Oocyte-specific deletion of Cdc42 has little effect on meiotic spindle organization and migration to the cortex but inhibits polar body emission, although homologous chromosome segregation occurs. The failure of cytokinesis is due to loss of polarized Arp2/3 accumulation and actin cap formation, and thus the defective contract ring.
Abstract
Mammalian oocyte maturation is distinguished by highly asymmetric meiotic divisions during which a haploid female gamete is produced and almost all the cytoplasm is maintained in the egg for embryo development. Actin-dependent meiosis I spindle positioning to the cortex induces the formation of a polarized actin cap and oocyte polarity, and it determines asymmetric divisions resulting in two polar bodies. Here we investigate the functions of Cdc42 in oocyte meiotic maturation by oocyte-specific deletion of Cdc42 through Cre-loxP conditional knockout technology. We find that Cdc42 deletion causes female infertility in mice. Cdc42 deletion has little effect on meiotic spindle organization and migration to the cortex but inhibits polar body emission, although homologous chromosome segregation occurs. The failure of cytokinesis is due to the loss of polarized Arp2/3 accumulation and actin cap formation; thus the defective contract ring. In addition, we correlate active Cdc42 dynamics with its function during polar body emission and find a relationship between Cdc42 and polarity, as well as polar body emission, in mouse oocytes.
INTRODUCTION
After birth, oocytes in mammalian ovaries are arrested at prophase of first meiosis, manifested by the germinal vesicle (GV) located at the center of the oocyte. Oocyte maturation, the final step of oogenesis, begins with germinal vesicle breakdown (GVBD) and ends with the first polar body emission, awaiting fertilization. During the program of oocyte maturation, two important processes must take place. First, during meiosis I, homologous chromosomes segregate, followed by second meiosis, which takes place after fertilization, to ensure haploid gamete production. Second, the two meiotic divisions must occur asymmetrically to allow almost all of the cytoplasm in the egg to support early embryogenesis (Fan and Sun, 2004). The asymmetric cell divisions depend on asymmetric positioning of the meiotic spindles, particularly the meiosis I (MI) spindle. During oocyte maturation, the centrally organized MI spindle migrates to the cortex, inducing formation of a polarized actin cap, as well as of oocyte polarity, and determines the position of two polar body emissions (Brunet and Verlhac, 2011). The migration of the MI spindle to the cortex is mainly dependent on microfilaments, which are regulated by actin nucleators formin-2 (Dumont et al., 2007; Azoury et al., 2008, 2011; Schuh and Ellenberg, 2008) and spire1/spire2 (Pfender et al., 2011).
Cell division cycle 42 (Cdc42) is a member of the Rho family of small GTPase proteins and plays pivotal roles in the establishment of cellular polarity. Cdc42 cooperates with the polarity-related complex Par3/Par6/aPKC and regulates polarity establishment, spindle migration/orientation, and asymmetric cell division in several systems, including the one-cell embryo in Caenorhabditis elegans (Gotta et al., 2001; Kay and Hunter, 2001), the neuroblast in Drosophila (Atwood et al., 2007), and epithelial polarity and morphogenesis (Wu et al., 2007; Jaffe et al., 2008). Actin cytoskeletal dynamics takes place downstream of Cdc42, and the regulators of actin dynamics are main effectors of Cdc42. The direct relationship between Cdc42 and actin dynamics, as well as its regulators, including WASP/WAVE family and Diaphanous-related proteins Dia1–3, are mainly localized to membrane protrusions (reviewed in Ridley, 2006). Little is known about Cdc42 regulation of actin dynamics in the establishment of polarity in other systems, although it has been reported that cofilin, which enhances actin dynamics, is regulated by Cdc42 in the establishment of neuronal polarity (Garvalov et al., 2007).
Cdc42 is required for coupling asymmetric spindle positioning with cytokinesis (Ma et al., 2006) and regulates the Arp2/3 complex–mediated membrane protrusion and polar body extrusion in Xenopus oocyte maturation (Zhang et al., 2008; Leblanc et al., 2011). By using GTPase-defective Cdc42 mutants, it has been reported that Cdc42 might be required for asymmetric spindle migration, homologous chromosome segregation, and polar body extrusion in mouse oocytes (Na and Zernicka-Goetz, 2006). Another study showed disrupted meiotic progression in oocytes incubated with Toxin B, which inhibits the binding activity of Rho GTPases (Bielak-Zmijewska et al., 2008).
In this study, we delete Cdc42 specifically in oocytes by Cre-loxP conditional knockout (Hu et al., 2012) and show that Cdc42 deletion leads to female infertility in mice. In contrast to the aforementioned in vitro reports, we find that in vivo Cdc42 deletion has little effect on spindle organization and migration to the cortex or homologous chromosome segregation, but it inhibits polar body emission by inhibiting the formation of the polarized actin cap and cytokinesis. In addition, we find a correlation of active Cdc42 with functions of Cdc42 during mouse oocyte maturation.
RESULTS
Generation of mutant mice with oocyte-specific deletion of Cdc42
To study how Cdc42 affects oocyte maturation in vivo, we generated mutant mice in which the Cdc42 gene was deleted in oocytes of growing follicles. The mutant mice (referred to as Cdc42loxP/loxP; ZCre+ mice) were generated by crossing Cdc42loxP/loxP mice (Wu et al., 2006) with transgenic mice expressing zona pellucida 3 (Zp3)-promoter–mediated Cre recombinase (Supplemental Figure S1A). By Western blot, we found that the expression of Cdc42 protein was almost completely absent in oocytes of mutant mice (Supplemental Figure S1B), showing successful deletion of the Cdc42 gene from oocytes. By reverse transcription (RT)-PCR, it was shown that exon 2 of Cdc42 (154 base pairs) was deleted in oocytes from Cdc42loxP/loxP; ZCre+ ovaries (Supplemental Figure S2). The Cdc42loxP/loxP mice combining transgenic mice expressing specific Cre have been used in many other systems (Wu et al., 2006; van Hengel et al., 2008; Kesavan et al., 2009; Lammermann et al., 2009), and they all show deletion of the Cdc42 gene and absence of Cdc42 protein.
