Abstract
The PROP bitter-taste phenotype is a marker for food preferences and eating behavior, and may associate with differences in body weight in children. Previous work has shown that PROP status in combination with eating attitudes are better predictors of weight status in preadolescents, than either factor alone. However, no studies have examined the role of PROP phenotypes in body weight change in children over time. The primary objective of this study was to investigate current weight status and change in weight status in children from preschool (baseline) to preadolescence as a function of eating attitudes and PROP phenotype. Other measures included self-reported food intakes and physical activity by activity monitor. Seventy-three lean (BMI %-ile = 57.7 ± 3.2%) children with mean age=10.3 ± 0.5 yrs, participated in the follow up. There were no group differences in energy intake, current BMI-percentile or change in BMI percentile from baseline by PROP phenotype in either boys or girls. However, there was a trend for non-taster girls to show a downward shift in BMI-percentile at follow up. Hierarchical regression analysis revealed that baseline BMI percentile and physical activity energy expenditure were the strongest predictors of current weight (28.5% variance), followed by child restraint, the taster x gender interaction, and the maternal BMI x maternal emotional eating interaction, accounting for 7.1%, 6.0% and 4.8% of variance in the model, respectively. These findings suggest that PROP status and eating attitudes are modest predictors of weight status in preadolescent children.
Keywords: 6-n-propylthiouracil, PROP phenotype, Eating attitudes, Body weight, Children
1.1 Introduction
The prevalence of pediatric obesity has increased three-fold during the past thirty years (Ogden, Carroll, Curtin, Lamb, & Flegal, 2010). Obesity in children is of extreme concern because weight status tends to track over time, and overweight children are 2–6 times more likely to become obese adults when compared to normal-weight children (Guo et al., 2000; Magarey, Daniels, Boulton, & Cockington, 2003). Eating patterns are also established early in the lifecycle, and once entrenched are difficult to change (Anzman, Rollins, & Birch, 2010). Thus, it is important to identify the critical determinants of eating behavior in children order to instill more healthful dietary habits early in life as well develop better primary prevention strategies for childhood obesity.
Food preferences are mediated, in part, by genetic predispositions (Keskitalo et al., 2007; Tornwall et al., 2012). Our laboratory has been examining the role of the 6-n-propylthiouracil (PROP) taste phenotype as a general index of food preferences and eating behaviors in children and adults (Tepper, 2008; Tepper et al., 2009). Individuals who are taste blind to PROP (i.e., non-tasters) perceive less intensity from a range of oral sensations including sweetness, bitterness, oral pungency and creaminess. In contrast, those who are moderately or extremely responsive to PROP bitterness (medium or super-tasters, respectively) perceive more intensity from these same oral sensations. These differences are apparent at an early age and are shown to influence food selection. Studies in young children have shown that in comparison to taster children, non-tasters are more likely to accept bitter-tasting vegetables and fruit juices (Bell & Tepper, 2006; Tepper, Keller, & Ullrich, 2003; Turnbull & Matisoo-Smith, 2002) and soy foods (Tsuji et al., 2012). In addition, non-taster children gave higher acceptability ratings to full-fat milk (Keller, Steinmann, Nurse, & Tepper, 2002)and they reportedly consumed more added fats in the diet than tasters (Keller & Tepper, 2004). The relative contribution of these dietary patterns to energy intake in PROP-classified children is unknown.
The involvement of the PROP bitter taste phenotype in body weight status in children is controversial. Two studies in preschool children reported that male non-tasters were heavier than male tasters, but the opposite (non-significant) trend was seen in girls (Keller et al., 2010; Keller & Tepper, 2004). Other studies reported no differences in body weight as a function of PROP status in this age group (Lumeng, Cardinal, Sitto, & Kannan, 2008). A large, population-based study in older children (7–18 yrs of age) of different ethnic and socioeconomic groups also found no differences in weight related to PROP status (Baranowski et al., 2010).
Parental characteristics such as weight status, eating attitudes and child feeding practices are known to play a critical role in the development of children’s food patterns and body weight (Francis, Ventura, Marini, & Birch, 2007; Hood et al., 2000; Magarey et al., 2003). Goldstein et al. (Goldstein, Daun, & Tepper, 2007) investigated the contribution of PROP taster phenotype and several maternal characteristics to these outcomes in a convenience sample of 9 year old children. Results showed that non-tasters consumed more energy than tasters, but no differences in body weight were found among the groups. Rather, maternal body mass index (BMI; kg/m2), restriction of child’s food intake and concern about child weight were positive predictors of children’s BMI percentile, whereas pressure to eat was a negative predictor. Maternal disinhibition (i.e., loss of eating control) was also associated with higher BMI percentile in girls but not boys(Goldstein et al., 2007). These data imply that maternal characteristics can potentially override the influence of PROP status on body weight in older children.
Pre-adolescence (age range= 9–14 yr) is a period of rapid growth and development as well as greater personal independence in food selection and lifestyle behaviors. These factors can impact the growth curve trajectories for BMI of children. Indeed, Berkey and co-workers (Berkey et al., 2000) reported that even modest changes in energy intake and physical activity modified the pattern of 1-yr weight change in adolescents in a national cohort of U.S. children. Individual differences in eating attitudes such as dietary restraint (i.e., conscious control of eating) or emotional eating which may have their origins in early childhood (Carper, Orlet Fisher, & Birch, 2000) also play a role in weight status and body satisfaction during preadolescence (Ledoux, Watson, Baranowski, Tepper, & Baranowski, 2011; Webber, Hill, Saxton, Van Jaarsveld, & Wardle, 2009) especially among girls (Shunk & Birch, 2004). Since all the studies on PROP status and adiposity in children have been cross-sectional, the influence of this phenotype on changes in body weight during childhood is unknown.
The objective of this study was to address this gap in knowledge. Children who participated in our studies as preschoolers and their mothers were invited to participate in a follow up study. Self-reported food intakes were collected, and current weight status and 6-yr change in weight from baseline (4.5 yrs of age) were also determined. Other data collected included maternal weight, and eating attitudes. The primary hypothesis was that PROP status would be inversely related to current energy intake and weight status of the children in the follow up cohort.
1.2 METHODS
1.2.1 Subjects and General Procedures
The subject pool for this follow up study consisted of children who attended the Rutgers University Preschool between 1999 and 2003, and had participated in one of three studies investigating the relationship among PROP taster status and food preferences and/or body weight when they were 4.2 ± 0.3 yrs of age (Bell & Tepper, 2006; Keller et al., 2002; Keller & Tepper, 2004) The total number of children who previously participated in the preschool studies was 154, and 148 of those children and their families were still living in the Central New Jersey area. Families were contacted by mail with an invitation for each former student and his/her mother to participate in the follow-up study. Mothers gave written, informed consent for themselves, and their child’s participation. Oral assent was also obtained from each child. The research protocols were approved by the Rutgers University Institutional Review Board. Participants were screened with a general health questionnaire to ensure that they had no medical conditions or recent illness, or that they were not taking medications that might interfere with taste perception.
