Abstract
Erosion of a peripancreatic artery into the pseudocyst as a result of enzymatic digestion of vessel wall gives rise to a pancreatic pseudoaneurysm (PSA), which is a rare complication seen in patients with chronic pancreatitis.1 Angiographic embolization as a treatment method for acute hemorrhage from pancreatic PSA has become increasingly popular. Here we report a unique case with bleeding from a giant pancreatic PSA where the single PSA had blood supply originating from the branches of both the celiac artery and superior mesenteric artery.
Keywords: Pancreatitis, Pseudoaneurysm, Gastrointestinal bleed, Embolization
1. Introduction
Pseudoaneurysms of the peripancreatic arteries are considered to be rare in patients with pancreatitis. An angiographic study reported a 10% occurrence of PSA in a series of 72 unselected patients with pancreatitis.2 Autodigestion caused by the leakage of pancreatic, proteolytic enzymes may weaken the arterial wall of adjacent visceral arteries (most commonly splenic artery in 30–50% followed by the gastroduodenal artery in 10–15% of the cases) and provoke development of PSA formation.3 Pancreatico-duodenal artery (PDA) pseudoaneurysm is an even uncommon vascular structure to be affected by this process.4 We present a case of chronic pancreatitis that had gastrointestinal tract bleeding from a PSA. Its transcathter embolization, perioperative complication and subsequent management are discussed in the following case report.
2. Case report
A 35-year-old male with a history of chronic and significant alcohol intake for the past 10 years and recurrent episodes of severe abdominal pain radiating to the back for the past four months presented to the gastro surgery department with a massive upper gastrointestinal bleed causing hematemesis and malena. The patient was clinically anemic, had icterus and a pulsatile lump in the upper abdomen. His heart rate was 122/min; blood pressure was 90/60 mmHg with cold extremities. His hemoglobin was low (7.0 g/dL), white cell count was 11,400/mm3 and platelet count was 85,000/mm3. The biochemical investigations of the patient revealed obstructive jaundice with coagulopathy (Table 1). Patient received two units packed red blood cell (PRBC) transfusion, one unit fresh frozen plasma and underwent upper gastrointestinal endoscopy that was unhelpful. Urgent CT imaging of the abdomen demonstrated atrophic pancreas with pancreatic head pseudoaneurysm causing common bile duct and main pancreatic duct compression and subsequent dilatation (Fig. 1). Due to the high risk of rupture, underlying coagulopathy and scar tissue arising out of chronic pancreatitis any surgical intervention was not feasible. Thus the patient was referred to us for visceral angiography and subsequent catheter-based intervention for occlusion of the pseudoaneurysm on an urgent basis. Diagnostic celiac angiography was performed using 5 French Judkins right catheter followed by selective cannulation of the gastroduodenal artery. Angiography revealed a giant pseudoaneurysm sac with a small neck arising from the superior pancreatico-duodenal artery very close to the branch-off of the right hepatic artery. During the procedure, the patient did not received any heparin in view of bleeding and coagulopathy arising out of obstructive jaundice. However, perioperatively it was realized that a thrombus probably formed at the catheter tip had migrated distally into the right hepatic artery (Fig. 2A). The patient was then given 5000 units heparin and subsequently coil embolization was performed with three 0.035 inches stainless steel coils of 3 mm diameter that were placed into the neck of the sac. There was immediate technical success with no further inflow into the PSA sac and a flowing hepatic artery was seen with a residual thrombus and contrast stain (Fig. 2B). The patient did well postoperatively and his hemoglobin improved to 10.0 gm% when three days later during the hospital stay he had another episode of severe gastrointestinal bleed. Surprisingly, this time it was a lower gastrointestinal bleed manifesting as hematochezia and his hemoglobin dropped to 8.0 gm%. Patient again received two units PRBC transfusion and underwent urgent visceral angiography. Celiac artery angiography confirmed no inflow into the sac from the superior pancreatico-duodenal artery, well flowing hepatic artery proper and a normal splenic artery. But selective angiography of the superior mesenteric artery that was not done previously revealed a communicating bleeder arising from the inferior pancreatico-duodenal artery to the inferior pole of the same aneurysmal sac (Fig. 3A). The bleeder was selectively engaged with a 3.0 French microferret (cook, USA) and one 0.018 inches stainless steel coil of 3 mm diameter was placed (Fig. 3B). Post procedure patient stayed well and a follow up CT scan was carried out two days later that showed minimal residual contrast and thrombosis of the pseudoaneurysmal sac. Patient demonstrated a decrease in the size of the abdominal lump, a gradual reduction in total bilirubin level to 5.0 gm% and an INR of 1.20 when he was discharged on medical management twelve days after the admission.