Infertility and impaired mature eggs in Cdc42loxP/loxP; ZCre+ mice
We found that the Cdc42loxP/loxP; ZCre+ females were completely infertile (Figure 1A). To investigate the possible reasons, we performed superovulation experiments. There was no difference between the numbers of ovulated oocytes from Cdc42loxP/loxP; ZCre+ and Cdc42loxP/loxP females (unpublished data). The oocytes ovulated from Cdc42loxP/loxP ovaries were normal mature eggs (Figure 1B) displaying the first polar body (Figure 1C, differential interference contrast, arrow, and immunofluorescence, PB) and a typical MII spindle. However, almost all of the ovulated oocytes from Cdc42loxP/loxP; ZCre+ ovaries had undergone GVBD but lacked the polar body (Figure 1C). Similar phenotypes were observed in in vitro maturation experiments (Figure 1D). We carried out chromosome-spread experiments (Hodges and Hunt, 2002) to analyze chromosome morphology in the ovulated oocytes. As expected, Cdc42loxP/loxP eggs displayed the typical monovalent MII chromosome array, whereas ovulated oocytes from Cdc42loxP/loxP; ZCre+ ovaries exhibited twice as many monovalent MII chromosomes (Figure 1C). As expected, the impaired polar body emission in Cdc42loxP/loxP; ZCre+ oocytes could be partially rescued by Cdc42 mRNA microinjection (Figure 1D and Supplemental Figure S3).
FIGURE 1:
Deletion of Cdc42 in mouse oocytes results in female infertility and impaired oocyte maturation. (A) Comparison of the total number of pups per female mouse during a period of 6 mo between Cdc42loxP/loxP; ZCre+ (n = 3; 51 ± 8) and Cdc42loxP/loxP (n = 3; 0 ± 0) mice. *P < 0.05. The data are represented as mean ± SEM. (B) Comparison of the rates of MII eggs with the first polar body from superovulated Cdc42loxP/loxP females (n = 4; 94 ± 4%) and Cdc42loxP/loxP; ZCre+ females (n = 4; 2 ± 2%). *p < 0.05. The data are represented as mean ± SEM. (C) differential interference contrast; representative images of ovulated oocytes; 20×. Arrow, the first polar body. IF, ovulated oocytes fixed and stained with anti–α-tubulin–FITC antibody (green) and counterstained with the fluorescent dye PI to visualize DNA (red). PB, the first polar body; bar, 50 μm. CS, chromosome spreads of ovulated oocytes, stained by PI; bar, 50 μm. Experiments were repeated at least three times; representative images are shown. (D) Comparison of the rates of MII eggs with the first polar body during in vitro maturation of Cdc42loxP/loxP GV oocytes, Cdc42loxP/loxP; ZCre+ GV oocytes, and Cdc42loxP/loxP; ZCre+ GV oocytes microinjected with Cdc42 mRNA(Rescue). Different letters (a, b, c) indicate statistically significance (p < 0.05).
Cdc42 deletion led to cytokinesis failure during oocyte maturation
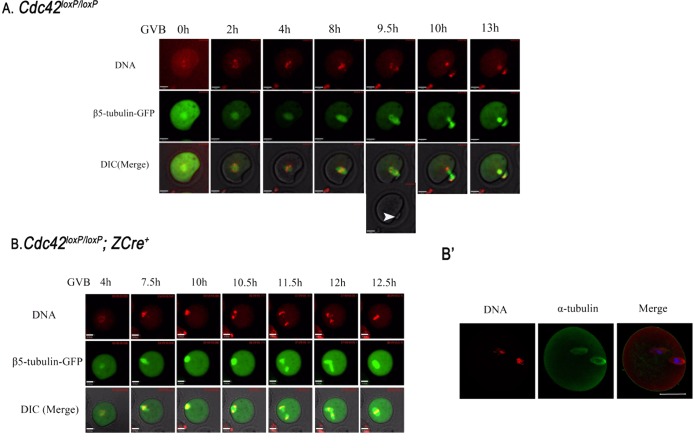
To determine the nature of the oocytes ovulated without polar bodies, we carried out live oocyte imaging experiments in which we monitored chromosome and spindle dynamics during oocyte maturation. As shown in Figure 2A (also see Supplemental Movie S1A), oocytes from Cdc42loxP/loxP ovaries underwent normal oocyte maturation. At 8 h of GVBD (Figure 2A, 8 h) the organized MI spindle had migrated and attached to the cortex. At 9.5 h, anaphase chromosomes were evident, associated with slight spindle elongation and membrane protrusion (Figure 2A, 9.5 h, arrowhead indicates protruding membrane; and Supplemental Movie S1A), followed by the first polar body emission. For Cdc42loxP/loxP; ZCre+ oocytes (Figure 2B and Supplemental Movie S1B), a well-formed and normal MI spindle (Supplemental Figures S4 and S5) reached the cortex at approximately the same time as control oocytes (Figure 2B, 7.5 h), and the oocytes proceeded to anaphase normally and timely (Figure 2B, 10.5 h, 21/30). In contrast, membrane protrusion did not occur as homologous chromosomes segregated (Figure 2B, 10.5 h), and the two separated sets of chromosomes remained inside the oocyte at all times (Figure 2B and Supplemental Movie S1B). Strikingly, two metaphase II spindles were organized around the two sets of separated homologous chromosomes, respectively (Figures B, 12 h, and B′, and Supplemental Movie S1B), and finally the two spindles incorporated into a single metaphase spindle (Figure 2B, 12.5 h, and Supplemental Movie S1B). For the Cdc42loxP/loxP; ZCre+ oocytes with GV near the cortex, the chromosomes stayed or moved slightly along the cortex (Supplemental Movie S5; 3/30) or moved to other areas near in the cortex (Supplemental Movie S6; 4/30) and then underwent a similar process as described.
FIGURE 2:
Cdc42 deletion inhibits polar body emission but does not affect the dynamics of chromosomes and spindles during oocyte maturation. DNA (chromosomes) was stained in blue with Hoechst 33342 (1:500,000 dilution), and the color was changed to red. Spindle is shown by fluorescent tubulin expressed by microinjected β5-tubulin–GFP mRNAs. Bar, 20 μM. (A) Chromosomes and spindle dynamics during maturation of a representative oocyte from Cdc42loxP/loxP female mice. Time points indicating the time lapse from GVBD occurrence in the oocyte are shown in Supplemental Movie S1A. Arrowhead indicates the protruding membrane accompanying homologue segregation. (B) Chromosomes and spindle images showing the phenotypes of Cdc42loxP/loxP; ZCre+ oocyte maturation. Time points indicating the time lapse from GVBD occurrence in the oocyte are shown in Supplemental Movie S1B. (B′) Representative images showing Cdc42loxP/loxP; ZCre+ oocyte with two spindles during in vitro maturation. Oocytes were fixed and stained with anti–α-tubulin–FITC antibody (green) and counterstained with the fluorescent dye PI to visualize DNA (red). Bar, 50 μM.
