Abstract
Technically difficult echocardiographic studies with suboptimal images remain a significant challenge in clinical practice despite advances in imaging technologies over the past decades. Use of microbubble ultrasound contrast for left ventricular opacification and enhancement of endocardial border detection during rest or stress echocardiography has become an essential component of the operation of the modern echocardiography laboratory. Contrast echocardiography has been demonstrated to improve diagnostic accuracy and confidence across a range of indications including quantitative assessment of left ventricular systolic function, wall motion analysis, and left ventricular structural abnormalities. Enhancement of Doppler signals and myocardial contrast echocardiography for perfusion remain off-label uses. Implementation of a contrast protocol is feasible for most laboratories and both physicians and sonographers will require training in contrast specific imaging techniques for optimal use. Previous concerns regarding the safety of contrast agents have since been addressed by more recent data supporting its excellent safety profile and overall cost-effectiveness.
Keywords: Contrast echocardiography, Laboratory protocols, Ultrasound settings
1. Introduction
Transthoracic echocardiography (TTE) remains a versatile and globally the most common cardiac diagnostic imaging modality. Numerous developments in ultrasound technology, including harmonic imaging and improvement in imaging frame rates up to 120 frames per sec, have greatly enhanced the diagnostic capabilities of the technique. However, there is still a need to improve image resolution when the acoustic windows are limited and endocardial definition suboptimal. This may result in potentially missed or incorrect diagnoses and consequential adverse outcomes or further inappropriate downstream investigations with both temporal and financial implications.1
Microbubble ultrasound contrast is now regarded as an essential tool in the day-to-day practice of the clinical echocardiography laboratory to overcome some of these limitations. The contemporary approved and appropriate indications for the use of ultrasound contrast agents include left ventricular opacification (LVO) and improvement of endocardial border detection (EBD), when ≥2 contiguous segments are not well-visualized without contrast enhancement.2–4 Some research and off-label use of contrast agents include augmentation of the spectral Doppler signal and assessment of myocardial perfusion. While the latter showed enormous potential in animal research studies, it has not translated into everyday clinical practice.
The commonly available second-generation echocardiographic contrast agents are Sonovue (Bracco Imaging), Optison (GE Healthcare) and Definity (Lantheus Medical Imaging) that is also marketed under the label of Luminity in Europe. While they are essentially similar in the way they enhance TTE image quality, each of these microbubble contrast agents have their own characteristics, which will be discussed later. Delivered intravenously, these microbubbles are sufficiently small (<10 μm; red blood cells are ∼6–8 μm for reference) to allow transpulmonary passage and therefore provide real-time imaging of blood flow through the left-heart. These microbubbles use high-molecular weight gases with low-solubility and the high elasticity shell to reduce acoustic destruction and thereby maintain the microbubble integrity (stability), prolong circulating time (persistence) or contrast effect, and maximize the non-linear contrast backscatter.5,6
The injected microbubbles provide multiple gas–liquid interfaces within the blood pool and thereby significantly increasing the backscatter of ultrasound waves from the insonating beam.2,7 These microbubbles undergo asymmetric oscillation (alternating compression and expansion with inverse changes in radius and stiffness) within the applied ultrasound field and essentially behave as non-linear scatterers. Real-time assessment with ultrasound contrast for LVO and improvement of EBD is conventionally performed with low-MI (usually <0.2) harmonic imaging. This reduces microbubble disruption, enhances the intracavitary contrast intensity, allows subtraction or filtering of linear tissue backscatter and minimizes tissue harmonics.8 The end result is enhancement of the endocardium that forms the border between the darker myocardium and bright intracavitary contrast.
2. Clinical utility and indications
The current consensus indications for contrast LVO in resting transthoracic echocardiography include (Table 1):
-
1)
Improvement of left ventricular (LV) EBD,
-
2)
Increased accuracy and reproducibility of ventricular volumetric assessments,
-
3)
Quantitative assessment of ejection fraction,
-
4)
Enhanced diagnostic confidence for LV structural abnormalities (including but not limited to apical thrombus, non-compaction and hypertrophic cardiomyopathy where near-field clutter and artifacts are problematic),
-
5)
Microbubble contrast is also clinically indicated in stress echocardiography when ≥2 contiguous segments are not well-visualized with the intent of improving interpretation of wall motion abnormalities and diagnostic accuracy,9
-
6)
Off-label use of microbubble contrast agents for MCE in perfusion imaging and Doppler signal enhancement will also be discussed briefly in this review.
