Highlights
► Recurrent invasive adenocarcinoma after definitive therapy for AIS is a rare occurrence. ► Because of its unpredictable nature, AIS requires long-term and close surveillance.
Keywords: Cervix, Adenocarcinoma in situ, Adenocarcinoma
Introduction
Cervical adenocarcinoma in situ (AIS) is a precursor to invasive cervical adenocarcinoma (Wright et al., 2007). Adenocarcinoma accounts for approximately 25% of cervical cancers in the United States, and the incidence of both AIS and invasive cervical adenocarcinoma has recently been increasing (Wright et al., 2007). AIS has challenges to managing conservatively as the lesion may be located high in the endocervical canal, be multicentric with “skip” lesions present, or have deep glandular involvement. The sensitivity of cervical cytology and ECC is only approximately 50% (Wolf et al., 1996), making it difficult to adequately monitor women with AIS who are managed conservatively. Furthermore, previous studies have shown that the incidence of residual AIS in hysterectomy specimens is approximately 52.8% with positive conization margins, and 20% with negative conization margins. In addition, approximately 5% of patients with positive margins and 0.7% of patients with negative margins have invasive adenocarcinoma at the time of hysterectomy (Salani et al., 2009; Ostor et al., 2000; Bull-Phelps et al., 2007; Im et al., 1995; McHale et al., 2001; Shin et al., 2000).
Given these challenges, the standard treatment for AIS in women who have completed childbearing is extrafascial hysterectomy. However, conservative management with cervical conization with negative margins is acceptable if future fertility is desired. If conservative management is planned and the margins of the specimen are involved or endocervical sampling obtained at the time of excision contains AIS, re-excision to increase the likelihood of complete excision is preferred. Once margins are negative, these patients are monitored closely with a combination of cervical cytology, HPV DNA testing, and colposcopy with endocervical sampling (Wright et al., 2007). Long‐term follow-up is recommended in all patients. There are a few published case reports of invasive adenocarcinoma occurring after hysterectomy for AIS, further illustrating the unpredictable nature of AIS and need for close surveillance (Miller et al., 2005; Krivak et al., 2000; Hopkins et al., 1988).
Case
A 34 year-old nulliparous woman was diagnosed with adenocarcinoma in situ of the cervix and underwent a cold knife cone biopsy of the cervix. The pathologic findings showed adenocarcinoma in situ and cervical intraepithelial neoplasia 3 with negative margins. She subsequently underwent a simple hysterectomy. Serial sectioning of the cervix showed no invasive disease or residual AIS in the cervix. She was followed with annual cytology, with no abnormalities noted. Fourteen years later, she presented to her primary care physician with left lower extremity edema. A computed tomography (CT) scan revealed diffuse lymphadenopathy in the left inguinal, iliac, and para-aortic areas, with the largest node measuring 4.9 × 2.8 cm (Fig. 1). There were no abnormal nodes in the right iliac region. She underwent excision of the enlarged left inguinal lymph nodes, which showed metastatic poorly differentiated carcinoma (Fig. 2). The carcinoma was diffusely positive for CK7, CK 8/18, and p16 by immunohistochemistry. There was focal staining for ER and PAX8, and rare cells stained for CK 5/6. In situ hybridization for high risk human papilloma virus (HPV) was positive. The tumor was felt to be consistent with metastatic poorly differentiated adenocarcinoma from the cervix, vagina, or vulva. Colposcopy of the vagina and vulva, as well as anoscopy, was unremarkable. Colonoscopy and upper endoscopy were negative. Positron emission tomography (PET) showed no other evidence of disease.
Fig. 1.
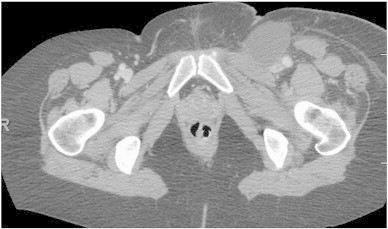
Metastatic adenocarcinoma. Left inguinal mass, measuring 4.9 × 2.8 cm, with biopsy consistent with metastatic poorly differentiated carcinoma.
Fig. 2.