Dynamics of active Cdc42 indicates its function during polar body emission
To determine Cdc42 functions in oocyte maturation, it is important to show the localization of Cdc42 during this process. Previous work showed that Cdc42 was mainly localized to meiotic spindles in oocytes during maturation (Bielak-Zmijewska et al., 2008). Cdc42-GTP binding indicates its active and functional state; however, the methods used previously did not distinguish between the two forms of Cdc42, and thus it was difficult to determine its function. To visualize active Cdc42, we used the Cdc42-binding domain of N-WASP (wGBD) fused to enhanced green fluorescent protein (eGFP; Pertz and Hahn, 2004) in the present experiments. wGBD binds specifically to active Cdc42 in Xenopus brain extracts (Li et al., 2002), and this has been used to visualize active Cdc42 in Xenopus eggs (Benink and Bement, 2005; Zhang et al., 2008) and mammalian cells (Kim et al., 2000).
For oocytes released from GV arrest, active Cdc42 appeared around the chromosomes and translocated to the cortex in synchrony with chromosomes (Figure 3A and Supplemental Movie S2A). In localizing close to the cortex, the ring-shaped active Cdc42 changed into an oval shape (Figure 3A, 3.5 h; i.e., 7.5 h after GVBD; also see Supplemental Movie S2A), and the leading edge gradually attached to the membrane to form a cap-like structure (18/20; Figure 3A, 4 h and 4 h 45 min, indicated by arrowhead and its enlarged image; also see Supplemental Movie S2A). When homologous chromosomes segregated, active Cdc42 appeared between the two sets of chromosomes (Figure 3A, 5 h 15 min). After polar body emission, the active Cdc42 was located near the chromosomes that were retained in the egg (Figure 3A, 5.5 h, and Supplemental Movie S2A). There was no specific signal of active Cdc42 throughout oocyte maturation in Cdc42loxP/loxP; ZCre+ oocytes (Figure 3B and Supplemental Movie S2B). The signal of active Cdc42 was confirmed by microinjection of Cdc42N17 mRNAs (Zheng et al., 2013) into control oocytes (Figure 3B′). As expected, the signal of active Cdc42 reappeared in Cdc42loxP/loxP; ZCre+ oocytes microinjected with Cdc42 mRNAs (Supplemental Movie S7), which could recover the oocyte's ability to emit the first polar body (Figure 1D).
FIGURE 3:
Dynamics of active Cdc42 during oocyte maturation. DNA (chromosomes) was stained in blue with Hoechst 33342 (1:500,000 dilution), and the color was changed to red. Cdc42 is shown by binding to GFP-wGBD expressed through microinjected GFP-wGBD mRNAs. Bar, 20 μM. (A) Active Cdc42 dynamics during Cdc42loxP/loxP oocyte maturation. Arrow, the active Cdc42 signal in the membrane overlying chromosomes. (B) The dynamics of GFP-wGBD in Cdc42loxP/loxP; ZCre+ oocyte maturation. (B′) Confirmation of the signal of active Cdc42 by comicroinjection of Cdc42N17 mRNAs and GFP-wGBD mRNAs into control oocytes. Snapshots were taken of microinjected oocytes at appropriate times after culture (BD 5 and 8.5 h, respectively); typical pictures are shown. DNA (chromosomes) was stained in blue with Hoechst 33342 (1:500,000 dilution), and the color was changed to red. Cdc42 is shown by binding to GFP-wGBD expressed through microinjected GFP-wGBD mRNAs. Bar, 20 μM.
Disruption of the polarized actin cap and polar body emission in Cdc42loxP/loxP; ZCre+ oocytes
On the basis of the dynamics of spindle/chromosomes and active Cdc42, we suspected that Cdc42 might play a role in the formation of the polarized actin cap and polar body emission. We carried out live oocyte imaging to determine actin dynamics especially in the actin cap by using monomeric G-actin: Alexa 568–G-actin. In Cdc42loxP/loxP oocytes, the chromosomes close to the cortex induced the formation of actin cap and indicated the establishment of polarity (Figure 4A, blue arrow, Supplemental Movie S3A, and Figure 4C). In contrast, there was no actin cap indicated by Alexa 568–G-actin when chromosomes became located at the cortex in mutant oocytes (11/12; Figure 4B, top, Supplemental Movie S3B, and Figure 4C). In addition, we fixed the oocytes from both Cdc42loxP/loxP; ZCre+ and Cdc42loxP/loxP ovaries at appropriate time points during maturation, and similar phenotypes were shown after phalloidin staining (Figure 4D). As expected, the cortex localization of Arp2 (Figure 4E, blue arrowhead) was impaired in Cdc42loxP/loxP; ZCre+ oocytes at MI stage (Figure 4E, yellow arrowhead).