1.2.2 Classification by PROP Taste Phenotype
As preschoolers, the children were classified as PROP tasters or non-tasters using an age-appropriate method (Keller et al., 2002; Keller & Tepper, 2004). In the current study, children were screened for PROP status using the paper disk method, previously tested for validity and reliability in both preadolescents and adults (Goldstein, Daun, & Tepper, 2005; Goldstein et al., 2007; Zhao, Kirkmeyer, & Tepper, 2003). Briefly, subjects place a filter paper disk impregnated with 1.0 mol/L NaCl on the tip of the tongue until it is thoroughly wet. They rate the taste intensity of the disk using the labeled magnitude scale (LMS), a 100-mm scale anchored with the phrases “barely detectable” to “strongest imaginable” (Green et al., 1996). This procedure is repeated with a second paper disk impregnated with 50 mmol/L PROP (6-propyl–2-thiouracil, P3755, Sigma-Aldrich). Subjects are instructed to rinse with spring water at room temperature before and in between tasting each paper disk. Subjects are categorized as non-tasters if they rate the PROP disk ≤ 13 mm on the LMS; they are categorized as super-tasters if they rate the PROP disk > 67 on the LMS. All others are classified as medium tasters (Zhao et al., 2003). NaCl ratings do not vary with PROP status in this method (Goldstein et al., 2005, 2007; Zhao et al., 2003). Therefore, NaCl ratings are used as a reference standard to clarify the taster status of subjects who give borderline ratings to PROP, although this situation is rare (~ 4% occurrence rate). This strategy is based on the rationale that non-tasters give higher ratings to NaCl than to PROP, medium tasters give equivalent ratings to both stimuli and super-tasters give higher ratings to PROP than NaCl. The PROP taste test was conducted twice. The correlation between the two PROP ratings was high (r=0.83; p<0.001) and the mean of the two ratings was used to classify the subjects.
1.2.3 TAS2R38 Genotype Analysis
TAS2R38 is the major gene controlling PROP taste sensitivity (Kim et al., 2003). Thus, children were also characterized byTAS2R38 genotypes as a check on the reliability of the PROP phenotyping. Cells were obtained by gently brushing the inside of the cheek with a swab (Epicentre, Madison WI), and genomic DNA was extracted using the extraction solution provided by the manufacturer (Epicentre). Alleles of the gene TAS2R38 (Accession # AF494231; rs713598 and rs172866) were genotyped for a variant site using allele-specific probes and primers purchased from Applied Biosystems (Foster City, CA). Samples were compared with a sequenced reference standard, and alleles that failed to cluster into one of three groups were re-genotyped as needed.
Although there are three variant sites in the gene associated with bitter sensitivity (A49P, V262A, and I296V), the last two are in perfect linkage disequilibrium in all human populations tested thus far, and therefore the third site (I296V) was not assayed but imputed (Mennella, Pepino, Duke, & Reed, 2011, 2012). Subjects were grouped by the first and second variant sites, A49P and V262A, respectively. Since these sites are not in perfect linkage disequilibrium, the haplotypes for the first two sites were imputed based on observed allele frequencies and knowledge gained through genotyping thousands of similar samples (Mennella et al., 2011, 2012).
Subjects who were homozygous for the bitter-insensitive allele are referred to as AV/AV, those who are heterozygous for the bitter-insensitive allele are referred to as PA/AV, and those who are homozygous for the bitter-sensitive allele are referred to as PA/PA. Those with the rare form (AA) display moderate sensitivity to PROP and are included in the PA/PA group.
1.2.4 Adiposity Measures
Each child’s height was measured to the nearest 0.25 in using a standard tape measure attached to a flat wall. Waist circumference was also measured by tape measure at the level of the umbilicus. Weight was measured to the nearest 0.1 kg with an electronic scale (WB-800; Best Wright, Brooklyn, NY). The children wore light clothing and no shoes for these measurements. Height, weight, gender and age in months were used to calculate body mass index (BMI) percentile and z-score for each child using online tools available from the U.S. Centers for Disease Control (http://apps.nccd.cdc.gov/dnpabmi/ and http://www.cdc.gov/growthcharts/zscore.htm). BMI percentile between the 85th and 95th percentile was defined as “overweight”; above the 95thpercentile was defined as “obese”. Triceps skin fold thickness was measured to the nearest 0.1 mm with a Lange caliper (Beta Technology Inc., Cambridge, MD). The measurements were taken midway between the acromion and olecranon on the posterior side of the upper arm. The average of three measurements was used. Maternal height and weight were measured in the same manner as for the children, and BMI (kg/m2) was calculated from these values.
1.2.5 Diet Assessments
Three 24-hour recalls were taken from each child, for one weekend day and two nonconsecutive weekdays. The 24-hour recall captures a retrospective look at food intake, and when averaged over three days, provides a reliable representation of habitual diet and intake patterns, and has been validated in children as young as 5–7 (Montgomery et al., 2005). The Nutrient Data System for Research (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) multiple-pass approach was used to collect diet information (Burrows, Martin, & Collins, 2010). The Foods Booklet available with the NDS program was used to illustrate portion size. Nutrient output from food recalls included energy intake (kcal), macronutrients (% energy), vitamins, minerals and non-nutritive substances such as caffeine and artificial sweeteners. We used 1.3 times basal metabolic rate (BMR)(Mifflin et al., 1990) as the cut-off for implausible diet reporting. Any child whose energy intake failed to reach this criterion was eliminated from the analysis. However, all of the children met this criterion, and none were eliminated.
1.2.6 Physical Activity
Physical activity level (PAL) was measured using the Actigraph GT1M activity monitor (ActiGraph LLC, Pensacola, FL). The activity monitor was worn around the waist for a consecutive 72hr, and was removed only for bathing or swimming. Each child kept a log of times that the activity monitor was removed for water sports, bathing, or other reasons. Using Actigraph GT1M software, the “combination equation” was used to derive energy expenditure for the duration of wearing the activity monitor. Total energy expenditure was averaged over the time the device was actually worn to derive average energy expenditure per 24-hour period.
1.2.7 Eating Attitudes
The Dutch Eating Behavior Questionnaire (DEBQ)(Van Strien, Frijters, Bergers, & Defares, 1986)was used to assess dietary restraint (conscious control of eating), emotional eating and external eating (responsiveness to external food cues) in both children and mothers. The DEBQ has previously been validated in children (Wardle et al., 1992). The questionnaire uses a 5-point scale where 1= never, 2=rarely, 3 = sometimes, 4 = often and 5 = very often. Mean scores were calculated for the three subscales.
1.2.8 Procedures
All data (with the exception of one food recall that was done by telephone) were collected at the children’s home during two sessions scheduled approximately 2 weeks apart. A single researcher (KNO) collected the anthropomorphic measurements and the diet information to reduce experimenter error. The researcher was certified in the use of the NDS software.
1.2.9 Statistical Analysis
PROP taster classifications obtained at baseline and follow-up were compared for test-retest reliability using Cohen’s Kappa statistic. Since the method used to screen these children for PROP status as preschoolers did not distinguish medium and super-tasters, these two groups were combined in the follow-up cohort into a single group (tasters) for this analysis.
Group differences in weight status and diet, and change in weight from baseline were analyzed by Analysis of Variance (ANOVA) with PROP taster status, gender and the taster by gender interaction as factors. A general linear model was used, and post-hoc comparisons were made using Tukey’s test, as appropriate.
Pearson’s Correlation coefficients were computed to examine associations between the prediction variables (e.g., child eating attitudes, maternal eating attitudes, maternal BMI) and the outcome variables of interest (energy intake and body weight). Variables were retained for the regression modeling if they achieved significance in the correlations at p<0.10. Age, gender, baseline BMI percentile and physical activity energy expenditure were included in all regression models.