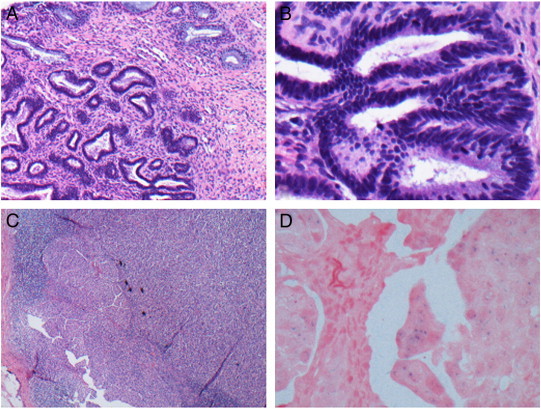
(A), (B) Adenocarcinoma in situ of the cervix. (A) Adenocarcinoma in situ of the endocervix is generally characterized at medium magnification by the preservation of normal glandular architecture, with an abrupt transition between normal and cytologically atypical cells. (B) At higher magnification, the features are evident, including nuclear enlargement, coarse chromatin, and stratification of nuclei. (C) Left inguinal lymph node with metastatic poorly differentiated adenocarcinoma of the cervix. (D) Positive high-risk human papilloma virus (HPV) DNA in situ hybridization.
The patient underwent chemotherapy with six cycles of intravenous carboplatinum and paclitaxel. Imaging at the completion of therapy showed significant improvement with residual small volume disease in the left inguinal, pelvic, common iliac, and para-aortic lymph nodes. She was therefore treated with intensity-modulated radiation therapy (IMRT) to the left inguinal, left pelvic, and para-aortic lymph nodes with a total dose of 50.4 Gy in 27 fractions, followed by a boost of 10 Gy in 5 fractions to PET positive nodes. Her left supraclavicular fossa was treated prophylactically with 40 Gy given over 20 fractions. She tolerated her treatment well with no serious adverse effects. However, a PET scan three months following the completion of therapy showed new right inguinal and right pelvic lymphadenopathy, all of which was outside the prior radiation field. She was then treated with IMRT to the new sites of disease in the left pelvis. Imaging three months after the completion of therapy showed recurrent disease with several PET positive nodes in the previously irradiated field, as well as new peritoneal disease. The patient is currently undergoing chemotherapy with carboplatinum and paclitaxel.
Discussion
Recurrent invasive adenocarcinoma after definitive therapy for AIS is a rare occurrence. This case describes the unpredictable course of cervical AIS. There have been three other published case reports in which invasive adenocarcinoma was diagnosed after definitive therapy for AIS. However, these cases all describe patients in whom either the cone specimen had positive margins or the hysterectomy specimen showed residual disease. Miller et al. (2005) describe a patient in whom AIS was found on a LEEP specimen with positive endocervical margins. She subsequently underwent an abdominal hysterectomy and bilateral salpingo-oopherectomy in whom the final pathology showed residual AIS, but no invasive component. She presented 28 months later with a pelvic side wall mass with biopsy showing invasive adenocarcinoma consistent with a cervical primary. Krivak et al. (2000) describe a patient with cervical AIS with positive endocervical margins on cold knife cone biopsy. She underwent a simple hysterectomy and bilateral salpingo-oopherectomy with no residual disease noted. Almost two years later, she presented with a vaginal cuff mass, with biopsy showing invasive adenocarcinoma consistent with an endocervical primary. Hopkins et al. (1988) reported a case of recurrent adenocarcinoma at the vaginal cuff extending to the pelvic sidewall with ureteral obstruction 8 years after hysterectomy in which the hysterectomy specimen contained residual AIS after cone biopsy, but no invasive carcinoma.
Our case and the others emphasize that even in patients who undergo definitive therapy with hysterectomy for AIS, recurrence with invasive adenocarcinoma may occur. The patient described in this report had negative cone biopsy margins for cervical AIS, and an extrafascial hysterectomy was performed that showed no evidence of residual disease in the cervix. The patient had a negative evaluation for other sources of invasive adenocarcinoma, including negative vulvar and vaginal colposcopy, anoscopy, colonoscopy and upper endoscopy. Furthermore, immunostaining suggested the tumor to be consistent with metastasis from a cervical primary. Although all pathology slides were re-reviewed, a focus of invasive disease on the hysterectomy specimen may not have been identified secondary to sampling issues. It is also possible that the carcinoma represented a de novo lesion that subsequently developed.