FIGURE 4:
Cdc42 deletion inhibits the formation of the polarized actin cap by impairing cortical localization of the Arp2/3 complex. (A, B) DNA (chromosomes) was stained in blue with Hoechst 33342 (1:500,000 dilution). Alexa 568–G-actin was microinjected into GV oocytes to show the dynamics of actin during oocyte maturation. Bar, 20 μM. (A) The dynamics of Alexa 568–G-actin during Cdc42loxP/loxP oocyte maturation. White and blue arrows show the actin cap G-actin in MI and MII oocytes, respectively. MI, metaphase I; MII, metaphase II. (B) The dynamics of Alexa 568–G-actin during Cdc42loxP/loxP; ZCre+ oocyte maturation. MI, metaphase I; AI, anaphase I. (C) Comparison of the density of G-actin in the actin cap, measured by Volocity software. M, density of G-actin in the membrane above the MI chromosomes. We chose three other sites not above the MI chromosomes in every measured oocyte, measured the density of G-actin, and regarded their average value as m. *p < 0.05. (D) Representative images of oocytes with one pole of MI spindle attached to the cortex from three independent experiments. The oocytes from both Cdc42loxP/loxP and Cdc42loxP/loxP; ZCre+ females were cultured for 9.5 h before being fixed for spindle and actin analysis. The 9.5 h was chosen because a few of the Cdc42loxP/loxP oocytes started to extrude the first polar body. Fixed oocytes were stained with anti–α-tubulin antibody (red), Alexa 488–phalloidin (green), and Hoechst 33342 (1:1000 dilution, blue) and viewed with confocal microscopy. Bar, 50 μM. (E) Impaired cortex localization of Arp2 in Cdc42loxP/loxP; ZCre+ oocytes at the MI stage. Blue arrowhead, Arp2 localized to the cortex in Cdc42loxP/loxP oocytes at the MI stage. Yellow arrowhead, absence of cortex localization of Arp2. Bar, 50 μM.
It was clearly observed that homologous chromosome segregation occurred in both Cdc42loxP/loxP and Cdc42loxP/loxP; ZCre+ oocytes, whereas there was no polar body emission in the latter, so the process of cytokinesis needed further exploration. By Alexa 488–phalloidin microinjection and live oocyte imaging, we showed the effects on cytokinesis in Cdc42loxP/loxP; ZCre+ oocytes. In control oocytes, the phalloidin signal was strong at the contractile ring (Figure 5A, white arrowhead, and Supplemental Movie S4A) between the two separated sets of chromosomes. For Cdc42loxP/loxP; ZCre+ oocytes, there was no obvious contractile ring between the two sets of chromosomes (8/10; Figure 5B, blue arrowhead, and Supplemental Movie S4B). The results from fixed oocytes further confirmed our observations. Actin participated in cytokinesis in control oocytes (Figure 5C, Cdc42loxP/loxP, arrow indicates contractile ring) and the final step of polar body emission. In Cdc42-deleted oocytes, the residual actin (Figure 5C, Cdc42loxP/loxP; ZCre+, arrowhead) indicated the intent-to-initiate but failed cytokinesis.
FIGURE 5:
Cdc42 deletion causes cytokinesis failure in mouse oocytes. (A, B) DNA (chromosomes) was stained in blue by Hoechst 33342 (1:500,000 dilution), and the color was changed into red. Alexa 488–phalloidin was microinjected into GV oocytes to show the stable F-actin dynamics during oocyte maturation, especially in cytokinesis. Bar, 20 μM. (A) The dynamics of Alexa 488–phalloidin during Cdc42loxP/loxP oocyte maturation. White arrowhead, contractile ring shown by Alexa 488–phalloidin. (B) The dynamics of Alexa 488–phalloidin during Cdc42loxP/loxP; ZCre+ oocyte maturation. Blue arrowhead, nearly no phalloidin signal between two sets of separated homologues. (C) Representative images of oocytes undergoing cytokinesis from three repeated experiments. Oocytes were stained with anti–α-tubulin antibody (red), Alexa 488–phalloidin (green), and Hoechst 33342 (1:1000 dilution, blue) and viewed with confocal microscopy. Arrow, contractile ring during polar body emission in Cdc42loxP/loxP oocyte; arrowhead, residual actin in the center of separating spindle in Cdc42loxP/loxP; ZCre+ oocyte. Bar, 50 μM.
DISCUSSION
In this study, we analyzed the functions of Cdc42 in mouse oocytes in vivo by conditional knockout technology. Oocyte-specific deletion of Cdc42 inhibits production of mature eggs and causes complete female infertility. Focusing on the process of oocyte maturation, we find that Cdc42 deletion blocks polar body emission by inhibiting formation of the polarized actin cap, which defines the protrusion site of the first polar body. Unlike conclusions derived from in vitro studies, we find that Cdc42 deletion has no effect on the initial steps of oocyte maturation, including spindle organization, spindle migration to the cortex, anaphase initiation, and homologous chromosome segregation. The inhibited polar body emission in Cdc42loxP/loxP; ZCre+ oocytes could be partially rescued by Cdc42 mRNA microinjection, which confirms the function of Cdc42.
Cdc42 plays pivotal roles in cytokinesis and polar body emission during oocyte maturation
In cell division, Cdc42 and its related proteins have been reported to play potential roles in regulation of spindle/chromosome behavior. Cdc42 is required for microtubule attachment to kinetochores (Yasuda et al., 2004) and subsequent chromosome segregation (Oceguera-Yanez et al., 2005) in HeLa cells. In Xenopus egg extracts, Cdc42 is involved in spindle assembly (Tatsumoto et al., 2003). Use of an in vitro approach showed (Na and Zernicka-Goetz, 2006) that disrupted Cdc42 causes spindle defects (abnormal bipolar spindles with multiple arrays of astral microtubules), impairs asymmetric spindle migration to the cortex, and inhibits homologous chromosome segregation. However, by using conditional knockout technology, we find that oocyte-specific deletion of Cdc42 in vivo does not impair normal bipolar spindle formation (Figure 2B, Supplemental Movie S1B, Supplemental Figures S4 and S5, and Figure 4D later in this article), asymmetric spindle positioning near the cortex (Figure 2B and Supplemental Movie S1B), and homologue separation at anaphase (Figures 2B, 3B, 4B, and 5, B and C, and Supplemental Movies S1B, S2B, S3B, S4B, S5, and S6), whereas it plays a role in the process of polar body emission. The previous study was associated with the problem that the in vitro approach lacks the efficiency test of Cdc42 mutants and does not eliminate toxicity caused by microinjection of excess mRNAs. Another in vitro study (Bielak-Zmijewska et al., 2008), in which inhibitor toxin B was used, supports our conclusion, but this inhibitor has binding activity to all the Rho-family proteins, which made it difficult to clarify the specific function of Cdc42.
