Highlights
►This case is an unusual presentation of a rare disease entity. ►The diagnosis of epithelioid trophoblastic tumor can be challenging resulting in delay in diagnosis. ►Epithelioid trophoblastic tumor can have a very aggressive course, especially in metastatic disease.
Keywords: ETT (epithelioid trophoblastic tumor), EB (endometrial biopsy), B Hcg (human chorionicgonadotrophin), ascites, nulligravid
Introduction
Epithelioid trophoblastic tumor (ETT) is an uncommon gestational trophoblastic tumor (Noh et al., 2008) which often presents as a discrete uterine mass following pregnancy with vaginal bleeding(Noh et al., 2008; Vencken et al., 2006). Here we report our experience in managing a nulligravid woman with an unusual presentation of this rare tumor type.
Case
A 45 year old African American female presented to the emergency department (ER) with vomiting, diarrhea and abdominal distention a day after having undergone an uneventful endometrial biopsy (EB) in the office for work-up of an abnormal pap smear revealing endometrial cells. Her initial evaluation in the ER revealed leukocytosis (28,000/ul), elevated β-hCG (443mIU/ml) and a fluid collection in the pelvis and abdomen with no adnexal mass visible on imaging. CT and MRI revealed a 6 cm mass in the right lobe of the liver, a large amount of perihepatic soft tissue density (S1) as well as complex fluid in the abdomen and pelvis. The uterus was unremarkable other than an incidental finding of fibroids (S2).
A culdocentesis was performed because of a concern for a ruptured ectopic pregnancy. Five milliliters of purulent fluid were removed with cultures significant for E coli. The patient then underwent a diagnostic laparoscopy. Finding were negative for peritonitis but 5 l of straw colored ascites were drained and peritoneal surfaces were found to be diffusely studded with disease. Omentum also appeared to be involved but the uterus and adnexa were normal. Histopathology of the omentum and peritoneal biopsies was consistent with ETT as described below.
Postoperatively, the patient was transferred to the surgical intensive care unit. Her WBC count continued to rise despite antibiotics, which was attributed to a leukemoid reaction. Her course was further complicated by increasing abdominal distension and development of bilateral pleural effusions. Thoracentesis and paracentesis were positive for malignant cells. Due to her poor performance status and diagnosis of metastatic ETT, she was felt to be a poor surgical candidate and was instead initiated on neoadjuvant chemotherapy with EMA/CO (etoposide, methotrexate, dactinomycin, cyclophosphamide, vincristine) on hospital day 13.
Her condition continued to deteriorate with worsening leukocytosis (WBC of 67,500/ul; peripheral blood smear revealing neutrophilia and monocytosis) and the development of a pulmonary embolism. She subsequently developed neutropenia and thrombocytopenia on chemotherapy day 2 and was intubated secondary to respiratory distress. Unfortunately the patient expired on hospital day 20, one week after the initiation of her chemotherapy.
Histopathology
The histopathology of the endometrial biopsy showed a relatively uniform growth of epithelioid trophoblasts containing a single hyperchromatic, pleomorphic nucleus surrounded by a hyaline-like matrix. The cells grew in nests and solid masses with evidence of necrosis and occasional abnormal mitotic figures. Similar findings were observed in the omental and peritoneal biopsies. (Fig 1) These cells were strongly positive for β-hCG (S3) and Ki-67 and negative for p63, human placental lactogen (hPL) (S4) and placental alkaline phosphatase (PLAP). These findings, coupled with the low serum β-hCG levels and lack of biphasic morphology encompassing cytotrophoblastic and syncytiotrophoblastic cells, made the neoplastic proliferation atypical to other malignant trophoblastic proliferations.
Fig. 1.
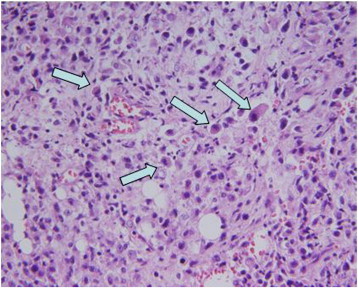
Endometrial biopsy (high power, 20x). Arrows highlight malignant epitheliod trophoblasts characterized by abundant eosinophilic cytoplasm and nuclear atypia.
Discussion
Epithelioid trophoblastic tumor is a rare form of gestational trophoblastic disease (GTD) that was recognized as a separate entity by Shih et al. in 1998 (Palmer et al., 2008). Most cases present in reproductive-age women with a prior gestation. The most common presenting symptom is abnormal uterine bleeding (Noh et al., 2008; Vencken et al., 2006; Palmer et al., 2008). This case posed a diagnostic challenge because of its unusual presenting characteristics. The above patient had no prior documented pregnancies and was consistently on contraceptive measures. When she presented to the ER with a positive β-hCG and complex pelvic fluid, ETT was not considered as part of the differential diagnosis. To the best of our knowledge, ETT has not been previously reported in a nulliparous female. Her presentation of abdominal distension with ascites is one of the rarest manifestations of ETT, which to our knowledge, has been reported in only one case in the literature (Noh et al., 2008). Although pyometra has been reported as an initial presentation (Vencken et al., 2006), the purulent fluid drained by culdocentesis in this case was thought to be secondary to bowel contamination.
