Abstract
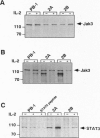
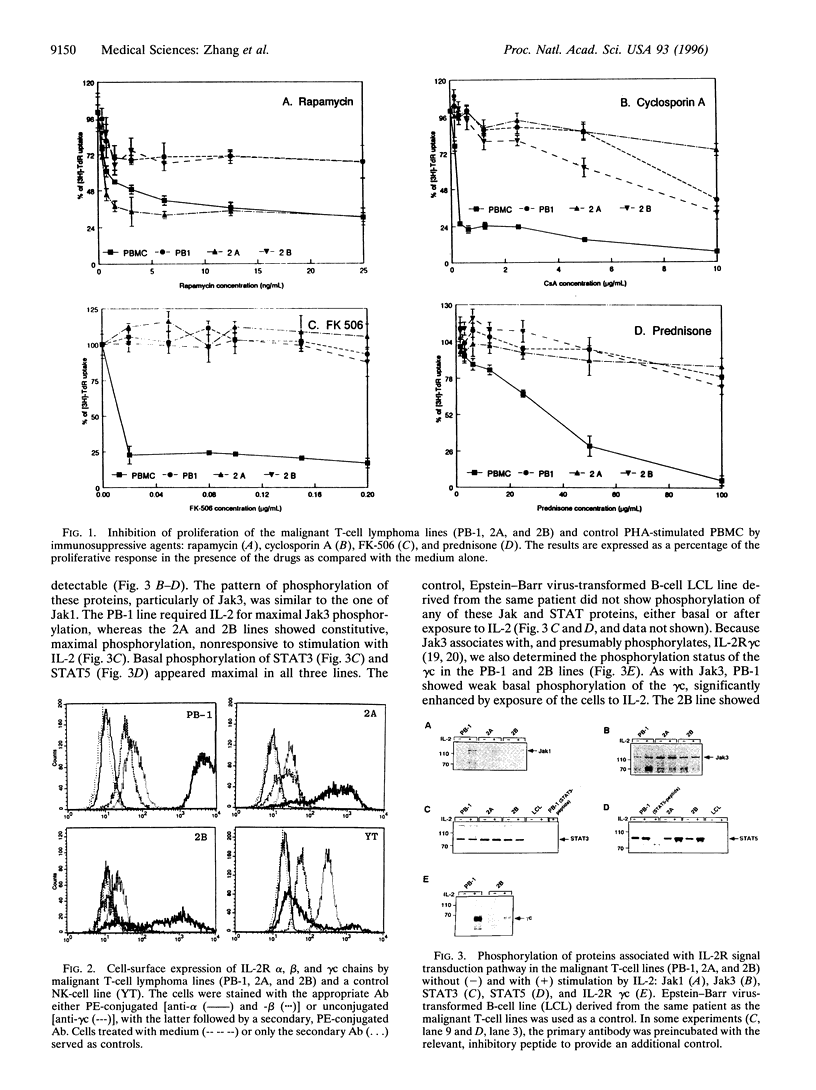
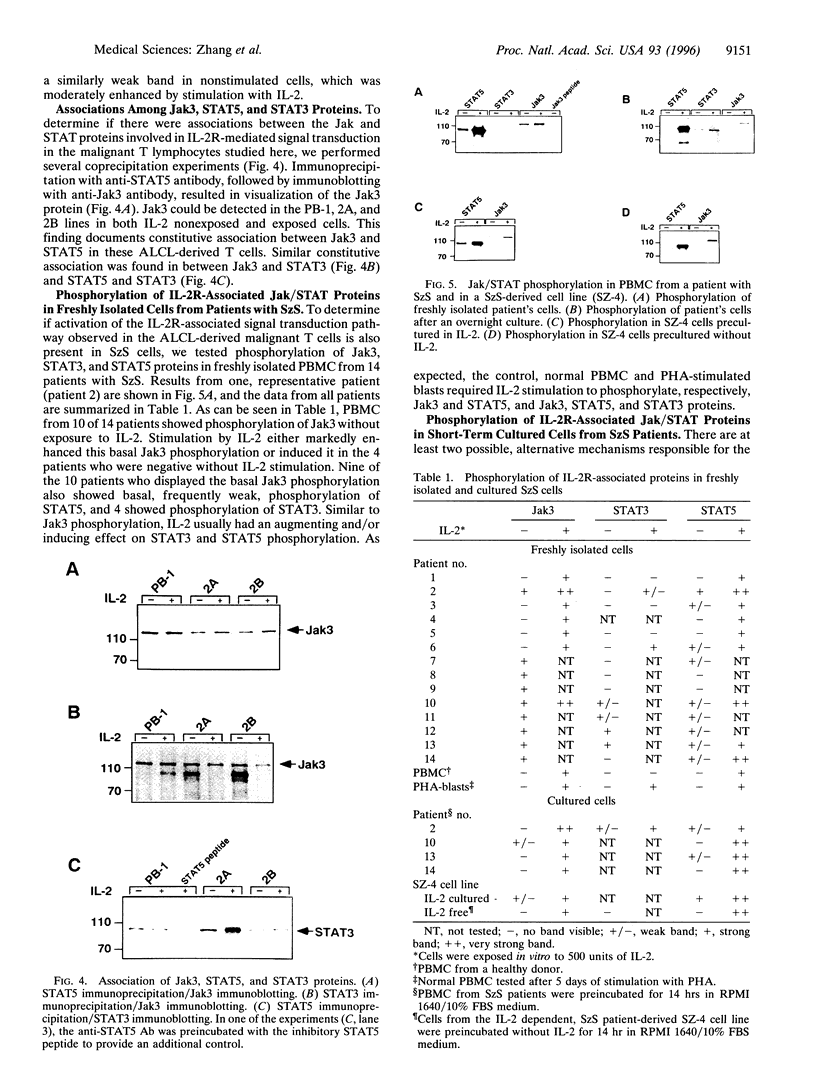
Signaling through the interleukin 2 receptor (IL-2R) involves phosphorylation of several proteins including Jak3, STAT5, and, in preactivated cells, STAT3. In the present study, we examined the functional status of the IL-2R-associated Jak/STAT pathway in malignant T lymphocytes from advanced skin-based lymphomas: anaplastic large T-cell lymphoma (ALCL) and Sezary syndrome (SzS). Proliferation of three ALCL cell lines (PB-1, 2A, and 2B) was partially inhibited by rapamycin, a blocker of some of the signals mediated by IL-2R, but not by cyclosporin A, FK-506, and prednisone, which suppress signals mediated by the T-cell receptor. All the cell lines expressed on their surface the high-affinity IL-2R (alpha, beta, and gamma c chains). They showed basal, constitutive phosphorylation, and coassociation of Jak3, STAT5, and STAT3. Weak basal phosphorylation of IL-2R gamma c was also detected. In regard to SzS, peripheral blood mononuclear cells from 10 of 14 patients showed basal phosphorylation of Jak3, accompanied by phosphorylation of STAT5 in 9 patients, and STAT3 in 4 patients. However, in vitro overnight culture of SzS cells without exogenous cytokines resulted in markedly decreased Jak3 and STAT5 phosphorylation, which could be reversed by stimulation with IL-2. This indicates that the basal phosphorylation of Jak3 and STAT5 in freshly isolated SzS cells is induced rather than constitutive. The basal activation of the Jak/STAT pathway involved in IL-2R signal transduction in ALCL and SzS cells reported here suggests that this pathway may play a role in the pathogenesis of cutaneous T-cell lymphomas, although the mechanism (induced versus constitutive) may vary between different lymphoma types.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. T., Lessin S., Ghosh S. K., Ju W., Vonderheid E. C., Nowell P., Murphy G., Elfenbein B., DeFreitas E. A clonal CD4-positive T-cell line established from the blood of a patient with Sézary syndrome. J Invest Dermatol. 1991 Jan;96(1):31–37. doi: 10.1111/1523-1747.ep12514693. [DOI] [PubMed] [Google Scholar]
- Auphan N., DiDonato J. A., Rosette C., Helmberg A., Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995 Oct 13;270(5234):286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Beadling C., Guschin D., Witthuhn B. A., Ziemiecki A., Ihle J. N., Kerr I. M., Cantrell D. A. Activation of JAK kinases and STAT proteins by interleukin-2 and interferon alpha, but not the T cell antigen receptor, in human T lymphocytes. EMBO J. 1994 Dec 1;13(23):5605–5615. doi: 10.1002/j.1460-2075.1994.tb06898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehncke W. H., Gerdes J., Wiese M., Kaltoft K., Sterry W. A majority of proliferating T cells in cutaneous malignant T cell lymphomas may lack the high affinity IL-2 receptor (CD25). Arch Dermatol Res. 1993;285(3):127–130. doi: 10.1007/BF01112913. [DOI] [PubMed] [Google Scholar]
- Cao X., Shores E. W., Hu-Li J., Anver M. R., Kelsall B. L., Russell S. M., Drago J., Noguchi M., Grinberg A., Bloom E. T. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995 Mar;2(3):223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Davis T. H., Morton C. C., Miller-Cassman R., Balk S. P., Kadin M. E. Hodgkin's disease, lymphomatoid papulosis, and cutaneous T-cell lymphoma derived from a common T-cell clone. N Engl J Med. 1992 Apr 23;326(17):1115–1122. doi: 10.1056/NEJM199204233261704. [DOI] [PubMed] [Google Scholar]