Two types of regression models were computed. First, multiple linear regression was used to predict current BMI%-ile. Models were computed for the whole cohort, and for boys and girls, separately. A forward, stepwise model was used. Next, hierarchical regression analysis was used as a multivariate approach for modeling current BMI%-ile. In this analysis, groups of variables are entered into the model as independent steps. We followed standard conventions where main effects and control variables were entered as the first step, followed by child variables, maternal variables, and interactions, in successive steps. Our approach was similar to that used by Sinton and Birch(Sinton & Birch, 2005)for modeling dieting behavior in girls. Details of the model building are described in the Results section. SAS software 9.2 for the personal computer (SAS Institute, Cary, NC) used for all analyses. Significance was set at p<0.05 for the study as a whole. However, the Bonferroni correction was used in the correlational analyses to adjust for the large number of comparisons made (p =0.05/17 comparisons for final p = 0.004).
1.3 RESULTS
1.3.1 General Subject Characteristics
Seventy-three children (45 boys; 28 girls) and their mothers (n=63) participated in the follow up study. The number of participating mothers was lower than the number of participating children due to the presence of 10 sibling pairs in the follow-up cohort. The overall participation rate was 49%. The mean age of the children at baseline (i.e., preschool) was 4.5 ±0.1 yrs. (range 3.5–4.8 yr) and mean age at follow-up was 10.3 ± 0.5 yrs (range 7–13 yrs). BMI percentile at baseline did not differ between the subset of children who participated in the follow-up and those who did not participate. Demographic characteristics of the follow-up cohort were as follows: the children were predominantly (86%) Caucasian; the majority (83.5%) of parents had a college or post-graduate degree; and household income exceeded $50,000/annum for 86% of households and exceeded $100,000/annum for 50% of households.
Mean BMI percentile at follow-up was 57.7 ± 3.2%. The prevalence of obesity (BMI for age ≥ 95th percentile) was 6.5% at baseline (14.3% in girls;6.6% in boys). The overall prevalence of obesity rose slightly to 8.2% at follow-up, with 6.1% of the girls and 11.5% of the boys classified as obese. The prevalence of overweight (BMI for age ≥ 85th and < 95th percentile) was 21.2% for the sample as a whole. Change in BMI percentile at follow-up was negatively correlated with baseline BMI percentile (r=−0.58, p<0.001) revealing a downward shift in BMI percentile score with increasing age. The children were moderately active. Mean daily physical activity energy expenditure estimated from Actigraph recordings over 3-days, was 225 ± 20 kcal/day for all children.
As expected, energy intakes differed by gender. Boys consumed an average of 1,988 kcal/day, whereas girls consumed an average of 1,667 kcal/day (F1,72= 5.05, p<0.01). Both genders consumed similar proportions of macronutrients which were consistent with current recommendations for children 9–13 yr of age (Food and Nutrition Board, 2005).
1.3.2PROP Taster Status
The distribution of taster groups in the follow-up cohort was: 25% non-tasters (n=18); 53% medium tasters (n=39); and 22% super-tasters (n=16). As expected, PROP intensity ratings varied among the groups (p<0.001, F2,72=199.1), with super-tasters giving the highest mean (±SEM) intensity ratings for PROP followed by medium tasters and non-tasters (86.5 ± 1.0, 47.2 ± 1.1, and 8.6 ± 0.7 mm, respectively). All three groups differed from each other (p< 0.05 by Tukey’s test). NaCl ratings also differed among the groups (p<0.05, F2,72=3.67), but this difference was due to the non-tasters who gave higher ratings to NaCl (49.4 ± 2.1 mm) than either the medium (29.6 ± 2.4 mm) or super-taster groups (31.8 ± 2.7 mm) (p<0.05). However, this difference did not impact the taster group classifications. These ratings were similar to those previously published for Caucasian children and adults (Calo et al., 2011; Goldstein et al., 2005, 2007).
To establish the test-retest reliability of the PROP taster group classifications, we combined the medium and super-tasters into a single group (tasters) and then compared the children’s classifications obtained at follow up to their classifications at baseline. Test-retest reliability of the PROP classifications was high (Cohen’s kappa coefficient= 0.76). A coefficient > 0.70 is considered reliable.
1.3.3 TAS2R38 Gene Analyses
Seventy-one children provided DNA samples. The allelic groupings were identified as follows: AV/AV (n=17), PA/AV or PA/AA (n=49) and PA/PA (n=5). Table 2 shows that no child with the AV/AV form was misclassified as a super-taster and likewise, no child with the PA/PA form was misclassified as a non-taster. The overall agreement between allelic group and phenotypic group was high based on the contingency coefficient of group membership (χ2=0.63; p<0.01) and the Spearman’s correlation co-efficient (r=0.72; p≤ 0.001).
Table 2.
| n | Non-taster | Medium Taster | Super-taster | |
|---|---|---|---|---|
| AV/AV | 17 | 14 (19.7) | 3 (4.0) | 0 (0.0) |
| AV/PA or AA/PA * | 49 | 2 (2.8) | 34 (47.9) | 13 (18.3) |
| PA/PA | 5 | 0 (0.0) | 1 (1.4) | 4 (5.6) |
Values in the table are cell counts with cell percentages in parenthesis.
Includes 7 children with the rare AA form which is associated with intermediate taste sensitivity to PROP (Timpson et al., 2007).
Contingency coefficient of group membership (χ2=0.63; p≤0.01)
1.3.4 Group Differences in Current Weight, and Weight Change from Baseline
There were no main effects of PROP taster status or gender on current BMI percentile or change in BMI percentile (Table 1). Additionally, waist circumference and triceps skin-fold thickness (data not shown) did not vary among PROP taster groups or between genders.
Table 1.
Characteristics of follow up children (top) and their mothers (bottom)1
| Children | |||||
|---|---|---|---|---|---|
|
| |||||
| Taster Status | Gender | ||||
| Non (n=18) | Medium (n=39) | Super (n=16) | Boys (n=45) | Girls (n=28) | |
| Age (yr) | 9.4 ± 0.4 | 10.6 ± 0.4 | 10.2 ± 0.4 | 10.3 ± 0.3 | 10.2 ± 0.4 |
| Current BMI Z-score | 0.2 ± 0.2 | 0.4 ± 0.2 | 0.1 ± 0.3 | 0.3 ± 0.1 | 0.2 ± 0.2 |
| Current BMI %ile | 55.0 ± 5.2 | 61.0 ± 4.4 | 52.7 ± 7.5 | 58.7 ± 3.9 | 56.1 ± 5.4 |
| Baseline BMI %ile | 63.8 ± 6.0 | 57.7 ± 4.8 | 55.1 ± 6.9 | 56.9 ± 4.2 | 61.4 ± 5.3 |
| Change in BMI %ile | −8.8 ± 6.5 | 2.9 ± 5.2 | −2.4 ± 7.4 | 1.8 ± 4.5 | −6.1 ± 5.8 |
| Physical activity EE (kcal/day) | 187.0 ± 23 | 227.6 ± 26 | 172.3 ± 29 | 213.2 ± 2.4 | 192.9 ± 18 |
| DEBQ | |||||
| Emotional eating | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.6 ± 0.1 | 1.7 ± 0.1 |
| External eating | 3.0 ± 0.2 | 2.8 ± 0.1 | 2.7 ± 0.2 | 2.9 ± 0.1 | 2.7 ± 0.2 |
| Restraint | 1.5 ± 0.2 | 1.8 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.1 | 1.9 ± 0.2 |
| Dietary Intake | |||||
| Energy (kcal/day) | 1885 ± 94 | 1887 ± 76 | 1786 ± 97 | 1988 ± 69 * | 1667 ± 55 |
| Energy (kcal/kg/day) | 62.9 ± 3.8 | 52.8 ± 2.3 | 57.2 ± 97 | 54.2 ± 2.2 | 53.5 ± 3.4 |
| % Fat | 30.1 ± 0.8 | 31.5 ± 0.9 | 31.3 ± 1.5 | 31.8 ± 0.8 | 30.1 ± 0.9 |
| % Carbohydrate | 57.7 ± 1.2 | 54.9 ± 1.1 | 55.9 ± 2.0 | 55.1 ± 1.0 | 57.0 ± 1.1 |
| % Protein | 13.5 ± 0.6 | 14.8 ± 0.4 | 14.1 ± 0.6 | 14.4 ± 0.4 | 14.2 ± 0.4 |
| Mothers | |||
|---|---|---|---|
|
| |||
| Taster Status | |||
| Non (n=13) | Medium (n=35) | Super (n=13) | |
| Age (yr) | 43.8 ± 1.1 | 43.8 ± 0.9 | 42.4 ± 1.5 |
| BMI (kg/m2) | 27.6 ± 1.9 | 25.1 ± 0.9 | 26.7 ± 1.3 |
| DEBQ | |||
| Emotional Eating | 2.4 ± 0.2 | 2.3 ± 0.1 | 2.3 ± 0.2 |
| External Eating | 3.0 ± 0.1 | 3.0 ± 0.1 | 3.3 ± 0.1 |
| Restraint | 3.2 ± 0.2 | 3.0 ± 0.1 | 3.0 ± 0.2 |
Values are means ± SEM.
