Abstract
Constrictive pericarditis following Coronary Artery Bypass Surgery is an uncommon disorder. We report a patient who developed constrictive pericarditis after Coronary Artery Bypass Grafting. After an unsuccessful trial of medical management and pericardial tapping, he underwent pericardiectomy via a left posterolateral thoracotomy.
Keywords: Constrictive pericarditis, Coronary Artery Bypass Grafting, Thoracotomy, Pericardiectomy
1. Case report
The patient was a 66-year-old normotensive, non-diabetic gentleman with normal left ventricular function, who underwent off-Pump CABG in January 2009, wherein he received three grafts. However, during sternal closure he had a sudden and unexplained hemodynamic collapse, necessitating emergent cardiopulmonary bypass (CPB) support. His subsequent post-operative course was uneventful and he was discharged on the 7th post-operative day. Pre-discharge echo showed minimal pericardial effusion posterolateral to the left ventricle (LV). After 16 months, he presented with shortness of breath, pedal edema and easy fatigability. 2D echocardiography revealed biatrial dilatation, small LV, marked pericardial effusion with fibrous strands, no constriction, marked thickening of posterolateral pericardium, normal LV ejection fraction and raised LVEDP. 300 ml of hemorrhagic fluid was drained under echocardiographic guidance. However, he again presented with similar complaints after six months. This time, echocardiography showed a large pericardial collection causing posterolateral compression of the LV, with pericardium thickened to 7 mm and features of constrictive physiology. MRI of thorax showed a mass 8 cm in diameter compressing the LV. Keeping in mind the clinical and imaging findings and the history of recurrence, it was decided to operate. He underwent preoperative cardiac catheterization and coronary angiography, which confirmed the echocardiographic findings and demonstrated patent grafts. Based on the MRI findings which suggested a left lateral compression of the LV (Fig. 2), and the presence of patent grafts, our surgical approach was left posterolateral thoracotomy. There was a large pleural effusion of about 2 L of straw colored fluid. The LV was encased by 1 cm thick pericardium with 500 ml of altered blood and partially-organized clots (Fig. 1). Pericardiectomy over the LV was done with thorough removal of the clots and fluid, leaving a strip of pericardium along the phrenic nerve. Post-operative echocardiography showed mild posterolateral pericardial collection with no constriction and the patient was discharged after 4 days.
Fig. 2.
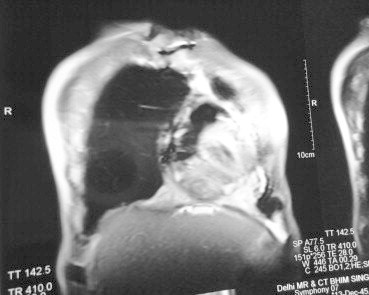
Preoperative MRI showing thickened pericardium.
Fig. 1.
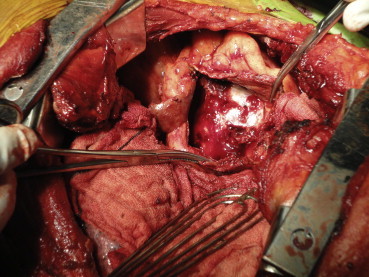
Operative photograph showing partially opened thickened pericardium.
