Abstract
Granulicatella adiacens, a recently nomenclatured bacterium, was considered as one of the nutritionally variant streptococci (NVS) and is a mouth commensal. It is redesignated as a streptococcus like bacterium since it differs from streptococci. We report a case of infective endocarditis (IE) caused by this fastidious and unusual bacteria in a 63-year-old man with rheumatic valvular heart disease. G. adiacens was isolated from four of his blood culture samples, which was sensitive to beta lactams, moderately sensitive to gentamicin and resistant to erythromycin and co-trimoxazole. Patient recovered completely on treatment with high dose of ampicillin and gentamicin for 28 days.
Keywords: Granulicatella adiacens, Streptococcus like bacteria, Infective endocarditis
1. Introduction
Infective endocarditis (IE) is a relatively uncommon but life-threatening disease for which cardiac valvular abnormalities are strong risk factors.1 Among the etiological agents of IE, streptococci come to 60–80%, of which, viridans streptococci contribute to 30–40%, enterococci 5–18% and other streptococci 15–25%.2 Most predominant pathogens of IE are bacterial species in the oral cavity and being a mouth commensal, the transmission of Granulicatella from the mouth should be noted as a possible cause of IE.1
The genus Granulicatella is one of the streptococcus like bacteria. In 1995, Kawamura and others in Japan relocated and renamed some NVS as Abiotrophia, based on 16S rRNA sequencing.3 Since then, Collins and Lawson4 have proposed that three species members of the genus Abiotrophia be reclassified in a new genus Granulicatella, as Granulicatella adiacens, balaenopterae and elegans and lately one more species G. para adiacens was also added.3 Granulicatella is seen as the normal flora of human mouth, genital and intestinal tracts. Abiotrophia and Granulicatella cause sepsis and bacteremia and are the causes of 5–6% of infective endocarditis. They are also associated with pulmonary, CNS and ocular infections. Though there are reports on IE caused by G. adiacens, to the best of our knowledge no such case has been reported from India.
2. Case report
A 63-year-old man with rheumatic valvular heart disease admitted in the cardiology department of our hospital with complaints of high grade irregular fever associated with chills and rigor of two months duration. He also had dyspnea of grade III, chest pain, cough and fatigue. The patient was a known case of rheumatic mitral regurgitation and mitral valve prolapse on regular treatment for diabetes mellitus and systemic hypertension. In 2004, he had a cerebellar infarct with subsequent hydrocephalus and underwent ventriculoperitonial shunt surgery. On examination his temperature was 101 °F, auscultation of the chest revealed a pan systolic murmur, abdominal examination showed hepatomegaly and mild splenomegaly. No other relevant findings were elicited. His blood investigations were as follows, hemoglobin – 9.4 g%, Total WBC count – 10,140 with P – 72% and L – 22%, platelet count – 1, 49,000 and ESR – 60 mm/h. Widal test was nonreactive. USG abdomen showed gall bladder calculus and moderate splenomegaly and echocardiogram revealed multiple vegetations attached to mitral valve (Fig. 1, echocardiogram picture showing vegetations). Subsequently a diagnosis of infective endocarditis was made.
Fig. 1.
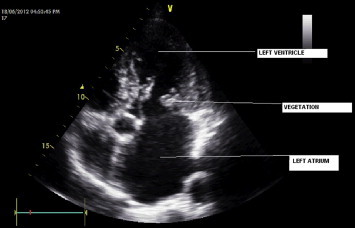
Echocardiogram picture showing vegetations.
On the day of admission, three blood culture samples were collected at an interval of half an hour from three different sites and were processed in BacT ALERT automated blood culture system (Bio Merieux). As he had high temperature, 102 °F, on the next day, another blood sample was also collected and processed in the same manner. All the four samples were positive within 48 h and the direct Gram smear from the broth showed Gram positive cocci arranged predominantly in pairs. Subcultures were done on human blood agar (HBA), chocolate agar (CA) and Mac-conkey's agar (MA). Direct sensitivity was performed on HBA. After overnight incubation, minute semi translucent, moist colonies appeared on HBA, CA and on the sensitivity plate. The colonies were non-hemolytic, Gram stain of which showed Gram variable cocci arranged in pairs, short chains and as tetrads. Some coccobacillary forms, and short club shaped bacilli were also observed (Fig. 2). The isolate was found to be catalase negative, bile esculin negative, optochin and bacitracin resistant. Satellitic type of growth around staphylococcus streak on BA plate was obtained. It failed to grow at 10 °C and on sheep BA (SBA).3 The identification of the isolate was confirmed as G. adiacens using Vitek 2 automatic bacterial identification system (Bio Merieux) and it was found to be sensitive to penicillin, ampicillin, cefotaxime, linezolid, clindamycin and vancomycin, moderately sensitive to gentamicin and resistant to co-trimoxazole and erythromycin by disc diffusion method.
Fig. 2.