- DiSanto J. P., Müller W., Guy-Grand D., Fischer A., Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995 Nov 15;86(10):3619–3639. [PubMed] [Google Scholar]
- Fujii H., Nakagawa Y., Schindler U., Kawahara A., Mori H., Gouilleux F., Groner B., Ihle J. N., Minami Y., Miyazaki T. Activation of Stat5 by interleukin 2 requires a carboxyl-terminal region of the interleukin 2 receptor beta chain but is not essential for the proliferative signal transmission. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5482–5486. doi: 10.1073/pnas.92.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J. G., Ahdieh M., Eisenman J., Shanebeck K., Grabstein K., Kumaki S., Namen A., Park L. S., Cosman D., Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994 Jun 15;13(12):2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J. G., Kumaki S., Ahdieh M., Friend D. J., Loomis A., Shanebeck K., DuBose R., Cosman D., Park L. S., Anderson D. M. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995 Aug 1;14(15):3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K. H., Eisenman J., Shanebeck K., Rauch C., Srinivasan S., Fung V., Beers C., Richardson J., Schoenborn M. A., Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994 May 13;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu Rev Immunol. 1995;13:369–398. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Kawamura M., Kirken R. A., Chen Y. Q., Blake T. B., Shibuya K., Ortaldo J. R., McVicar D. W., O'Shea J. J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994 Jul 14;370(6485):151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Takeshita T., Kondo M., Ishii N., Nakamura M., Van Snick J., Sugamura K. Sharing of the IL-2 receptor gamma chain with the functional IL-9 receptor complex. Int Immunol. 1995 Jan;7(1):115–120. doi: 10.1093/intimm/7.1.115. [DOI] [PubMed] [Google Scholar]
- Kondo M., Takeshita T., Ishii N., Nakamura M., Watanabe S., Arai K., Sugamura K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993 Dec 17;262(5141):1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Noguchi M., Russell S. M., McBride O. W. The molecular basis of X-linked severe combined immunodeficiency: the role of the interleukin-2 receptor gamma chain as a common gamma chain, gamma c. Immunol Rev. 1994 Apr;138:61–86. doi: 10.1111/j.1600-065x.1994.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Lin J. X., Migone T. S., Tsang M., Friedmann M., Weatherbee J. A., Zhou L., Yamauchi A., Bloom E. T., Mietz J., John S. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995 Apr;2(4):331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- Macchi P., Villa A., Giliani S., Sacco M. G., Frattini A., Porta F., Ugazio A. G., Johnston J. A., Candotti F., O'Shea J. J. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature. 1995 Sep 7;377(6544):65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- Merz H., Fliedner A., Orscheschek K., Binder T., Sebald W., Müller-Hermelink H. K., Feller A. C. Cytokine expression in T-cell lymphomas and Hodgkin's disease. Its possible implication in autocrine or paracrine production as a potential basis for neoplastic growth. Am J Pathol. 1991 Nov;139(5):1173–1180. [PMC free article] [PubMed] [Google Scholar]