Scale ranges for the DEBQ subscales are 1–5.
significant difference between genders at p<0.05.
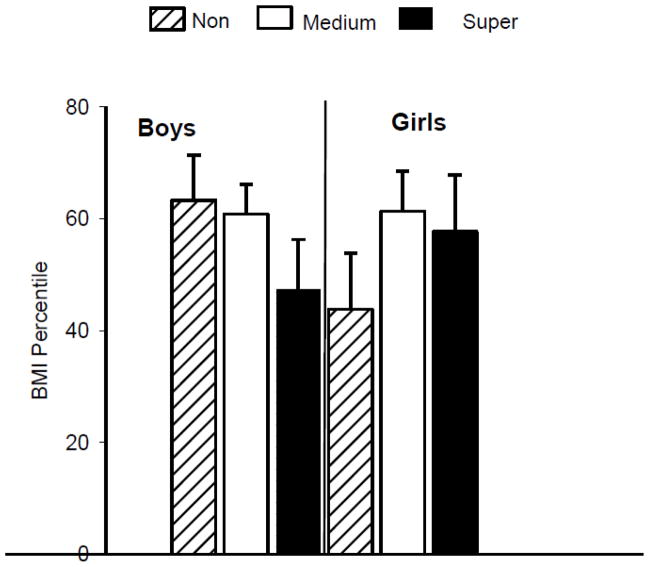
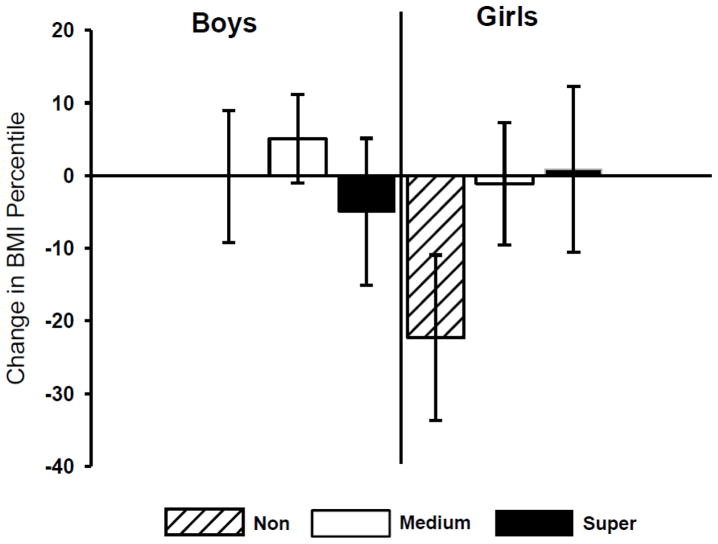
Figure 1 shows the current BMI percentile of the children by PROP status and gender, and Figure 2 shows the change in BMI percentile relative to baseline in these same groups. Neither of these interactions reached statistical significance, but directional trends were evident. Current BMI percentile in boys was inversely related to PROP responsiveness, whereas girls showed the opposite pattern (Figure 1). These patterns are consistent with those previously reported by Keller and co-workers (Keller et al., 2010; Keller & Tepper, 2004). Boys showed no change in BMI percentile relative to baseline for any taster group (Figure 2). However, non-taster girls showed a downward shift in BMI percentile between baseline and follow up that was not evident in the other groups of girls.
Figure 1.
Current BMI percentile by gender and PROP phenotype. There were no differences in BMI percentile by PROP phenotype among boys or girls. Among boys there were 11 non-tasters; 25 medium tasters; and 9 super-tasters. Among girls there were 7 non-tasters, 14 medium tasters; and 7 super-tasters.
Figure 2.
Change in BMI percentile from preschool to preadolescence by gender and PROP phenotype. There were no differences among the groups for gender, PROP phenotype or their interaction. Among boys there were 11 non-tasters; 25 medium tasters; and 9 super-tasters. Among girls there were 7 non-tasters, 14 medium tasters; and 7 super-tasters.
1.3.5 Eating Attitudes, Weight Status and Energy Intake
Child external and emotional eating scores from the DEBQ were normally distributed. Child restraint was positively skewed, so a log transformation was used to correct for skewness. There were no differences in child dietary restraint, external eating or emotional eating by taster status or between genders (Table 1). Child restraint showed a positive association with both baseline and current BMI percentiles (r=0.33; p<0.01 and r=0.37; p<0.01, respectively), but both comparisons failed to reach the Bonferroni-corrected cutoff criterion (p<0.004). There was a modest, non-significant association between child external eating and energy intake (r=0.25; p=0.04). None of the eating attitudes were related to energy consumption. Also, reported energy intake did not vary among PROP taster groups (see Table 1).
Maternal BMI did not vary with mothers’ taster status, dietary restraint or external eating scores (Table 1). However, mothers with a BMI > 25 kg/m2 had higher emotional eating scores than mothers with a BMI ≤ 25 kg/m2 (2.5 ± 0.1 vs. 2.1± 0.2, respectively; p <0.05). Maternal emotional eating was also associated with greater maternal BMI (r=0.42, p<0.001).
Only a few of the maternal variables were related to outcomes in the children. Specifically, maternal BMI and maternal dietary restraint were related to children’s current BMI percentile (r=0.32; p<0.01 for both), but these relationships failed to reach the Bonferroni-corrected criterion (p=0.004).
1.3.6 Regression Modeling to Predict Current BMI Percentile
Stepwise, multiple linear regression revealed six variables influencing BMI percentile for the cohort as a whole (See Table 3). The positive predictors were baseline BMI%-ile, physical activity energy expenditure and child restraint; the negative predictors were age, gender and the taster by gender interaction. In the gender-specific models, restraint and taster status were positive predictors of BMI%-ile in girls. In boys, restraint and physical activity energy expenditure were positive predictors in the model, and age was a negative predictor. All three models accounted for 43–53% of the variance in BMI%-ile in these children.
