pen” (a writing instrument).” This is a case in which the result of processing the first part of the sentence (e.g., a sequence of prior stimuli) creates a contextual representation that can bias future behavior (e.g., semantic interpretation of the word “pen) [10]
- Episodic Memory
The ability to learn and then subsequently retrieve information about new “episodes” in one's life. Episodes can refer to events, experiences, situations, or new associations, and can be linguistic or non-linguistic in content.
- Executive Control
A collection of processes involved in supporting a range of functions thought to be critical for effective cognitive function, including processes such as goal maintenance, set-shifting, and inhibition.
- Neurotransmitter System
Types of chemicals in the brain that support communication between neurons across synapses. Some examples include the dopamine, serotonin, acetylcholine, glutamate and norepinephrine systems.
- Proactive Control
A form of cognitive processing in which goal relevant information is actively maintained in a form that biases attention, perception and action towards the achievement of that goal [17]
- Psychosis
A mental disturbance in which thoughts, emotions, and perceptions lose touch with reality. Individuals experiencing psychosis can have delusions, hallucinations, and disorganized speech and behavior, as well as other types of alterations (e.g., cognitive and motivational impairments). The psychiatric disorder referred to as schizophrenia involves psychosis, but psychosis can also occur in other psychiatric disorders such as Major Depression and Bipolar Disorder.
- Working Memory
The ability to temporarily maintain and manipulate information [34]
Contributor Information
Deanna M. Barch, Washington University In St. Louis
Alan Ceaser, Washington University In St. Louis.
References
- 1.Nuechterlein KH, et al. Neurocognitive predictors of work outcome in recent- onset schizophrenia. Schizophr Bull. 2011;37(2):S33–40. doi: 10.1093/schbul/sbr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesholam-Gately RI, et al. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- 3.Forbes NF, et al. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- 4.Barch DM, et al. CNTRICS final task selection: executive control. Schizophr Bull. 2009;35:115–135. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards BG, et al. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Front Hum Neurosci. 2010;4:32. doi: 10.3389/fnhum.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesh TA, et al. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barch DM, et al. Selective deficits in prefrontal cortex regions in medication naive schizophrenia patients. Arch Gen Psychiatry. 2001;50:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 8.Braver TS, et al. Cognition and control in schizophrenia: A computational model of dopamine and prefrontal function. Biol Psychiat. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JD, et al. Context-processing deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psych. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 10.Barch DM, Braver TS. Cognitive control in schizophrenia: Psychological and neural mechanisms. In: Engle RW, et al., editors. Cognitive limitations in aging and psychopathology. Cambridge University Press; 2007. pp. 122–159. [Google Scholar]
- 11.Snitz BE, et al. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald AW, 3rd, et al. Functional magnetic resonance imaging study of cognitive control in the healthy relatives of schizophrenia patients. Biol Psychiat. 2006;60:1241–1249. doi: 10.1016/j.biopsych.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald AW, 3rd, et al. A convergent-divergent approach to context processing, general intellectual functioning, and the genetic liability to schizophrenia. Neuropsychology. 2005;19:814–821. doi: 10.1037/0894-4105.19.6.814. [DOI] [PubMed] [Google Scholar]
- 14.Braver TS, et al. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway AR, et al., editors. Variation in Working Memory. Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- 15.Braver TS, et al. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddon JE, Killcross S. Contextual control of choice performance: behavioral, neurobiological, and neurochemical influences. Ann NY Acad Sci. 2007;1104:250–269. doi: 10.1196/annals.1390.000. [DOI] [PubMed] [Google Scholar]
- 17.Braver TS. The variable nature of cognitive control: A dual-mechanisms framework. Trends Cog Sci. doi: 10.1016/j.tics.2011.12.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minzenberg MJ, et al. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbalat G, et al. Organization of cognitive control within the lateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry. 2009;66:377–386. doi: 10.1001/archgenpsychiatry.2009.10. [DOI] [PubMed] [Google Scholar]
- 20.Holmes AJ, et al. Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophr Res. 2005;76:199–206. doi: 10.1016/j.schres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald A, et al. Specificity of prefrontal dysfunction and context processing deficts to schizophrenia in a never medicated first-episode psychotic sample. Am J Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald AW, 3rd, et al. Imaging genetic liability to schizophrenia: systematic review of FMRI studies of patients' nonpsychotic relatives. Schizophr Bull. 2009;35:1142–1162. doi: 10.1093/schbul/sbn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fusar-Poli P, et al. Neurofunctional correlates of vulnerability to psychosis: A systematic review and meta-analysis. Neurosci Biobehav R. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Yoon JH, et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fornito A, et al. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiat. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole MW, et al. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiat. 2011;70:43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Repovs G, et al. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiat. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res. 2005;77:43–58. doi: 10.1016/j.schres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 29.McClure MM, et al. Pergolide treatment of cognitive deficits associated with schizotypal personality disorder: continued evidence of the importance of the dopamine system in the schizophrenia spectrum. Neuropsychopharmacol. 2010;35:1356–1362. doi: 10.1038/npp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis DA, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minzenberg MJ, et al. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacol. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho RY, et al. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. P Natl Acad Sci. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psych. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 34.Baddeley AD. The episodic buffer: A new component of working memory? Trends Cog Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 35.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Ne. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 36.Barch DM. The cognitive neuroscience of schizophrenia. In: Cannon T, Mineka S, editors. Annual Review of Clinical Psychology. American Psychological Association; 2005. pp. 321–353. [DOI] [PubMed] [Google Scholar]
- 37.Kim J, et al. Maintenance and manipulation of information in schizophrenia: further evidence for impairment in the central executive component of working memory. Schizophr Res. 2004;68:173–187. doi: 10.1016/S0920-9964(03)00150-6. [DOI] [PubMed] [Google Scholar]
- 38.Horan WP, et al. Verbal working memory impairments in individuals with schizophrenia and their first-degree relatives: findings from the Consortium on the Genetics of Schizophrenia. Schizophr Res. 2008;103:218–228. doi: 10.1016/j.schres.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith EE, et al. Intact and impaired cognitive-control processes in schizophrenia. Schizophr Res. 2011;126:132–137. doi: 10.1016/j.schres.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Galletly CA, et al. Impaired updating of working memory in schizophrenia. Int J Psychophysiol. 2007;63:265–274. doi: 10.1016/j.ijpsycho.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Thakkar KN, Park S. Impaired Passive Maintenance and Spared Manipulation of Internal Representations in Patients With Schizophrenia. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quee PJ, et al. Working memory in schizophrenia: a systematic study of specific modalities and processes. Psychiat Res. 2011;185:54–59. doi: 10.1016/j.psychres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Hill SK, et al. Salience of working-memory maintenance and manipulation deficits in schizophrenia. Psychol Med. 2010;40:1979–1986. doi: 10.1017/S003329171000019X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Snellenberg JX, et al. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- 45.Glahn DC, et al. Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barch DM, Csernansky JG. Abnormal parietal cortex activation during working memory in schizophrenia: verbal phonological coding disturbances versus domain-general executive dysfunction. Am J Psychiatry. 2007;164:1090–1098. doi: 10.1176/ajp.2007.164.7.1090. [DOI] [PubMed] [Google Scholar]
- 47.Anticevic A, et al. Working Memory Encoding and Maintenance Deficits in Schizophrenia: Neural Evidence for Activation and Deactivation Abnormalities. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Driesen NR, et al. Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry. 2008;64:1026–1034. doi: 10.1016/j.biopsych.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karlsgodt KH, et al. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 50.Henseler I, et al. Disturbed functional connectivity within brain networks subserving domain-specific subcomponents of working memory in schizophrenia: relation to performance and clinical symptoms. J Psychiatr Res. 2010;44:364–372. doi: 10.1016/j.jpsychires.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Basar-Eroglu C, et al. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Barr MS, et al. Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophr Res. 2010;121:146–152. doi: 10.1016/j.schres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 53.Schlosser RG, et al. Inefficient executive cognitive control in schizophrenia is preceded by altered functional activation during information encoding: an fMRI study. Neuropsychologia. 2008;46:336–347. doi: 10.1016/j.neuropsychologia.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Johnson MR, et al. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiat. 2006;60:11–21. doi: 10.1016/j.biopsych.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Edin F, et al. Mechanism for top-down control of working memory capacity. P Natl Acad Sci USA. 2009;106:6802–6807. doi: 10.1073/pnas.0901894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eichenbaum H, et al. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Front Neurosci. 2009;3:166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Achim AM, Lepage M. Is associative recognition more impaired than item recognition memory in schizophrenia? A meta-analysis. Brain Cognition. 2003;53:121–124. doi: 10.1016/s0278-2626(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 59.Titone D, et al. Transitive inference in schizophrenia: Impairments in relational memory organization. Schizophr Res. 2004;68:235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- 60.Coleman MJ, et al. Reinforcement ambiguity and novelty do not account for transitive inference deficits in schizophrenia. Schizophr Bull. 2010;36:1187–1200. doi: 10.1093/schbul/sbp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. J Neurosci. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hannula DE, Ranganath C. The eyes have it: hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hannula DE, et al. Use of eye movement monitoring to examine item and relational memory in schizophrenia. Biol Psychiat. 2010;68:610–616. doi: 10.1016/j.biopsych.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams LE, et al. Eye-movement behavior reveals relational memory impairment in schizophrenia. Biol Psychiat. 2010;68:617–624. doi: 10.1016/j.biopsych.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Erp TG, et al. Remember and know judgments during recognition in chronic schizophrenia. Schizophr Res. 2008;100:181–190. doi: 10.1016/j.schres.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Danion JM, et al. Conscious recollection in autobiographical memory: An investigation in schizophrenia. Conscious Cogn. 2005;14:535–547. doi: 10.1016/j.concog.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Cur Top Behav Neuro. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- 68.Janowsky JS, et al. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav Neuro. 1989;103:548–560. doi: 10.1037//0735-7044.103.3.548. [DOI] [PubMed] [Google Scholar]