- Meydan N., Grunberger T., Dadi H., Shahar M., Arpaia E., Lapidot Z., Leeder J. S., Freedman M., Cohen A., Gazit A. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996 Feb 15;379(6566):645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- Migone T. S., Lin J. X., Cereseto A., Mulloy J. C., O'Shea J. J., Franchini G., Leonard W. J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995 Jul 7;269(5220):79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Kawahara A., Fujii H., Nakagawa Y., Minami Y., Liu Z. J., Oishi I., Silvennoinen O., Witthuhn B. A., Ihle J. N. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994 Nov 11;266(5187):1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Liu Z. J., Kawahara A., Minami Y., Yamada K., Tsujimoto Y., Barsoumian E. L., Permutter R. M., Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995 Apr 21;81(2):223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Nielsen M., Svejgaard A., Skov S., Odum N. Interleukin-2 induces tyrosine phosphorylation and nuclear translocation of stat3 in human T lymphocytes. Eur J Immunol. 1994 Dec;24(12):3082–3086. doi: 10.1002/eji.1830241225. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Nakamura Y., Russell S. M., Ziegler S. F., Tsang M., Cao X., Leonard W. J. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993 Dec 17;262(5141):1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Yi H., Rosenblatt H. M., Filipovich A. H., Adelstein S., Modi W. S., McBride O. W., Leonard W. J. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993 Apr 9;73(1):147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- Nosaka T., van Deursen J. M., Tripp R. A., Thierfelder W. E., Witthuhn B. A., McMickle A. P., Doherty P. C., Grosveld G. C., Ihle J. N. Defective lymphoid development in mice lacking Jak3. Science. 1995 Nov 3;270(5237):800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- Ohbo K., Suda T., Hashiyama M., Mantani A., Ikebe M., Miyakawa K., Moriyama M., Nakamura M., Katsuki M., Takahashi K. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood. 1996 Feb 1;87(3):956–967. [PubMed] [Google Scholar]
- Paul W. E., Seder R. A. Lymphocyte responses and cytokines. Cell. 1994 Jan 28;76(2):241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Peuchmaur M., Emilie D., Crevon M. C., Solal-Celigny P., Maillot M. C., Lemaigre G., Galanaud P. IL-2 mRNA expression in Tac-positive malignant lymphomas. Am J Pathol. 1990 Feb;136(2):383–390. [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Johnston J. A., Noguchi M., Kawamura M., Bacon C. M., Friedmann M., Berg M., McVicar D. W., Witthuhn B. A., Silvennoinen O. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994 Nov 11;266(5187):1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Keegan A. D., Harada N., Nakamura Y., Noguchi M., Leland P., Friedmann M. C., Miyajima A., Puri R. K., Paul W. E. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993 Dec 17;262(5141):1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Tayebi N., Nakajima H., Riedy M. C., Roberts J. L., Aman M. J., Migone T. S., Noguchi M., Markert M. L., Buckley R. H. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995 Nov 3;270(5237):797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- Scheinman R. I., Cogswell P. C., Lofquist A. K., Baldwin A. S., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995 Oct 13;270(5234):283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- Schlegelberger B., Himmler A., Gödde E., Grote W., Feller A. C., Lennert K. Cytogenetic findings in peripheral T-cell lymphomas as a basis for distinguishing low-grade and high-grade lymphomas. Blood. 1994 Jan 15;83(2):505–511. [PubMed] [Google Scholar]
- Sigal N. H., Dumont F. J. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- Suchi T., Lennert K., Tu L. Y., Kikuchi M., Sato E., Stansfeld A. G., Feller A. C. Histopathology and immunohistochemistry of peripheral T cell lymphomas: a proposal for their classification. J Clin Pathol. 1987 Sep;40(9):995–1015. doi: 10.1136/jcp.40.9.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamura K. [Structure and function of IL-2 receptor subunits]. Hum Cell. 1994 Mar;7(1):1–5. [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995 Apr 14;268(5208):251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- Thomis D. C., Gurniak C. B., Tivol E., Sharpe A. H., Berg L. J. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995 Nov 3;270(5237):794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- Vonderheid E. C., Sobel E. L., Nowell P. C., Finan J. B., Helfrich M. K., Whipple D. S. Diagnostic and prognostic significance of Sézary cells in peripheral blood smears from patients with cutaneous T cell lymphoma. Blood. 1985 Aug;66(2):358–366. [PubMed] [Google Scholar]
- Vowels B. R., Cassin M., Vonderheid E. C., Rook A. H. Aberrant cytokine production by Sezary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol. 1992 Jul;99(1):90–94. doi: 10.1111/1523-1747.ep12611877. [DOI] [PubMed] [Google Scholar]
- Wasik M. A., Morimoto C. Differential effects of cytokines on proliferative response of human CD4+ T lymphocyte subsets stimulated via T cell receptor-CD3 complex. J Immunol. 1990 May 1;144(9):3334–3340. [PubMed] [Google Scholar]
- Wasik M. A. Preferential interaction of the CD4+29+/45RA-subset of human CD4+ T lymphocytes with an antibody against the cell-membrane ganglioside GD3. Scand J Immunol. 1992 Apr;35(4):421–428. doi: 10.1111/j.1365-3083.1992.tb02877.x. [DOI] [PubMed] [Google Scholar]
- Wieselthier J. S., Koh H. K. Sézary syndrome: diagnosis, prognosis, and critical review of treatment options. J Am Acad Dermatol. 1990 Mar;22(3):381–401. doi: 10.1016/0190-9622(90)70054-l. [DOI] [PubMed] [Google Scholar]
- Willemze R., van Vloten W. A., Hermans J., Damsteeg M. J., Meijer C. J. Diagnostic criteria in Sézary's syndrome: a multiparameter study of peripheral blood lymphocytes in 32 patients with erythroderma. J Invest Dermatol. 1983 Nov;81(5):392–397. doi: 10.1111/1523-1747.ep12521991. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Silvennoinen O., Miura O., Lai K. S., Cwik C., Liu E. T., Ihle J. N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994 Jul 14;370(6485):153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Teshigawara K., Nikaido T., Fukui K., Noma T., Honjo T., Takigawa M., Sasaki M., Minato N., Tsudo M. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J Immunol. 1985 Mar;134(3):1623–1630. [PubMed] [Google Scholar]