Cdc42 plays a pivotal role in cytokinesis by regulating contractile ring formation through mediating the actin cytoskeleton, and this function is conserved in yeast (Merla and Johnson, 2000), Xenopus embryos (Drechsel et al., 1997), and mammalian cells (Dutartre et al., 1996; Zhu et al., 2011). RhoA is one of the master keys for cytokinesis, and it is necessary for Cdc42 to collaborate with RhoA in the process of cytokinesis. During Xenopus oocyte maturation, Cdc42 cooperates with RhoA to complete cytokinesis (Zhang et al., 2008). The residual actin at the center of the spindle (Figure 5C, Cdc42loxP/loxP; ZCre+, arrowhead) indicates the intent-to-initiate but failed cytokinesis, indicating that deleted Cdc42 alone could prevent polar body emission. Epithelial cell transforming protein 2 is the guanine exchange nucleotide factor that activates Rho GTPases (Tatsumoto et al., 1999), and its depletion in mouse oocytes also results in inhibited polar body emission and produces oocytes with two MII spindles or one MI spindle (probably MII spindle with 40 univalents; Elbaz et al., 2010), resembling the phenotypes observed in this study (Figure 2, B and B′, and Supplemental Movie S1B). In addition, polar body emission resembles protrusion formation, and protrusion is closely related to the establishment of cell polarity, which may be regulated by Cdc42. The relationships between protrusion, cell polarity, and Cdc42 will be discussed subsequently.
The dynamics of active Cdc42 during oocyte maturation and functions in the establishment of polarity
Oocyte maturation includes highly asymmetric cell divisions and polar body extrusion that is closely correlated with the establishment of polarity. The marker for polarity—the polarized actin cap—determines the sites for the first and second polar body emissions, and the actin cap is induced by the asymmetrically positioned MI spindle. Actin is required for asymmetric spindle positioning. Cdc42 is also associated with the spindle's asymmetric position/rotation in many systems; however, Cdc42 is not necessary for this process (Figures 2B and 5B and Supplemental Movies S1B and S4B), although active Cdc42 moves to the cortex along with chromosomes (Figure 3A and Supplemental Movies S2A) during mouse oocyte maturation, which is also reported in two recent articles (Dehapiot et al., 2013; Zheng et al., 2013). Our results indicate that the asymmetrically positioned spindle/chromosomes leads the distribution of active Cdc42 to the cortex and that the cause-and-effect relationship of Cdc42 and polarity establishment is contrary to that in several other important and widely studied systems, as mentioned in the Introduction, in which Cdc42 in combination with Par3/Par6/PKC establishes polarity, regulates asymmetric spindle position/orientation, and causes asymmetric cell division. During oocyte maturation, the localization of both Par3 (Duncan et al., 2005) and Par6 (Vinot et al., 2004) becomes asymmetric upon spindle migration and during polar body emission, similar to the dynamics of active Cdc42 (Figure 3A and Supplemental Movie 2A), indicating their function in the formation of the polarized actin cap but not in the regulation of spindle migration.
Polarity establishment by Cdc42 involves protrusion in several widely studied systems: Cdc42 controls the polarization of membrane protrusions in fibroblast migration (Cau and Hall, 2005), regulates epidermal growth factor–stimulated protrusions in MTLn3 carcinoma cells (El-Sibai et al., 2007, 2008), and plays similar roles in other cell lines (Cox et al., 2001). Of interest, Cdc42-regulated polarity mediates a special process of “protrusion” budding in yeast, resembling the protrusion in polar body extrusion during Xenopus oocyte maturation (Zhang et al., 2008). During polar body extrusion, the protrusion is mediated by actin dynamics regulated by the Arp2/3 complex in Xenopus oocytes (Leblanc et al., 2011), and the Arp2/3 protein complex has been widely reported as an actin nucleator downstream of Cdc42. In our study, Cdc42 deletion leads to impaired cortex localization of Arp2 (Figure 4E, yellow arrowhead). However, the functions of Cdc42 and the Arp2/3 complex differ. During mouse oocyte maturation, Arp2/3 inhibition results in disruption of the actin cap by inhibiting spindle migration to the cortex (Sun et al., 2011), which is in contrast to the phenotype resulting from Cdc42 deletion (Figures 2B and 4B and Supplemental Movies S1B and S3B). In addition, Arp2/3 inhibition leads to failed cytokinesis in oocytes in which the migration of the spindle to the cortex is not affected, producing oocytes arrested at telophase (for the oocytes in which the migration is inhibited, symmetric division occurs; Sun et al., 2011), which is different from the phenotypes resulting from Cdc42 deletion (Figures 2B, 3B, 4B, and 5B and Supplemental Movies S1B, S2B, S3B, S4B, S5, and S6). For MII eggs, Arp2/3 inhibition disrupts the actin cap and established polarity, causing MII spindle detachment from the cortex (Yi et al., 2011). Although it appears that active Cdc42 signal does not exist in the cortex overlying MII chromosomes in our study (Figure 3A and Supplemental Movie S2A), Dehapiot et al. (2013) report the existence of active Cdc42 in MII eggs and its function in the second polar body emission. In our study, we propose that Cdc42 may cooperate with downstream Arp2/3 to function in the formation of the actin cap, the marker of polarity establishment, which is conserved in mouse and Xenopus oocytes (Zhang et al., 2008).
In conclusion, our study for the first time clarifies the functions of Cdc42 in oocyte maturation by an in vivo approach. Cdc42 is required for the formation of the polarized actin cap induced by asymmetrically positioned spindle/chromosomes and for membrane protrusion, as well as for cytokinesis during polar body emission. The data provide new insights into the relationship between Cdc42 and polarity in an important polarity-related biological event—oocyte maturation and fertilization. It will be interesting to determine Cdc42-related proteins and networks that play a role in polarity events in the cortex during oocyte development.
MATERIALS AND METHODS
All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise indicated.
Mice (Mus musculus)
Homologous Cdc42loxP/loxP mice were intercrossed with Zp3-Cre transgenic mice (C57BL6 background) from the Jackson Laboratory to generate Cdc42loxP/loxP; ZCre+ animals (C57BL6 and 129 mixed background). Control animals were littermates possessing two loxP-flanked alleles without Cre (Cdc42loxP/loxP). Mice were maintained in alternating 12-h light/dark cycles. Animal care and use were carried out in accordance with the Animal Research Committee Guidelines of the Institute of Zoology, Chinese Academy of Sciences, China.