Table 3.
| Entire Cohort | Siblings Removed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter Estimate | Standard Error | F Value | Pr> F | Adjusted r2 | Parameter Estimate | Standard Error | F Value | Pr> F | Adjusted r2 | ||
| BOTH GENDERS (n=73) | 0.53 | BOTH GENDERS (n=63) | 0.44 | ||||||||
| Intercept | 1.55 | 0.57 | 7.43 | <0.01 | Intercept | 1.45 | 0.59 | 7.40 | <0.01 | ||
| Gender | −1.5 | 0.52 | 8.4 | <0.01 | Gender | −1.46 | 0.41 | 8.0 | <0.01 | ||
| Child restraint | 0.59 | 0.2 | 8.43 | <0.01 | Child restraint | 0.50 | 0.2 | 8.43 | <0.01 | ||
| Taster x gender | −0.86 | 0.36 | 5.8 | <0.05 | Taster x gender | −0.71 | 0.27 | 5.8 | <0.05 | ||
| Age (mo) | −0.01 | 0 | 8.14 | <0.01 | Age (mo) | −0.01 | 0 | 7.7 | <0.01 | ||
| Baseline BMI%-ile | 0.19 | 0.08 | 5.27 | <0.05 | Baseline BMI%-ile | 0.26 | 0.1 | 5.8 | <0.05 | ||
| Physical activity EE | 0.7 | 0.28 | 9.29 | <0.01 | Physical activity EE | 0.9 | 0.31 | 9.8 | <0.01 | ||
| GIRLS (n=28) | 0.43 | GIRLS (n=24) | 0.36 | ||||||||
| Intercept | 6.13 | 16.04 | 0.15 | 0.71 | Intercept | 5.33 | 18.7 | 0.10 | 0.8 | ||
| Child restraint | 10.34 | 4.63 | 5 | 0.04 | Child restraint | 9.8 | 5.7 | 4.6 | 0.05 | ||
| Taster | 17.88 | 6.13 | 8.51 | 0.01 | Taster | 17.0 | 6.93 | 8.1 | 0.01 | ||
| BOYS (n=45) | 0.45 | BOYS (n=39) | 0.40 | ||||||||
| Intercept | 67.67 | 17.61 | 14.76 | <0.01 | Intercept | 66.4 | 17.8 | 13.9 | <0.01 | ||
| Dietary Restraint | 10.65 | 4.68 | 5.19 | <0.01 | Dietary Restraint | 10.2 | 4.6 | 5.10 | <0.01 | ||
| Baseline BMI%-ile | 0.22 | 0.12 | 3.39 | 0.07 | Baseline BMI%-ile | 0.20 | 0.12 | 3.0 | 0.08 | ||
| Age (mo) | −.41 | 0.14 | 9.17 | <0.01 | Age (mo) | −0.39 | 0.17 | 8.0 | <0.05 | ||
| Physical activity EE | 0.052 | 0.024 | 4.81 | <0.05 | Physical activity EE | 0.04 | 0.02 | 4.1 | <0.05 | ||
Stepwise forward selection was used; variables that were not significant predictors at α=0.05 were removed from the model.
Models were calculated for the whole cohort (n=73; left panel) and after random elimination of one member of each sib-pair (n=63; right panel).
The follow up cohort included 10 sibling pairs. To rule out the possibility that family relatedness was influencing the models, we randomly eliminated one sibling from each pair (4 girls; 6 boys), and recalculated the stepwise models (see Table 3). The models remained stable; only the percent of variance in BMI%-ile explained by the models was reduced to 33–44%.
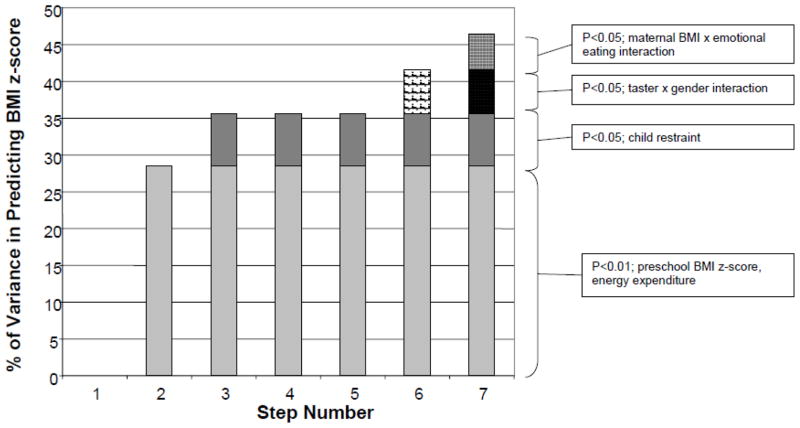
Hierarchical regression (for the full cohort) was used to examine the independent effects of groups of variables entered into the prediction model (see Table 4). In step 1, age, taster status, and gender were entered into the model and together did not account for significant variance. Within step 1, gender and taster status did not meet the probability cutoff of p<0.05, but since the interaction between the two was of interest, they were retained in the model. In step 2, baseline BMI percentile and physical activity energy expenditure were entered, and together accounted for 28.5% of the variance. Child restraint was entered as step 3, and accounted for an additional 7.1% of variance in BMI percentile. Neither maternal BMI (step 4) nor maternal emotional eating and restraint (step 5) accounted for significant variance in the model. In step 6, the taster by gender interaction was entered, which accounted for an additional 6% of variance. The interaction between maternal BMI and maternal external eating was entered as step 7 and accounted for a final 4.3% of the variance in BMI percentile. All together, the model accounted for 46.4% of the variance in child BMI percentile. The proportion of variance accounted for by each step of the model is depicted in Figure 3.
Table 4.
| Step | Variables | Parameter Estimate | p-value | Variance (%) |
|---|---|---|---|---|
| 1 | Age Taster Gender |
NS | ---- | |
| 2 | Baseline BMI%-ile Physical activity EE |
0.241 0.003 |
<0.01 | 28.5 |
| 3 | Child restraint | 0.125 | <0.05 | 7.1 |
| 4 | Maternal BMI | 0.14 | ---- | |
| 5 | Maternal restraint Maternal emotional |
NS | ---- | |
| 6 | Taster x gender | 0.542 | <0.05 | 6.0 |
| 7 | MaternalBMI x Maternal emotional | 0.061 | <0.05 | 4.8 |
| Overall | 46.4% |
Each step shows percent of variance, and p-value for the step, with individual parameter estimates for variables that make a significant contribution to the step.
Model represents the full cohort (n=73).
Figure 3.
Steps of the hierarchical regression model for predicting BMI z-score. The hierarchical regression model is shown in Table 3
1.4 DISCUSSION
The objective of this study was to investigate the contribution of PROP taster status and eating attitudes to weight status at follow up in a cohort of children we originally studied as preschoolers. PROP phenotype was not associated with current weight or change in weight from baseline based on group comparisons. However, multiple regression revealed that for children of both sexes, child restrained eating and the taster by gender interaction was associated with current BMI%-ile. Hierarchical regression analysis showed that these same variables (child eating restraint and the PROP by gender interaction), as well as the interaction between maternal BMI and maternal emotional eating each made modest contributions to BMI percentile at age 10 after controlling for baseline BMI percentile and current physical activity. Overall, this model accounted for 46.4% of the variance in current BMI percentile with the PROP interaction and eating attitudes contributing a total of 18% of variance to the model (steps 3, 6 & 7). Thus, PROP phenotype in combination with gender contributed at least as well to current weight status as did child and maternal eating attitudes which have been well studied in conjunction with childhood weight status and obesity development (Francis et al., 2007; Shunk & Birch, 2004; Webber et al., 2009).