Table 1.
Current approveda indications for contrast echocardiography.
LV opacification during resting transthoracic echocardiography in difficult-to-image patients for:
|
Doppler signal enhancement, myocardial perfusion, and use of contrast echocardiography during interventional procedures are currently regarded as off-label uses for echocardiographic contrast agents.
2.1. Left ventricular structure and function
2.1.1. Quantification of left ventricular systolic function
Quantitative evaluation of LV systolic function in the form of the LV ejection fraction (LVEF) is one cornerstone for the initial diagnosis of heart failure and remains a significant prognosticator of survival. Many currently used chemotherapeutic agents have an increased risk of early or delayed cardiovascular toxicities and regular surveillance of LVEF is a critical part of continuing care.10 It has been repeatedly demonstrated that contrast-enhanced echocardiography for LVO improves LVEF correlation with radionuclide ventriculography and cardiac magnetic resonance imaging (cMRI), and decreases the overall intra- and interobserver variability.1,11–16
2.1.2. Endocardial border definition and wall motion assessment (resting and stress echocardiography)
Up to 20% of routine transthoracic echocardiograms may have poor EBD and could be regarded as non-diagnostic.1,17,18 Patient factors contributing to these difficult images include co-existent chronic obstructive airways disease, chest wall-deformities, and body habitus (obesity). Studies performed in the emergency department or on mechanically ventilated patients in the intensive care setting also pose significant challenges from the perspective of image quality. Multiple studies have demonstrated that microbubble contrast combined with harmonic imaging for LVO improves the diagnostic accuracy, confidence and interobserver agreement in assessment of regional systolic function or myocardial thickening in these technically difficult-to-image patients.19–22 Kitzman et al demonstrated that contrast-enhanced images resulted in the conversion of 48% of non-diagnostic examinations (defined as ≥4 of 6 non-evaluable segments in a single apical view) into “salvaged” studies (where ≤1 poorly visualized segment remained on the same comparative view) following LVO.23 These salvage rates have been reported to be higher in intensive care unit (ICU) patients who were mechanically ventilated.24–26
The assessment of regional wall motion (segmental myocardial thickening) that forms the basis of interpretation of stress echocardiography is subjective and highly dependent on optimal endocardial definition. The same patient factors contributing to less than ideal images are often further exaggerated during stress. Technically suboptimal studies have been reported in up to 30% of exercise stress echocardiograms (ESE) and the inter-institutional agreement (positive or negative) for dobutamine stress echocardiograms (DSE) ranges from 43% with poor images to 100% in those with the highest quality.27,28 Contrast administration enhances 77–95% of poorly visualized segments during DSE and has been shown to improve the diagnostic accuracy and readers' interpretive confidence.29–33
2.1.3. Delineation of left ventricular structural abnormalities
Despite the close anatomical position of the LV apex to the ultrasound transducer “footprint” on the chest wall, the apical region can be challenging to image due to foreshortening and high prevalence of near-field artefacts. Contrast for LVO offers significant advantages in visualizing the appearance of the apex.
The accurate detection of an apical LV thrombus is critical due to the potential for systemic embolization and devastating outcomes. Standard non-contrast enhanced echocardiography may be non-diagnostic in a significant proportion (up to 46% in one retrospective analysis) of patients and may be difficult to distinguish from apical trabeculae, papillary muscles, false tendons, tumors (albeit uncommon) or artefacts (clutter or reverberations).34 Contrast administration can convert up to 90% of these suboptimal studies into diagnostic images and anticoagulation therapy may then be initiated or withdrawn as appropriate. Apical thrombi typically appear as dark irregular intraluminal filling defects in comparison to the relatively “smooth” ventricular endocardial border as outlined by the bright intracavitary contrast (Figs. 1 and 2). Equally, the addition of microbubble contrast to routine 2D harmonic imaging can rule out the presence of an apical thrombus, and avoid unnecessary long-term anticoagulation (Fig. 3). Apical aneurysms can also be clearly outlined and definitively diagnosed by LVO.
Fig. 1.