Table 1.
Biochemical investigations of the patient revealing obstructive jaundice, elevated INR and non-specifically elevated serum amylase level.
| Blood urea nitrogen | 55 mg/dL |
| Serum creatinine | 1.4 mg/dL |
| Total serum bilirubin | 14.0 mg/dL (mixed conjugated and unconjugated hyperbilirubinemia |
| Aminotransferase (SGOT/SGPT) | 98/68 U/L (normal < 40) |
| Serum alkaline phosphatase | 4 times the normal value |
| International Normalized Ratio (INR) | 3.5 |
| Serum amylase | 185 U/L (normal, 20–80) |
| Serum lipase | 55 U/L (normal, 0–190) |
Fig. 1.
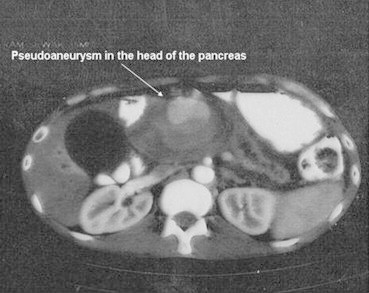
Preoperative CT scan showing enhancing mass in the head of the pancreas.
Fig. 2.
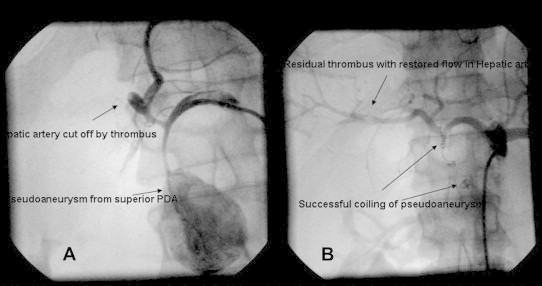
(A): Dislodgement of thrombus at guide tip with hepatic artery cut off and filling of pseudoaneurysmal sac with contrast proximally. (B): Complete isolation of pseudoaneurysmal sac with flowing hepatic artery and minimal thrombus postoperatively. Embolization coils are seen in situ.
Fig. 3.
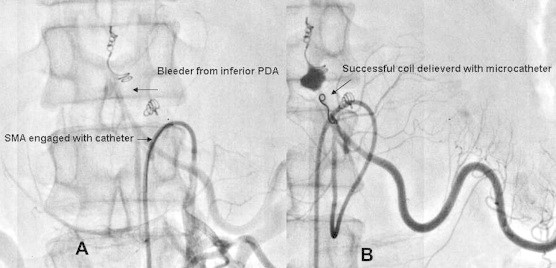
(A): GI bleeder arising from inferior pancreatico-duodenal artery and supplying the lower pole of aneurysmal sac. (B): Selective intubation of lower pole feeder with microferret catheter and subsequent coil placement.