Oocyte collection and culture
The experiments used 4- to 8-wk-old female mice. For in vitro maturation, GV oocytes were collected and cultured in M2 medium under liquid paraffin oil at 37°C in an atmosphere of 5% CO2 in air. To obtain MII eggs, mice were induced to superovulate by injection of 8 IU of pregnant mare's serum gonadotropin followed 48 h later by injection of 8 IU of human chorionic gonadotropin. At 14–18 h of human chorionic gonadotropin injection, mice were killed, and the oviductal ampullae were broken to release the cumulus–oocyte complexes. Eggs were freed of cumulus cells by exposure to 300 μg/ml hyaluronidase. Oocytes or MII eggs were collected for immunofluorescence staining, microinjection, or immunoblot analysis.
RT-PCR
One hundred fifty GV oocytes from Cdc42loxP/loxP or Cdc42loxP/loxP; ZCre+ ovaries were used to extract the total RNA using the RNeasy micro purification kit (Qiagen, Hilden, Germany), and first-strand cDNA generation was performed following the cDNA synthesis kit instructions (Takara, Otsu, Shiga, Japan). RT-PCR was carried out to assess the expression of Cdc42 mRNA based on the following primers: forward, TTCCGCGGGCACCCAACCAT; reverse, TGTGTGAGG GCAGAGCACTC.
Plasmids, mRNA transcription, microinjection, live oocyte imaging
The first-strand cDNA in the foregoing from Cdc42loxP/loxP oocytes was used for PCR cloning. The full length of Cdc42 cDNA was cloned by PCR using the following pair of primers: F1, CGCGGATCCATGCAGACAATTAAGTGTG; and R1, TGCTCTAGATCATAGCAGCACACACCT. For in vitro transcription reaction, the Cdc42 cDNA was subcloned into the pCS2+ vector. The pCS2+ -Cdc42 plasmid was linearized by SalI. The plasmids pCS2+ -Cdc42, pRN3-β5-tubulin-GFP, and pCS2+ -GFP-wGBD were used for the production of Cdc42 mRNA, β5-tubulin-GFP mRNA, and GFP-wGBD mRNA, respectively, by in vitro transcription kit (Ambion, Austin, TX). Microinjection of mRNAs, Alexa 568–G actin (Invitrogen, Carlsbad, CA), and Alexa 488–phalloidin (Invitrogen) into GV oocytes was performed using a Nikon Diaphot ECLIPSE TE 300 (Nikon UK, Kingston upon Thames, Surrey, United Kingdom) inverted microscope equipped with Narishige MM0202N hydraulic three-dimensional micromanipulators (Narishige, Sea Cliff, NY) and completed within 30 min. After 1–2 h of culture (except for Cdc42 mRNA) the microinjected oocytes were used for live oocyte imaging on an UltraVIEW VoX confocal imaging system (PerkinElmer, Waltham, MA).
Immunofluorescence and confocal microscopy
For single staining of α-tubulin, oocytes were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4) for at least 30 min at room temperature. After being permeabilized with 0.5% Triton X-100 at room temperature for 20 min, oocytes were blocked in 1% bovine serum albumin (BSA)–supplemented PBS for 1 h and incubated overnight at 4°C with 1:200 anti–α-tubulin–fluorescein isothiocyanate (FITC) antibody. After three washes in PBS containing 0.1% Tween-20 and 0.01% Triton X-100, the oocytes were stained with propidium iodide (PI; 10 μg/ml in PBS). Then the oocytes were mounted on glass slides and examined with a confocal laser scanning microscope (LSM 710; Carl Zeiss, Jena, Germany).
For double staining of α-tubulin and actin, after fixation, permeabilization, and blocking as described, oocytes were incubated overnight at 4°C with 1:100 rabbit anti–α-tubulin antibody (Cell Signaling Technology, Danvers, MA). After three washes in PBS containing 0.1% Tween- 20 and 0.01% Triton X-100 for 5 min each, the oocytes were labeled with 1:100 tetramethylrhodamine isothiocyanate–conjugated goat anti-rabbit immunoglobulin G (IgG) for 1 h at room temperature. After three washes, oocytes were blocked in 1% BSA-supplemented PBS for 1 h and incubated overnight at 4°C with 1:200 Alexa 488–phalloidin. After three washes in PBS, the oocytes were stained with Hoechst 33342 (1:1000 dilution). Then the oocytes were mounted on glass slides and examined with a confocal laser scanning microscope (Zeiss LSM 710).
For single staining of Arp2, oocytes were fixed in fixation solution (2% formaldehyde, 0.5% Triton X-100, 1 μM Taxol, 10 μg/ml aprotinin, 10 mM Tris, 150 mM NaCl) for 20 min at 37°C and then incubated in blocking solution (2% BSA, 2% fetal bovine serum, 0.1 M glycine, 0.01% Triton X-100, 10 mM Tris, pH 7.5, 150 mM NaCl). Rabbit anti-Arp2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) incubation was carried out at 4°C overnight after permeabilizing with 0.5% Triton X-100 for 20 min and blocking with 1% BSA in PBS. After three washes in PBS containing 0.1% Tween-20 and 0.01% Triton X-100 for 5 min each, the oocytes were labeled with 1:100 FITC-conjugated goat anti-rabbit IgG for 1 h at room temperature. After three washes in PBS containing 0.1% Tween-20 and 0.01% Triton X-100, the oocytes were stained with PI (10 μg/ml in PBS). Then the oocytes were mounted on glass slides and examined with a confocal laser scanning microscope (Zeiss LSM 710).
Immunoblot analysis
Samples each containing 200 mouse oocytes were collected in SDS sample buffer and heated for 5 min at 100°C. The proteins were separated by SDS–PAGE and then electrically transferred to polyvinylidene fluoride membranes. After transfer, membranes were blocked in TBST buffer (TBS containing 0.1% Tween-20) containing 5% skimmed milk for 2 h, followed by incubation overnight at 4°C with 1:500 mouse monoclonal anti-Cdc42 antibody (BD Biosciences, Franklin Lakes, NJ) and 1:1000 mouse monoclonal anti–β-actin antibody (Proteintech, Chicago, IL). After three washes, 10 min each in TBST, the membranes were incubated for 1 h at 37°C with 1:1000 horseradish peroxidase–conjugated goat anti-mouse IgG. Finally, the membranes were processed using an enhanced chemiluminescence detection system (Amersham, Piscataway, NJ).