The diet and body weight characteristics of our sample deserve comment. At 4.5 yrs of age, these children maintained a healthy weight, and they continued to track within the healthy weight range at follow up. Thus, for the most part, the children we studied remained lean with a markedly lower prevalence of obesity (8.2%) than reported for non-Hispanic White children in the general population (16.9%)(Ogden et al., 2010). Reported energy consumption in boys was 1,988 kcal/day, which is consistent with the recommended 2,079 kcal/day for boys9–13 yrs of age (Food and Nutrition Board, 2005). Girls consumed 1,667 kcal/day, which was 300 kcal/day less than recommended (1,970 kcal/day). Underreporting of food intake is more common in females than in males, particularly among Caucasians (Klesges, Eck, & Ray, 1995) and the possibility that girls underreported their food intakes cannot be ruled out in our study. Nevertheless, girls tended to show a negative change in BMI percentile between baseline and follow up, and non-taster girls were more likely to show this trend than the other groups (Figure 2). The decline in BMI%-ile in girls could reflect the rapid increase in linear growth (growth spurts) that accompanies puberty. Since girls mature earlier than boys (Aksglaede, Olsen, Sorensen, & Juul, 2008), the timing of our data collection at 10.2 yrs might have captured this period more effectively in girls than in boys.
Another possible explanation is that dieting to lose weight, which is common in preadolescent girls, may have played a role in these trends. Dieting could explain the apparent mismatch between recommended energy requirements and reported intakes in girls. Unfortunately, our study did not collect information on dieting behavior in the children which could have addressed this question directly. It is important to stress, however, that none of the weight shifts observed in our study were statistically significant. This may be due, in part, to the large amount of variability or ‘noise’ associated with this measure. This variability is not surprising since the growth trajectories of children tend to fluctuate over time, accelerating at some time points and decelerating at others (Centers for Disease Control, 2000). A longitudinal study by Ventura et al. (Ventura, Loken, & Birch, 2009) revealed four distinct BMI trajectories in girls. In two groups of girls with higher BMI percentiles at 5–9 yrs of age, one group continued to show accelerated weight gain to age 15 years and the other showed a downward deflection in their weight trajectories. Two other groups tracked at the 50th and 60th BMI percentile, respectively. Thus, our observation of a downward shift in BMI percentile in some of the girls we studied is consistent with patterns observed in girls by other workers.
Restrained eating was a particularly important determinant of weight status in this group of children. Child restraint score was positively associated with current BMI percentile in the mixed-gender, and female-only regression models. Our data agree with several studies showing that restrained eating is correlated with greater adiposity in children (Ledoux et al., 2011; van Strien & Oosterveld, 2008; Wardle et al., 1992). Interestingly, higher weight in boys in our sample was associated with maternal rather than child dietary restraint suggesting that the mother’s own eating style may be a risk factor for excess weight gain in her child. Previous research has suggested a link between parental eating attitudes and weight status in children. For example, Hood et al. (Hood et al., 2000) showed that parental restrained eating was associated with greater body fat change in young children between 3 and 5 yrs of age in the Framingham cohort. The combination of high parental restraint and disinhibition (i.e., loss of control over eating) led to the highest body fat increases in children. Although there is also ample evidence that maternal eating attitudes are particularly powerful mediators of children’s eating behavior and body weight, especially between mothers and daughters (Cutting, Fisher, Grimm-Thomas, & Birch, 1999; Francis et al., 2007; Vogels et al., 2006), we also found evidence for these relationships in male children.
A previous study by Goldstein et al. (Goldstein et al., 2007) in a slightly younger cohort (mean age = 9.0 +/− 0.2 yrs) reported higher energy intakes in non-taster children relative to super-taster children, but no group differences in weight status as a function of PROP phenotype. Regression analysis also revealed that maternal BMI and child feeding practices rather than PROP status were the primary predictors of child weight in this earlier investigation. The lack of agreement between these two studies is difficult to reconcile. Nevertheless, the earlier study did not include a measure of physical activity which is a major determinant of energy balance. Further, regression models in the present study controlled for both physical activity energy expenditure and early childhood weight. Both variables are considered strong predictors of weight status in children.
There is a lack of consensus in the literature on the role of PROP status in body weight in children. One possible explanation for this lack of consistency is that the influence of PROP on eating behavior and weight may be variable across childhood, having stronger or weaker effects at different stages of development. This mechanism may explain the uneven effects of the FTO (fat mass and obesity related) gene on adiposity development in children. A meta-analysis based on eight cohorts of children from European descent showed that a variant in a single locus of FTO was associated with lower BMI in infancy but a higher BMI in early childhood (Sovio et al., 2011). This was due to an earlier adiposity rebound in affected children that permitted greater accretion of body fat starting at a younger age (Sovio et al., 2011). Whether the PROP phenotype exerts variable effects on food selection and weight status across life stages is not presently known. It is intriguing however, that Goldstein et al. (Goldstein et al., 2007) found no association between PROP status and body weight in 9-yr old children, but in a separate study these same authors found a strong negative association between PROP status and adiposity in the mothers of these same children (Goldstein et al., 2005). These data suggest that PROP status may have a greater influence on body weight later in the life cycle, particularly in the context of a sedentary lifestyle. Conversely, Mennella and co-workers (Mennella, Pepino, & Reed, 2005) observed a strong association between PROP tasting and sweet preferences in 5 to 10 yr old children, but not in the mothers of these children suggesting that the influence of PROP status on sweet preferences waned with increasing age. The lack of availability of longitudinal data sets on PROP tasting has hampered the investigation of this question which should receive more attention in the future.
To our knowledge, no studies have assessed the long-term stability of PROP taster classifications in children at different developmental stages. Our results showed high test-retest reliability of our PROP screening and classification procedures even though we used different methods for preschool screening (Keller et al., 2002; Keller & Tepper, 2004) than we used for follow up testing (Zhao et al., 2003). For the most part, taster and non-taster children were similarly classified at baseline and at age 10, and allelic groupings derived from genotyping confirmed the validity of the PROP classifications. Demonstrating that PROP classifications are stable and reliable is an important first step for implementing longitudinal studies on PROP tasting across the lifecycle.
The current study had several weaknesses. First, our study sample was small, limited to children from middle and high income households, and the majority of the children were Caucasian. Although the gender-specific analyses (Table 3) are informative, small sample size dictates that the results should be interpreted with caution. The extent to which our findings will generalize to other groups in the population is unclear. Baranowski et al. (Baranowski et al., 2010) recently reported no association between PROP status and BMI in low and middle income families from diverse ethnic groups (African-American, Hispanic and Caucasian). It is possible that economic factors exert a greater influence than PROP status on body weight in children with lower socioeconomic status. Second, the percentage of non-taster children in the followup cohort was lower (25%) than in the origin baseline sample (36%). We do not know the weight status of children who declined to participate in the follow up, but it is plausible that children who are overweight would be less likely to participate in a study in which their body weights, food intake and physical activity will be scrutinized than children who maintain a normal weight. Lower representation of non-tasters in our dataset might have reduced weight variability of our sample and the ability to detect differences.