3. Discussion
Acute hemorrhage from a PSA is the most rapidly fatal complication of chronic pancreatitis. The mortality rate of untreated patients reaches 90 to even a 100%. Even with the most aggressive treatment, the mortality is still at 12–50%.5 Spontaneous thrombosis of these lesions is rarely reported.6 A PSA can bleed into a contiguous viscus, peritoneum, or pancreatic duct. The definitive management requires demonstration of PSA. Dual phase CT scan, CT angiography, and magnetic resonance cholangiopancreatography can help in the diagnosis of pancreatic PSA.7 The two primary methods of treatment of bleeding pancreatic PSA are either surgery or transcatheter occlusion. Debate still exists about the best methods of treatment. The results of Bergert et al suggest that the mortality rate is not affected by the mode of treatment in chronic pancreatitis. As a consequence, a successful embolization requires no further treatment.8 Efficacy for transcatheter embolization in pseudoaneurysms caused by pancreatitis ranges from 67–100%.9
Due to the lack of interventional radiology specialty in Indian setting, an interventional cardiologist often receives the emergency call for the possibility of transcatheter embolization of a visceral bleeder. Our case report highlights dilemmas faced by an interventional cardiologist during the management of such cases. First issue relates to the use of heparin during these procedures when use of heparin is feared as a dual edged sword in the presence of underlying coagulopathy and an ongoing gastrointestinal bleeding. As selective cannulation of a visceral bleeder requires guiding catheter manipulation, heparin should be given to the patients to avoid the formation of thrombus at catheter tip by contact activation pathway. Second, as pancreatic head is a site for pancreatico-duodenal arcade formed by the superior and inferior PDA, both celiac and SMA angiography should be done during the primary evaluation of a gastrointestinal bleeder in such cases. Multiple feeders to the visceral pseudoaneurysm are common and a pre procedure CT – angiography in the first instance could also have avoided a second procedure. Surgical treatment of pancreatic PSA should be limited to patients for whom less invasive catheter-based embolization is not technically feasible, for patients who have undergone a failed embolization, or for recurrent PSA after successful embolization. Minimal invasiveness, intra-aneurysmatic thrombosis, aneurysm size regression, and the low rate of complications are the main advantages of transcatheter intervention over surgical treatment.10 This tale of two bleeds and one thrombus taught us many lessons and was a leap in our understanding of the catheter-based management of a bleeding visceral pseudoaneurysm.
4. Conclusion
Angiographic embolization has increasingly become the favored therapeutic option in pancreatic PSA bleed for patients who are at increased surgical risk. The patients should undergo prompt initial angiographic evaluation with selective visceral angiography of both celiac and superior mesenteric artery. The need to give anticoagulant during these procedures must be realized. The angiographic principle of occluding the PSA upstream and downstream should be adopted.
Conflicts of interest
All authors have none to declare.
References
- 1.Von Flue M., Kocher T., Herzog U. Hemorrhage from pseudocysts caused by pseudoaneurysms in chronic pancreatitis. Diagnosis and management. Helv Chir Acta. 1993;59:785–789. [PubMed] [Google Scholar]
- 2.White A.F., Baum S., Buranasiri S. Aneurysms secondary to pancreatitis. AJR. 1976;127:393–396. doi: 10.2214/ajr.127.3.393. [DOI] [PubMed] [Google Scholar]
- 3.Stabile B.E., Wilson S.E., Debas H.T. Reduced mortality from bleeding pseudocysts and pseudoaneurysms caused by pancreatitis. Arch Surg. 1983;118:45. doi: 10.1001/archsurg.1983.01390010035009. [DOI] [PubMed] [Google Scholar]
- 4.Carr J.A., Cho J.-S., Shepard A.D. Visceral pseudoaneurysm due to pancreatic pseudocysts: rare but lethal complications of pancreatitis. J Vasc Surg. 2000;32:722–730. doi: 10.1067/mva.2000.110055. [DOI] [PubMed] [Google Scholar]
- 5.Law N.M., Freeman M.L. Emergency complications of acute and chronic pancreatitis. Gastroenterol Clin North Am. 2003;32:1169–1194. doi: 10.1016/s0889-8553(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 6.De Ronde T., Vanbeers B., De Canniere L., Trigaux J.P., Melange M. Thrombosis of splenic artery pseudoaneurysm complicating pancreatitis. Gut. 1993;34:1271–1273. doi: 10.1136/gut.34.9.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyoki Y., Hakamada K., Narumi S. Hemosuccus pancreaticus: problems and pitfalls in diagnosis and treatment. World J Gastroenterol. 2008;14:2776–2779. doi: 10.3748/wjg.14.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergert H., Hinterseher I., Kersting S., Leonhardt J., Bloomenthal A., Saeger H.D. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery. 2005;137:323–328. doi: 10.1016/j.surg.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.De Perrot M., Berney T., Buhler L., Delgadillo X., Mentha G., Morel P. Management of bleeding pseudoaneurysms in patients with pancreatitis. Br J Surg. 1999;86:29–32. doi: 10.1046/j.1365-2168.1999.00983.x. [DOI] [PubMed] [Google Scholar]
- 10.Golzarian J., Nicaise N., Deviere J. Transcatheter embolization of pseudoaneurysms complicating pancreatitis. Cardiovasc Intervent Radiol. 1997;20:435–440. doi: 10.1007/s002709900189. [DOI] [PubMed] [Google Scholar]