Chromosome spread
Briefly, the zona pellucida of oocytes was removed in Tyrode's solution, and then the oocytes were dispersed on a coverslip that contained the fixation solution (1% paraformaldehyde, 3 mM dithiothreitol, and 0.18% Triton X-100, pH 9.2), followed by slow drying of the coverslip in air. The spreads were stained with PI (10 μg/ml in PBS) and examined with a confocal laser scanning microscope (Zeiss LSM 710).
Statistics
All percentages from at least three repeated experiments were expressed as mean ± SEM. Data were analyzed by paired-samples t test. p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Zhengjun Chen (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China) for transferring the Cdc42loxP/loxP mice. We also thank X. Johné Liu (Ottawa Health Research Institute, Ottawa, Canada) for the plasmids pRN3- β5-tubulin-GFP and pCS2+ -GFP-wGBD. We thank Ping Zheng (Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China) for the Cdc42N17 plasmid. We are grateful for technical assistance from Lijuan Wang, Hua Qin, Shiwen Li, and Jian-Dong Dong. This work was supported by the National Basic Research Program of China (2012CB944404, 2011CB944501) and the National Natural Science Foundation of China (30930065, 31201078, 31371451).
Abbreviations used:
- BSA
bovine serum albumin
- Cdc42
cell division cycle 42
- DIC
differential interference contrast
- eGFP
enhanced green fluorescent protein
- FITC
fluorescein isothiocyanate
- GUBD
germinal vesicle breakdown
- GV
germinal vesicle
- IgG
immunoglobulin G
- MII
metaphase II
- PBS
phosphate-buffered saline
- RT
reverse transcription
- Zp3
zona pellucida 3
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E13-03-0123) on October 16, 2013.
REFERENCES
- Atwood SX, Chabu C, Penkert RR, Doe CQ, Prehoda KE. Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6 aPKC. J Cell Sci. 2007;120:3200–3206. doi: 10.1242/jcs.014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoury J, Lee KW, Georget V, Hikal P, Verlhac MH. Symmetry breaking in mouse oocytes requires transient F-actin meshwork destabilization. Development. 2011;138:2903–2908. doi: 10.1242/dev.060269. [DOI] [PubMed] [Google Scholar]
- Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol. 2008;18:1514–1519. doi: 10.1016/j.cub.2008.08.044. [DOI] [PubMed] [Google Scholar]
- Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168:429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak-Zmijewska A, Kolano A, Szczepanska K, Maleszewski M, Borsuk E. Cdc42 protein acts upstream of IQGAP1 and regulates cytokinesis in mouse oocytes and embryos. Dev Biol. 2008;322:21–32. doi: 10.1016/j.ydbio.2008.06.039. [DOI] [PubMed] [Google Scholar]
- Brunet S, Verlhac MH. Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update. 2011;17:68–75. doi: 10.1093/humupd/dmq044. [DOI] [PubMed] [Google Scholar]
- Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- Cox EA, Sastry SK, Huttenlocher A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol Biol Cell. 2001;12:265–277. doi: 10.1091/mbc.12.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehapiot B, Carriere V, Carroll J, Halet G. Polarized Cdc42 activation promotes polar body protrusion and asymmetric division in mouse oocytes. Dev Biol. 2013;377:202–212. doi: 10.1016/j.ydbio.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- Dumont J, Million K, Sunderland K, Rassinier P, Lim H, Leader B, Verlhac MH. Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev Biol. 2007;301:254–265. doi: 10.1016/j.ydbio.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Duncan FE, Moss SB, Schultz RM, Williams CJ. PAR-3 defines a central subdomain of the cortical actin cap in mouse eggs. Dev Biol. 2005;280:38–47. doi: 10.1016/j.ydbio.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Dutartre H, Davoust J, Gorvel JP, Chavrier P. Cytokinesis arrest and redistribution of actin-cytoskeleton regulatory components in cells expressing the Rho GTPase CDC42Hs. J Cell Sci. 1996;109(Pt 2):367–377. doi: 10.1242/jcs.109.2.367. [DOI] [PubMed] [Google Scholar]
- El-Sibai M, Nalbant P, Pang H, Flinn RJ, Sarmiento C, Macaluso F, Cammer M, Condeelis JS, Hahn KM, Backer JM. Cdc42 is required for EGF-stimulated protrusion and motility in MTLn3 carcinoma cells. J Cell Sci. 2007;120:3465–3474. doi: 10.1242/jcs.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sibai M, Pertz O, Pang H, Yip SC, Lorenz M, Symons M, Condeelis JS, Hahn KM, Backer JM. RhoA/ROCK-mediated switching between Cdc42- and Rac1-dependent protrusion in MTLn3 carcinoma cells. Exp Cell Res. 2008;314:1540–1552. doi: 10.1016/j.yexcr.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz J, Reizel Y, Nevo N, Galiani D, Dekel N. Epithelial cell transforming protein 2 (ECT2) depletion blocks polar body extrusion and generates mouse oocytes containing two metaphase II spindles. Endocrinology. 2010;151:755–765. doi: 10.1210/en.2009-0830. [DOI] [PubMed] [Google Scholar]
- Fan HY, Sun QY. Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol Reprod. 2004;70:535–547. doi: 10.1095/biolreprod.103.022830. [DOI] [PubMed] [Google Scholar]
- Garvalov BK, Flynn KC, Neukirchen D, Meyn L, Teusch N, Wu X, Brakebusch C, Bamburg JR, Bradke F. Cdc42 regulates cofilin during the establishment of neuronal polarity. J Neurosci. 2007;27:13117–13129. doi: 10.1523/JNEUROSCI.3322-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta M, Abraham MC, Ahringer J. CDC-42 controls early cell polarity and spindle orientation in C. elegans. Curr Biol. 2001;11:482–488. doi: 10.1016/s0960-9822(01)00142-7. [DOI] [PubMed] [Google Scholar]
- Hodges CA, Hunt PA. Simultaneous analysis of chromosomes and chromosome-associated proteins in mammalian oocytes and embryos. Chromosoma. 2002;111:165–169. doi: 10.1007/s00412-002-0195-3. [DOI] [PubMed] [Google Scholar]