4.1 Conclusions
This is the first study to address changes in body weight with age in children classified by PROP bitter-taste phenotype. Results showed that the interaction between PROP status and gender had a modest influence on the body weights of children, accounting for roughly the same fraction of variance in BMI percentile as child and maternal eating attitudes. PROP status is the most-studied phenotype in taste research, but it is clearly not the only genetic taste factor involved in food preference and eating behavior. For example, liking and use frequency for sweet and fatty foods are heritable traits (Keskitalo et al., 2008; Keskitalo et al., 2007) that may have indirect effects on body weight (Keskitalo, 2008). Emerging evidence also suggests that variation in the gene controlling the fatty acid binding protein (CD36) which is found in taste cells in animals and humans (Simons, Kummer, Luiken, & Boon, 2011), may play a role in human oral fat perception and preference (Keller et al., 2012). It is likely that other genes and phenotypes will be discovered that participate in the pathway linking oral perception with preference, food selection and body weight. Multivariate modeling of taste genetic factors along with environmental and behavioral determinants of food intake will become increasingly important for understanding weight maintenance and obesity development in childhood. These findings are preliminary and are useful for informing the design of larger, prospective studies to more thorough investigate the role of taste genes in pediatric obesity.
Research Highlights.
We examined the role of PROP status and eating attitudes as predictors of weight status in preadolescents during a 6 yr follow up.
There were no group differences in current BMI-percentile or change in BMI%-ile from baseline by PROP phenotype.
Baseline BMI%-ile and physical activity were the strongest predictors of weight status (28.5% variance)
Child restraint, and 2 interactions (taster x gender and maternal BMI x emotional eating), also accounted for 18% variance.
These data suggest that PROP status and eating attitudes are modest predictors of weight status in preadolescents.
Acknowledgments
These studies were conducted, in partial fulfillment of the M.S. degree in Food Science by KNO. The authors thank Harriet Worobey, director of the Rutgers Nutritional Sciences Preschool for assistance with contacting families for this follow-up study, and Dr. Danielle Reed and Ms. Kirsten J. Mascioli of the Monell Chemical Senses Center for performing the genotype analyses.BJT conceptualized the research and designed the study; KNO conducted the research and analyzed the data; KNO and BJT wrote the paper and had primary responsibility for final content; both authors read and approved the final manuscript. The study was supported by the National Cancer Institute (CA1166766) and by Hatch Act Funds administered through the New Jersey Agricultural Experiment Station. The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksglaede L, Olsen LW, Sorensen TI, Juul A. Forty years trends in timing of pubertal growth spurt in 157,000 Danish school children. PLoS One. 2008;3(7):e2728. doi: 10.1371/journal.pone.0002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzman SL, Rollins BY, Birch LL. Parental influence on children’s early eating environments and obesity risk: implications for prevention. Int J Obes (Lond) 2010;34(7):1116–1124. doi: 10.1038/ijo.2010.43. [DOI] [PubMed] [Google Scholar]
- Baranowski JC, Baranowski T, Beltran A, Watson KB, Jago R, Callie M, et al. 6-n-Propylthiouracil sensitivity and obesity status among ethnically diverse children. Public Health Nutr. 2010;13(10):1587–1592. doi: 10.1017/S1368980009993004. [DOI] [PubMed] [Google Scholar]
- Bell KI, Tepper BJ. Short-term vegetable intake by young children classified by 6-n-propylthoiuracil bitter-taste phenotype. Am J Clin Nutr. 2006;84(1):245–251. doi: 10.1093/ajcn/84.1.245. [DOI] [PubMed] [Google Scholar]
- Berkey C, Rockett H, Field A, Gillman M, Frazier A, Camargo J, CA, et al. Activity, dietary intake, and weight changes in a longitudinal study of preadolescent and adolescent boys and girls. Pediatrics. 2000;105(4):e56. doi: 10.1542/peds.105.4.e56. [DOI] [PubMed] [Google Scholar]
- Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc. 2010;110(10):1501–1510. doi: 10.1016/j.jada.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Calo C, Padiglia A, Zonza A, Corrias L, Contu P, Tepper BJ, et al. Polymorphisms in TAS2R38 and the taste bud trophic factor, gustin gene co-operate in modulating PROP taste phenotype. Physiol Behav. 2011;104:1065–1071. doi: 10.1016/j.physbeh.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Carper JL, Orlet Fisher J, Birch LL. Young girls’ emerging dietary restraint and disinhibition are related to parental control in child feeding. Appetite. 2000;35(2):121–129. doi: 10.1006/appe.2000.0343. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Growth Charts for the United States. 2000 Retrieved June 10, 2011, from http://www.cdc.gov/growthcharts.
- Cutting TM, Fisher JO, Grimm-Thomas K, Birch LL. Like mother, like daughter: familial patterns of overweight are mediated by mothers’ dietary disinhibition. Am J Clin Nutr. 1999;69(4):608–613. doi: 10.1093/ajcn/69.4.608. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board, N. A. o. S. . Dietary reference intakes for energy, carbohydrate, fiber, fats, fatty acids, cholesterol, protein and amino acids (macronutrients) Washington, D.C: National Academy Press; 2005. pp. 107–264. [DOI] [PubMed] [Google Scholar]
- Francis LA, Ventura AK, Marini M, Birch LL. Parent overweight predicts daughters’ increase in BMI and disinhibited overeating from 5 to 13 years. Obesity (Silver Spring) 2007;15(6):1544–1553. doi: 10.1038/oby.2007.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein GL, Daun H, Tepper BJ. Adiposity in middle-aged women is associated with genetic taste blindness to 6-n-propylthiouracil. Obes Res. 2005;13(6):1017–1023. doi: 10.1038/oby.2005.119. [DOI] [PubMed] [Google Scholar]
- Goldstein GL, Daun H, Tepper BJ. Influence of PROP taster status and maternal variables on energy intake and body weight of pre-adolescents. Physiol Behav. 2007;90(5):809–817. doi: 10.1016/j.physbeh.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21(3):323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Guo S, Huang C, Maynard L, Demerath E, Towne B, Chumlea W, et al. Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity: the Fels Longitudinal Study. Int J Obes Relat Metab Disord. 2000;24(12):1628–1635. doi: 10.1038/sj.ijo.0801461. [DOI] [PubMed] [Google Scholar]
- Hood MY, Moore LL, Sundarajan-Ramamurti A, Singer M, Cupples LA, Ellison RC. Parental eating attitudes and the development of obesity in children. The Framingham Children’s Study. Int J Obes Relat Metab Disord. 2000;24(10):1319–1325. doi: 10.1038/sj.ijo.0801396. [DOI] [PubMed] [Google Scholar]