- 69.Wagner AD, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 70.Blumenfeld RS, et al. Putting the pieces together: the role of dorsolateral prefrontal cortex in relational memory encoding. J Cogn Neurosci. 2011;23:257–265. doi: 10.1162/jocn.2010.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonner-Jackson A, et al. Episodic memory in schizophrenia: the influence of strategy use on behavior and brain activation. Psychiat Res. 2008;164:1–15. doi: 10.1016/j.pscychresns.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonner-Jackson A, Barch DM. Strategic manipulations for associative memory and the role of verbal processing abilities in schizophrenia. J Int Neurosci Soc. 2011;17:796–806. doi: 10.1017/S1355617711000749. [DOI] [PubMed] [Google Scholar]
- 74.Ragland JD, et al. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. Am J Psychiat. 2005;162:1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mathews JR, Barch DM. Episodic memory for emotional and nonemotional words in schizophrenia. Cognition Emotion. 2004;18:721–740. [Google Scholar]
- 76.Ragland JD, et al. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166:863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ragland JD, et al. Neural correlates of relational and item-specific encoding during working and long-term memory in schizophrenia. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Craddock N, et al. Psychosis genetics: modeling the relationship between schizophrenia, bipolar disorder, and mixed (or “schizoaffective”) psychoses. Schizophr Bull. 2009;35:482–490. doi: 10.1093/schbul/sbp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Depp CA, et al. Neurocognitive impairment in middle-aged and older adults with bipolar disorder: comparison to schizophrenia and normal comparison subjects. J Affect Disord. 2007;101:201–209. doi: 10.1016/j.jad.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hill SK, et al. Neuropsychological dysfunction in antipsychotic-naive first-episode unipolar psychotic depression. Am J Psychiatry. 2004;161:996–1003. doi: 10.1176/appi.ajp.161.6.996. [DOI] [PubMed] [Google Scholar]
- 81.Krabbendam L, et al. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80:137–149. doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Gooding DC, Tallent KA. Spatial working memory performance in patients with schizoaffective psychosis versus schizophrenia: a tale of two disorders? Schizophr Res. 2002;53:209–218. doi: 10.1016/s0920-9964(01)00258-4. [DOI] [PubMed] [Google Scholar]
- 83.Smith MJ, et al. Bridging the gap between schizophrenia and psychotic mood disorders: Relating neurocognitive deficits to psychopathology. Schizophr Res. 2009;107:69–75. doi: 10.1016/j.schres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heinrichs RW, et al. Are schizophrenia and schizoaffective disorder neuropsychologically distinguishable? Schizophr Res. 2008;99:149–154. doi: 10.1016/j.schres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Reichenberg A, et al. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Insel T, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiat. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 87.Fuller RL, et al. Impaired visual working memory consolidation in schizophrenia. Neuropsychology. 2009;23:71–80. doi: 10.1037/a0013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gold JM, et al. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psych. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- 89.Gold JM, et al. Impaired top-down control of visual search in schizophrenia. Schizophr Res. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gold JM, et al. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Arch Gen Psychiatry. 2010;67:570–577. doi: 10.1001/archgenpsychiatry.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hahn B, et al. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biol Psychiat. 2010;68:603–609. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cowan N. The Magical Mystery Four: How is Working Memory Capacity Limited, and Why? Curr Dir Psychol Sci. 2010;19:51–57. doi: 10.1177/0963721409359277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pisella L, et al. Impaired working memory for location but not for colour or shape in visual neglect: a comparison of parietal and non-parietal lesions. Cortex. 2004;40:379–390. doi: 10.1016/s0010-9452(08)70132-1. [DOI] [PubMed] [Google Scholar]
- 94.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- 95.Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- 96.Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cog Affect Behav Ne. 2005;5:144–155. doi: 10.3758/cabn.5.2.144. [DOI] [PubMed] [Google Scholar]
- 97.Lisman J. Working memory: the importance of theta and gamma oscillations. Curr Biol. 2010;20:R490–492. doi: 10.1016/j.cub.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 98.Dickinson D, et al. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 99.Antonova E, et al. The relationship of structural alterations to cognitive deficitsin schizophrenia: a voxel-based morphometry study. Biol Psychiat. 2005;58:457–467. doi: 10.1016/j.biopsych.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 100.Minzenberg MJ, Carter CS. Developing treatments for impairedcognition in schizophrenia. Trends Cog Sci in press. [Google Scholar]