2. Discussion
Constrictive pericarditis as a complication following CABG is rare and was first reported in 1972.1 Constrictive pericarditis is caused by adhesions and fibrous contracture of the pericardial sac. This impairs normal diastolic ventricular filling, causing a fall in stroke volume and elevation of systemic and pulmonary venous pressures. Fatigue, dyspnea and signs of congestive cardiac failure result. At our institution, at which almost 500 major cardiac surgical procedures—mainly CABGs – are performed annually; this was the first such case. While it is true that such cases eventually requiring pericardiectomy are rare, the true incidence of pericardial constriction post-surgery may be underestimated. Mild cases may mimic low cardiac output syndrome, with low systemic pressures and high venous pressures, especially post-valve surgeries, or following CABG with severe LV dysfunction. Patients with mild disease may respond well to diuretic treatment given for heart failure and thus never undergo investigations to establish the diagnosis, which requires a high degree of clinical suspicion.2 Definitive factors that predispose to the development of constriction in patients after cardiac surgery are still not well known. Studies on animal models have demonstrated that pericardial adhesions will develop if spilled blood comes into contact with an injured serosal surface.3 The volume of blood spillage during surgery is variable, but the initiation of inflammation and fibrosis depends on the presence of pericardial damage. Post-pericardiotomy syndrome (PPS) has been postulated as a potential cause and has been found to occur in up to 30% of patients after cardiac surgery.4 The presence of PPS does not necessarily predict which patients will develop constriction, and its absence does not preclude the development of constriction. However, PPS is an indication of inflammation in and around the pericardium, and perhaps these patients should be watched more closely for development of constrictive pericarditis. Definitive management of this condition remains re-do surgery with pericardiectomy. Patients who have undergone previous surgery, especially CABG, pose a greater risk with patent grafts lying just beneath the sternum, which are susceptible to injury during the procedure. Lee et al have recommended a non-midline sternotomy approach, to avoid damage to patent grafts. Patients in their series underwent pericardial stripping via right, left and bilateral thoracotomies, depending on the extent of constriction.5 Our patient underwent CABG over two years ago, which had required CPB support. It is well known that CPB is associated with increased risk of bleeding complications and inflammatory response. Removal of drainage tubes following CABG might have been followed by gradual oozing from the raw surfaces caused by the surgery, which could have led to inflammation, fibrosis and constriction. Imaging studies revealed a marked thickening on the left lateral aspect of the heart. This correlates with the post-operative echocardiography findings of a posterolateral collection. Keeping in mind the site of compression and the fact that the patient was post-CABG with patent grafts, it was decided to use a left posterolateral thoracotomy. Apart from obviating the need for any extra precaution with regard to the grafts, this approach avoided the morbidity associated with a re-do sternotomy and very importantly, allowed for a satisfactory decompression of the LV, which was bearing the brunt of the constrictive process.
3. Conclusion
Based on the experience of this case, we conclude that pericardiectomy via lateral thoracotomy is a safe and effective approach in cases of post-CABG constrictive pericarditis, in view of the presence of patent grafts, and added morbidity of a re-do sternotomy. Also, this uncommon complication has to be considered in all post-cardiac surgery patients presenting with signs of unexplained myocardial failure, before ascribing them to the underlying cardiac disorder, or to post-cardiac surgery LV dysfunction.
Conflicts of interest
All authors have none to declare.
References
- 1.Kendall M.E., Rhodes G.R., Wolfe W. Cardiac constriction following aorta to coronary bypass surgery. J Thorac Cardiovasc Surg. 1972;64:142–153. [PubMed] [Google Scholar]
- 2.Killian D.M., Furiasse J.G., Scanlon P.J. Constrictive pericarditis after cardiac surgery. Am Heart J. 1989;118:563–568. doi: 10.1016/0002-8703(89)90273-1. [DOI] [PubMed] [Google Scholar]
- 3.Cliff W.J., Grobetz J., Ryan G.B. Postoperative pericardial adhesions. The role of mild serosal injury and spilled blood. J Thorac Cardiovasc Surg. 1973;65:744–750. [PubMed] [Google Scholar]
- 4.Drusin L.M., Engle M.A., Hagstrom J.W.C. The postpericardiotomy syndrome. A six year epidemiologic study. N Engl J Med. 1965;272:597–602. doi: 10.1056/NEJM196503252721201. [DOI] [PubMed] [Google Scholar]
- 5.Lee C., Yang S., Yang H. Pericardiectomy through a right anterior thoracotomy for constrictive pericarditis after coronary artery bypass grafting. J Med Sci. 2007;27(2):085–088. [Google Scholar]