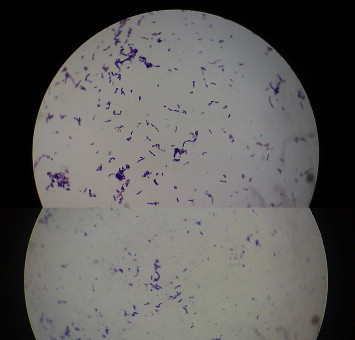
Gram stained smear of bacterial colony showing pleomorphic Gram positive cocci, 1000×.
After collecting the fourth blood sample, the patient was started on ampicillin 2 g IV 4th hourly and gentamicin 60 mg IV 8th hourly for two weeks and then tapered gradually over a period of another two weeks. His temperature came down on initiation of the therapy and he was completely afebrile after 7 days. Rest of the hospital stay was uneventful and the blood counts and ESR came down gradually. The patient was discharged after one month of admission and he was doing well on review after one month.
3. Discussion
Among the members of the genus Granulicatella, G. adiacens appears to have higher degree of infectivity, and this has been attributed to its capacity to bind to the cardiac valvular tissue. IE due to NVS is indolent in onset and pre existing valvular pathology is, as in our case, frequent.2 Nevertheless, classic endocarditis signs such as digital clubbing, petechiae and Osler nodes are rare. The most common valves affected are the aortic and the mitral valves with detectable vegetations being observed in about 64% of cases.5 Long-term combination therapy with penicillin and an aminoglycoside is usually recommended. Vancomycin is an effective alternative against resistant strains. In spite of in vitro results, treatment failure is observed in about 41% of cases, and almost 27% require prosthetic valve replacement, especially due to congestive heart failure or major systemic emboli,6 relapse and death are also common, 17–27%.2
Since G. adiacens is a bacterium which is nutritionally deficient, requiring pyridoxal and l-cysteine as growth factors, it is difficult to obtain growth of this bacterium on ordinary culture media like SBA even from specimens and blood culture broths showing Gram positive cocci in direct smears. It may fail to grow on conventional blood culture media, since many of them lack pyridoxal unlike the automated blood culture broths like BACTEC or BacT ALERT. Hence G. adiacens is considered as one of the causes of culture negative IE.
Granulicatella and Abiotrophia are more resistant to antibiotics than viridans streptococci. Most strains are sensitive to clindamycin, erythromycin, rifampicin and vancomycin. Notably, the present susceptibility profile is in agreement with those of other NVS isolates; many of the previous strains were also susceptible to penicillin whereas they exhibited variable susceptibility to aminoglycosides.7,8
Due to the clinical significance and the exacting cultural requirements of this bacterium, it is essential that the clinicians and the microbiologists should consider this bacterium in culture negative cases of IE.
Conflicts of interest
All authors have none to declare.
References
- 1.Ohara-Nemoto Yuko, Kishi Kayo, Satho Mamoru. Infective endocarditis caused by Granulicatella elegans originating in the oral cavity. J Clin Microbiol. 2005;43:1405–1407. doi: 10.1128/JCM.43.3.1405-1407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler Vance G., Michael Scheld W., Jr., Bayer Arnold S. Endocarditis and intravascular infections, chapter 74. In: Mandell G.L., Bennett J.E., Dolin R., editors. 6th ed. vol. 1. Churchill Livingstone; Philadelphia, USA: 2005. pp. 988–989. (Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases). [Google Scholar]
- 3.Koneman E.W., Allen S.D., Janda W.M., Schreckenberger P.C., Winn W.C. 6th ed. Lippincott William and Wilkins; Philadelphia, USA: 2006. Colour Atlas and Text Book of Diagnostic Microbiology. [Google Scholar]
- 4.Collins M.D., Lawson P.A. The genus Abiotrophia (Kawamura et al.) is not monophyletic: proposal of Granulicatella gen. nov., Granulicatella adiacens comb. nov, Granulicatella elegans comb. nov. and Granulicatella balaenopterae comb. nov. Int J Syst Evol Microbiol. 2000;50:365–369. doi: 10.1099/00207713-50-1-365. [DOI] [PubMed] [Google Scholar]
- 5.Perkins A., Osorio S., Serrano M. A case of endocarditis due to Granulicatella adiacens. Clin Microbiol Infect. 2003;9:576–577. doi: 10.1046/j.1469-0691.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- 6.Brouqui P., Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev. 2001;14:177–207. doi: 10.1128/CMR.14.1.177-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruoff K.L. Nutritionally variant streptococci. Clin Microbiol Rev. 1991;4:184–190. doi: 10.1128/cmr.4.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuohy M.J., Procop G.W., Washington J.A. Antimicrobial susceptibility of Abiotrophia adiacens and Abiotrophia defectiva. Diagn Microbiol Infect Dis. 2000;38:189–191. doi: 10.1016/s0732-8893(00)00194-2. [DOI] [PubMed] [Google Scholar]