As seen in this case and previous reports, the risk of developing invasive disease can extend many years after definitive therapy for AIS. It has been difficult to delineate treatment recommendations for patients based on the numerous retrospective cohorts published concerning AIS management. Moreover, this case highlights the concerns over the definitive management of adenocarcinoma in situ of the cervix as well as the adequacy and frequency of follow-up. Physicians should educate patients on the symptoms of recurrence and to report them to their physicians promptly. This case should alert physicians to the possibility that AIS of the cervix may recur several years after definitive therapy and patients may present with metastatic disease.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent form is available for review by the Editor-in-Chief of this journal upon request.
References
- Bull-Phelps S.L., Garner E.I., Walsh C.S., Gehrig P.A., Miller D.S., Schorge J.O. Fertility-sparing surgery in 101 women with adenocarcinoma in situ of the cervix. Gynecol. Oncol. 2007;107(2):316–319. doi: 10.1016/j.ygyno.2007.06.021. (Nov) [DOI] [PubMed] [Google Scholar]
- Hopkins M.P., Roberts J.A., Schmidt R.W. Cervical adenocarcinoma in situ. Obstet. Gynecol. 1988;71:842–844. [PubMed] [Google Scholar]
- Im D.D., Duska L.R., Rosenshein N.B. Adequacy of conization margins in adenocarcinoma in situ of the cervix as a predictor of residual disease. Gynecol. Oncol. 1995;59(2):179–182. doi: 10.1006/gyno.1995.0003. (Nov) [DOI] [PubMed] [Google Scholar]
- Krivak T.C., Retherford B., Voskuil S., Rose G.S., Alagoz T. Recurrent invasive adenocarcinoma after hysterectomy for cervical adenocarcinoma in situ. Gynecol. Oncol. 2000;77(2):334–335. doi: 10.1006/gyno.2000.5761. (May) [DOI] [PubMed] [Google Scholar]
- McHale M.T., Le T.D., Burger R.A., Gu M., Rutgers J.L., Monk B.J. Fertility sparing treatment for in situ and early invasive adenocarcinoma of the cervix. Obstet. Gynecol. 2001;98(5 Pt 1):726–731. doi: 10.1016/s0029-7844(01)01544-7. (Nov) [DOI] [PubMed] [Google Scholar]
- Miller B., Dunn J., Dalrymple J., Krivak T.C. Pelvic sidewall adenocarcinoma after definitive therapy for cervical adenocarcinoma in situ. Gynecol. Oncol. 2005;99(2):489–492. doi: 10.1016/j.ygyno.2005.06.044. (Nov) [DOI] [PubMed] [Google Scholar]
- Ostor A.G., Duncan A., Quinn M., Rome R. Adenocarcinoma in situ of the uterine cervix: an experience with 100 cases. Gynecol. Oncol. 2000;79(2):207–210. doi: 10.1006/gyno.2000.5957. (Nov) [DOI] [PubMed] [Google Scholar]
- Salani R., Puri I., Bristow R.E. Adenocarcinoma in situ of the uterine cervix: a metaanalysis of 1278 patients evaluating the predictive value of conization margin status. Am. J. Obstet. Gynecol. 2009;200:182.e1–182.e5. doi: 10.1016/j.ajog.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Shin C.H., Schorge J.O., Lee K.R., Sheets E.E. Conservative management of adenocarcinoma in situ of the cervix. Gynecol. Oncol. 2000;79(1):6–10. doi: 10.1006/gyno.2000.5962. (Oct) [DOI] [PubMed] [Google Scholar]
- Wolf J.K., Levenback C., Malpica A., Morris M., Burke T., Mitchell M.F. Adenocarcinoma in situ of the cervix: significance of cone biopsy margins. Obstet. Gynecol. 1996;88(1):82–86. doi: 10.1016/0029-7844(96)00083-X. (Jul) [DOI] [PubMed] [Google Scholar]
- Wright T.C., Massad S.L., Dunton C.J. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am. J. Obstet. Gynecol. 2007;197:340–345. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]