Extrauterine presentations of ETT are relatively rare. This highlighted case had no abnormality detected in the uterus. This is unlike most reported cases where lesions have been noted in the uterus, cervix or the adnexa (Noh et al., 2008; Vencken et al., 2006; Coulson et al., 2000). The unexplained leukocytosis seen in the present case was thought to be related to a leukemoid reaction. The WBC trended down after the EMA/CO regimen was initiated with rapid development of neutropenia. A literature review highlights a case with ETT and leukocytosis who later developed plasmacytoma 6 months after surgical treatment (Vencken et al., 2006). Whether the leukocytosis in this case was related to a future development of plasmacytoma is unknown.
Histologically, ETT is derived from chorionic-type intermediate trophoblasts with dual cell populations (intermediate and syncytiotrophoblastic cells). Immunostains for trophoblastic proteins hPL, hCG and PLAP will most often be positive in ETT. Furthermore, immunostains will also be positive for E-cadherin and epidermal growth factor receptor given the epithelial origin of ETT. Because of its epitheliod nature, ETT may be confused with squamous cell carcinoma. The latter, however, is characterized by keratin pearls and intercellular bridges (Palmer et al., 2008).
ETT may present as metastatic disease in up to 35% of cases (Palmer et al., 2008). The majority of metastases involve the lung, liver or vagina though rare sites such as brain, spine and gallbladder have been reported. ETT has been shown to have a very aggressive course with a previous reported mortality rate of 13% (Palmer et al., 2008). This patient presented with stage IV disease with a liver mass, peritoneal carcinomatosis and malignant peritoneal and pleural cytology. ETT had a very aggressive course in this patient as she succumbed to her disease 20 days after presentation. One reason for the dismal prognosis in this case could be the delay in diagnosis due to her atypical clinical picture. Another could have been the failure to manage her surgically with a hysterectomy, hepatic lobectomy and debulking. ETT appears to not be as chemosensitive as other GTDs, making surgical resection the preferred treatment modality (Urabe et al., 2007).
In summary, we report a case of ETT which challenged the treating physicians and had a very aggressive course. More cases with similar morphology need to be identified and published so that we may have a better understanding of this disease process.
Conflict of interest statement
The authors have no conflicts of interest.
Contributor Information
Bagaria Madhu, Email: mbagari1@hfhs.org.
Rasool Nabila, Email: nrasool1@hfhs.org, dr_nabila16@yahoo.com.
Appendix A. Supplementary data
S1:MRI of the abdomen showing hepatic mass and perihepatic fluid
S2. MRI of the abdomen showing complex fluid in the pelvis and uterus with the fibroid
S3. Beta HCG positive tumor cells
S4. Negative hPL staining of the tumor cells.
References
- Noh H.T., Lee K.H., Lee M.A. Epithelioid trophoblastic tumor of paracervix and parametrium. Int. J. Gynecol. Cancer. 2008;18:843–846. doi: 10.1111/j.1525-1438.2007.01086.x. [DOI] [PubMed] [Google Scholar]
- Vencken P.M., Ewing P.C., Zweemer R.P. Epithelioid trophoblastic tumor: a case report and review of literature. J. Clin. Pathol. 2006;59(12):1307–1308. doi: 10.1136/jcp.2005.030734. (Dec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J.E., Macdonald M., Wells M., Hancock B.W., Tidy J.A. Epithelioid trophoblastic tumor: a review of the literature. J. Reprod. Med. 2008;53(7):465–475. (Jul) [PubMed] [Google Scholar]
- Coulson L.E., Christina S.K., Zaloudek C. Epithelioid trophoblastic tumor of the uterus in a postmenopausal women: a case report and review of literature. Am. J. Surg. Pathol. 2000;24:1558–1562. doi: 10.1097/00000478-200011000-00014. [DOI] [PubMed] [Google Scholar]
- Urabe S., Fujiwara H., Miyoshi H., Arihiro K., Soma H., Yoshihama I., Mineo S., Kudo Y. Epithelioid trophoblastic tumor of the lung. J. Obstet. Gynaecol. Res. 2007;33(3):397–401. doi: 10.1111/j.1447-0756.2007.00545.x. (Jun) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1:MRI of the abdomen showing hepatic mass and perihepatic fluid
S2. MRI of the abdomen showing complex fluid in the pelvis and uterus with the fibroid
S3. Beta HCG positive tumor cells
S4. Negative hPL staining of the tumor cells.