- Hu MW, Wang ZB, Schatten H, Sun QY. New understandings on folliculogenesis/oogenesis regulation in mouse as revealed by conditional knockout. J Genet Genomics. 2012;39:61–68. doi: 10.1016/j.jgg.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–633. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AJ, Hunter CP. CDC-42 regulates PAR protein localization and function to control cellular and embryonic polarity in C. elegans. Curr Biol. 2001;11:474–481. doi: 10.1016/s0960-9822(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Kesavan G, Sand FW, Greiner TU, Johansson JK, Kobberup S, Wu X, Brakebusch C, Semb H. Cdc42-mediated tubulogenesis controls cell specification. Cell. 2009;139:791–801. doi: 10.1016/j.cell.2009.08.049. [DOI] [PubMed] [Google Scholar]
- Kim SH, Li Z, Sacks DB. E-cadherin-mediated cell-cell attachment activates Cdc42. J Biol Chem. 2000;275:36999–37005. doi: 10.1074/jbc.M003430200. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Renkawitz J, Wu X, Hirsch K, Brakebusch C, Sixt M. Cdc42-dependent leading edge coordination is essential for interstitial dendritic cell migration. Blood. 2009;113:5703–5710. doi: 10.1182/blood-2008-11-191882. [DOI] [PubMed] [Google Scholar]
- Leblanc J, Zhang X, McKee D, Wang ZB, Li R, Ma C, Sun QY, Liu XJ. The small GTPase Cdc42 promotes membrane protrusion during polar body emission via ARP2-nucleated actin polymerization. Mol Hum Reprod. 2011;17:305–316. doi: 10.1093/molehr/gar026. [DOI] [PubMed] [Google Scholar]
- Li Z, Aizenman CD, Cline HT. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- Ma C, Benink HA, Cheng D, Montplaisir V, Wang L, Xi Y, Zheng PP, Bement WM, Liu XJ. Cdc42 activation couples spindle positioning to first polar body formation in oocyte maturation. Curr Biol. 2006;16:214–220. doi: 10.1016/j.cub.2005.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merla A, Johnson DI. The Cdc42p GTPase is targeted to the site of cell division in the fission yeast Schizosaccharomyces pombe. Eur J Cell Biol. 2000;79:469–477. doi: 10.1078/0171-9335-00073. [DOI] [PubMed] [Google Scholar]
- Na J, Zernicka-Goetz M. Asymmetric positioning and organization of the meiotic spindle of mouse oocytes requires CDC42 function. Curr Biol. 2006;16:1249–1254. doi: 10.1016/j.cub.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Oceguera-Yanez F, Kimura K, Yasuda S, Higashida C, Kitamura T, Hiraoka Y, Haraguchi T, Narumiya S. Ect2 and MgcRacGAP regulate the activation and function of Cdc42 in mitosis. J Cell Biol. 2005;168:221–232. doi: 10.1083/jcb.200408085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertz O, Hahn KM. Designing biosensors for Rho family proteins—deciphering the dynamics of Rho family GTPase activation in living cells. J Cell Sci. 2004;117:1313–1318. doi: 10.1242/jcs.01117. [DOI] [PubMed] [Google Scholar]
- Pfender S, Kuznetsov V, Pleiser S, Kerkhoff E, Schuh M. Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr Biol. 2011;21:955–960. doi: 10.1016/j.cub.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Schuh M, Ellenberg J. A new model for asymmetric spindle positioning in mouse oocytes. Curr Biol. 2008;18:1986–1992. doi: 10.1016/j.cub.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Sun SC, Wang ZB, Xu YN, Lee SE, Cui XS, Kim NH. Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PLoS One. 2011;6:e18392. doi: 10.1371/journal.pone.0018392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumoto T, Sakata H, Dasso M, Miki T. Potential roles of the nucleotide exchange factor ECT2 and Cdc42 GTPase in spindle assembly in Xenopus egg cell-free extracts. J Cell Biochem. 2003;90:892–900. doi: 10.1002/jcb.10750. [DOI] [PubMed] [Google Scholar]
- Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol. 1999;147:921–928. doi: 10.1083/jcb.147.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hengel J, D'Hooge P, Hooghe B, Wu X, Libbrecht L, De Vos R, Quondamatteo F, Klempt M, Brakebusch C, van Roy F. Continuous cell injury promotes hepatic tumorigenesis in cdc42-deficient mouse liver. Gastroenterology. 2008;134:781–792. doi: 10.1053/j.gastro.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Vinot S, Le T, Maro B, Louvet-Vallee S. Two PAR6 proteins become asymmetrically localized during establishment of polarity in mouse oocytes. Curr Biol. 2004;14:520–525. doi: 10.1016/j.cub.2004.02.061. [DOI] [PubMed] [Google Scholar]
- Wu X, Li S, Chrostek-Grashoff A, Czuchra A, Meyer H, Yurchenco PD, Brakebusch C. Cdc42 is crucial for the establishment of epithelial polarity during early mammalian development. Dev Dyn. 2007;236:2767–2778. doi: 10.1002/dvdy.21309. [DOI] [PubMed] [Google Scholar]
- Wu X, Quondamatteo F, Lefever T, Czuchra A, Meyer H, Chrostek A, Paus R, Langbein L, Brakebusch C. Cdc42 controls progenitor cell differentiation and beta-catenin turnover in skin. Genes Dev. 2006;20:571–585. doi: 10.1101/gad.361406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, Ishizaki T, Narumiya S. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature. 2004;428:767–771. doi: 10.1038/nature02452. [DOI] [PubMed] [Google Scholar]
- Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, Li R. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol. 2011;13:1252–1258. doi: 10.1038/ncb2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ma C, Miller AL, Katbi HA, Bement WM, Liu XJ. Polar body emission requires a RhoA contractile ring and Cdc42-mediated membrane protrusion. Dev Cell. 2008;15:386–400. doi: 10.1016/j.devcel.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Baibakov B, Wang XH, Dean J. PtdIns(3,4,5)P3 is constitutively synthesized and required for spindle translocation during meiosis in mouse oocytes. J Cell Sci. 2013;126:715–721. doi: 10.1242/jcs.118042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang J, Moriguchi K, Liow LT, Ahmed S, Kaverina I, Murata-Hori M. Proper regulation of Cdc42 activity is required for tight actin concentration at the equator during cytokinesis in adherent mammalian cells. Exp Cell Res. 2011;317:2384–2389. doi: 10.1016/j.yexcr.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.