- Keller KL, Liang LC, Sakimura J, May D, van Belle C, Breen C, et al. Common Variants in the CD36 Gene Are Associated With Oral Fat Perception, Fat Preferences, and Obesity in African Americans. Obesity (Silver Spring) 2012 doi: 10.1038/oby.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KL, Reid A, MacDougall MC, Cassano H, Song JL, Deng L, et al. Sex differences in the effects of inherited bitter thiourea sensitivity on body weight in 4–6-year-old children. Obesity (Silver Spring) 2010;18(6):1194–1200. doi: 10.1038/oby.2009.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KL, Steinmann L, Nurse RJ, Tepper BJ. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite. 2002;38(1):3–12. doi: 10.1006/appe.2001.0441. [DOI] [PubMed] [Google Scholar]
- Keller KL, Tepper BJ. Inherited taste sensitivity to 6-n-propylthiouracil in diet and body weight in children. Obes Res. 2004;12(6):904–912. doi: 10.1038/oby.2004.110. [DOI] [PubMed] [Google Scholar]
- Keskitalo K, Tuorila H, Spector TD, Cherkas LF, Knaapila A, Kaprio J, et al. The Three-Factor Eating Questionnaire, body mass index, and responses to sweet and salty fatty foods: a twin study of genetic and environmental associations. Am J Clin Nutr. 2008;88(2):263–271. doi: 10.1093/ajcn/88.2.263. [DOI] [PubMed] [Google Scholar]
- Keskitalo K, Tuorila H, Spector TD, Cherkas LF, Knaapila A, Silventoinen K, et al. Same genetic components underlie different measures of sweet taste preference. Am J Clin Nutr. 2007;86(6):1663–1669. doi: 10.1093/ajcn/86.5.1663. [DOI] [PubMed] [Google Scholar]
- Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299(5610):1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Eck LH, Ray JW. Who underreports dietary intake in a dietary recall? Evidence from the Second National Health and Nutrition Examination Survey. J Consult Clin Psychol. 1995;63(3):438–444. doi: 10.1037//0022-006x.63.3.438. [DOI] [PubMed] [Google Scholar]
- Ledoux T, Watson K, Baranowski J, Tepper BJ, Baranowski T. Overeating styles and adiposity among multiethnic youth. Appetite. 2011;56(1):71–77. doi: 10.1016/j.appet.2010.11.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng JC, Cardinal TM, Sitto JR, Kannan S. Ability to taste 6-n-propylthiouracil and BMI in low-income preschool-aged children. Obesity (Silver Spring) 2008;16(7):1522–1528. doi: 10.1038/oby.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Predicting obesity in early adulthood from childhood and parental obesity. Int J Obes Relat Metab Disord. 2003;27(4):505–513. doi: 10.1038/sj.ijo.0802251. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Duke FF, Reed DR. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2011;11:60. doi: 10.1186/1471-2156-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Duke FF, Reed DR. Psychophysical dissection of genotype effects on human bitter perception. Chem Senses. 2012;36(2):161–167. doi: 10.1093/chemse/bjq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115(2):e216–222. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Reilly JJ, Jackson DM, Kelly LA, Slater C, Paton JY, et al. Validation of energy intake by 24-hour multiple pass recall: comparison with total energy expenditure in children aged 5–7 years. Br J Nutr. 2005;93(5):671–676. doi: 10.1079/bjn20051405. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- Shunk JA, Birch LL. Girls at risk for overweight at age 5 are at risk for dietary restraint, disinhibited overeating, weight concerns, and greater weight gain from 5 to 9 years. J Am Diet Assoc. 2004;104(7):1120–1126. doi: 10.1016/j.jada.2004.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons PJ, Kummer JA, Luiken JJ, Boon L. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 2011;113(8):839–843. doi: 10.1016/j.acthis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Sinton MM, Birch LL. Weight status and psychosocial factors predict the emergence of dieting in preadolescent girls. Int J Eat Disord. 2005;38(4):346–354. doi: 10.1002/eat.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovio U, Mook-Kanamori DO, Warrington NM, Lawrence R, Briollais L, Palmer CNA, et al. Association between Common Variation at the FTO Locus and Changes in Body Mass Index from Infancy to Late Childhood: The Complex Nature of Genetic Association through Growth and Development. PLoS Genet. 2011;7(2):e1001307. doi: 10.1371/journal.pgen.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper BJ. Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu Rev Nutr. 2008;28:367–388. doi: 10.1146/annurev.nutr.28.061807.155458. [DOI] [PubMed] [Google Scholar]
- Tepper BJ, Keller KL, Ullrich NV. Genetic variation in taste and preferences for bitter and pungent foods: Implications for chronic disease risk. In: Hofmann TL, Ho CT, Pickenhagen W, editors. Challenges in Taste Chemistry and Biology. 2003. pp. 60–74. American Chemical Society Symposium Series 867. [Google Scholar]
- Tepper BJ, White EA, Koelliker Y, Lanzara C, d’Adamo P, Gasparini P. Genetic variation in taste sensitivity to 6-n-propylthiouracil and its relationship to taste perception and food selection. Ann N Y Acad Sci. 2009;1170:126–139. doi: 10.1111/j.1749-6632.2009.03916.x. [DOI] [PubMed] [Google Scholar]
- Timpson NJ, Heron J, Day IN, Ring SM, Bartoshuk LM, Horwood J, et al. Refining associations between TAS2R38 diplotypes and the 6-n-propylthiouracil (PROP) taste test: findings from the Avon Longitudinal Study of Parents and Children. BMC Genet. 2007;8:51. doi: 10.1186/1471-2156-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornwall O, Silventoinen K, Keskitalo-Vuokko K, Perola M, Kaprio J, Tuorila H. Genetic contribution to sour taste preference. Appetite. 2012;58(2):687–694. doi: 10.1016/j.appet.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Nakamura K, Tamai Y, Wada K, Sahashi Y, Watanabe K, et al. Relationship of intake of plant-based foods with 6-n-propylthiouracil sensitivity and food neophobia in Japanese preschool children. Eur J Clin Nutr. 2012;66(1):47–52. doi: 10.1038/ejcn.2011.127. [DOI] [PubMed] [Google Scholar]
- Turnbull B, Matisoo-Smith E. Taste sensitivity to 6-n-propylthiouracil predicts acceptance of bitter-tasting spinach in 3–6-y-old children. Am J Clin Nutr. 2002;76(5):1101–1105. doi: 10.1093/ajcn/76.5.1101. [DOI] [PubMed] [Google Scholar]
- Van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained eating, emotional and external eating behavior. Int J Eat Disord. 1986;5:295–315. [Google Scholar]
- van Strien T, Oosterveld P. The children’s DEBQ for assessment of restrained, emotional, and external eating in 7- to 12-year-old children. Int J Eat Disord. 2008;41(1):72–81. doi: 10.1002/eat.20424. [DOI] [PubMed] [Google Scholar]
- Ventura AK, Loken E, Birch LL. Developmental trajectories of girls’ BMI across childhood and adolescence. Obesity (Silver Spring) 2009;17(11):2067–2074. doi: 10.1038/oby.2009.123. [DOI] [PubMed] [Google Scholar]
- Vogels N, Posthumus DL, Mariman EC, Bouwman F, Kester AD, Rump P, et al. Determinants of overweight in a cohort of Dutch children. Am J Clin Nutr. 2006;84(4):717–724. doi: 10.1093/ajcn/84.4.717. [DOI] [PubMed] [Google Scholar]
- Wardle J, Marsland L, Sheikh Y, Quinn M, Fedoroff I, Ogden J. Eating style and eating behaviour in adolescents. Appetite. 1992;18(3):167–183. doi: 10.1016/0195-6663(92)90195-c. [DOI] [PubMed] [Google Scholar]
- Webber L, Hill C, Saxton J, Van Jaarsveld CH, Wardle J. Eating behaviour and weight in children. Int J Obes (Lond) 2009;33(1):21–28. doi: 10.1038/ijo.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Kirkmeyer SV, Tepper BJ. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol Behav. 2003;78(4–5):625–633. doi: 10.1016/s0031-9384(03)00057-x. [DOI] [PubMed] [Google Scholar]