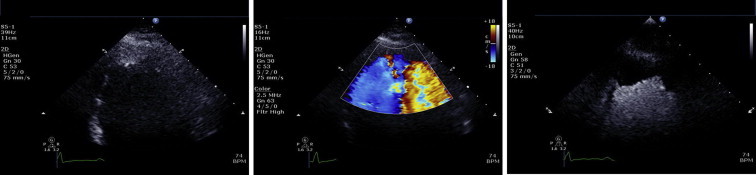
Two-dimensional echocardiographic images from a standard apical 4-chamber view at a reduced depth to focus on the LV apex. The non-contrast enhanced image demonstrates an echodensity within the apex that may represent a possible apical thrombus. Colour flow Doppler with a reduced scale was used in an attempt to further delineate this echodensity but did not offer any significant additional diagnostic information. Contrast enhancement clearly shows a large apical filling defect void of any contrast infiltration consistent with a large LV apical thrombus.
Fig. 2.
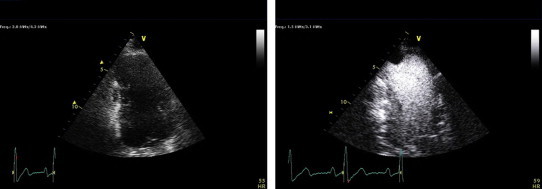
Technically difficult study with an aneurysmal apex on the non-contrast enhanced apical 2-chamber image. Contrast administration then reveals a well-defined apical thrombus that would not have been detectable otherwise.
Fig. 3.
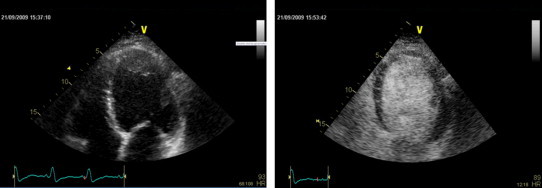
Two-dimensional echocardiographic apical 4-chamber view demonstrating a dilated globular LV with a possible apical thrombus on the non-contrast enhanced image. The administration of intravenous echocontrast was able to exclude the presence of an intraluminal filling defect and therefore avoiding unnecessary anticoagulation.
The differential diagnosis that needs to be delineated includes the aforementioned apical thrombi, apical tumors, isolated left ventricular non-compaction, apical displacement of the papillary muscles, and endomyocardial fibrosis. The management decisions and prognosis for all these diagnoses are very disparate. Contrast-enhanced echocardiography has been demonstrated to establish the diagnosis of apical HCM where routine imaging is unclear or non-diagnostic (Fig. 4).35,36 The classic end-diastolic “spade-like” appearance of the LV apex on left ventriculography can be reproduced non-invasively as an alternative to cMRI.37 Fig. 5 is an example of contrast echocardiography revealing the appearance of prominent trabeculae with deep recesses involving the apex and mid-inferolateral walls typical of left ventricular non-compaction.
Fig. 4.
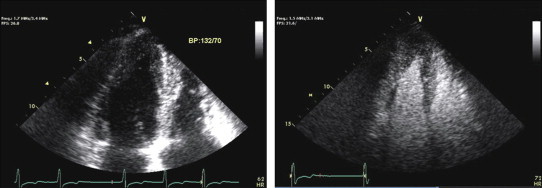
Two-dimensional non-contrast enhanced echocardiographic image suggestive of apical regional wall thickening and then following injection of Definity echocontrast showing the typical spade-shape appearance of the LV apex as described in apical HCM.
Fig. 5.
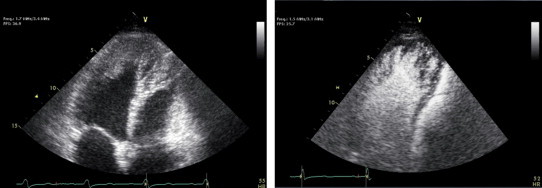
Non-contrast enhanced apical view of the LV with an ill-defined echodensity in the apex extending down along the lateral wall toward the mid cavity. Contrast enhancement reveals prominent trabeculae with deep intertrabecular recesses consistent with non-compaction cardiomyopathy.
LV pseudoaneurysms that occur as a result of a post-myocardial infarction free wall rupture may be difficult to distinguish from an aneurysm with underlying thinned and scarred myocardium. Echocardiography will often identify the echo-free collection but may encounter difficulty in localizing the small neck or discontinuity in the myocardium (either by 2D, color or spectral Doppler) when the acoustic window or images are suboptimal and potentially underestimate the true maximal cavity diameter when thrombus is present. Given the propensity of pseudoaneurysms for spontaneous fatal rupture definitive diagnosis is critical to expedite surgical intervention. Contrast LVO has been utilized and reported to provide incremental diagnostic power to detect pseudoaneurysms as evidenced by direct visualization of contrast extravasation through the narrow neck into the pericardial space (Fig. 6).38–40
Fig. 6.
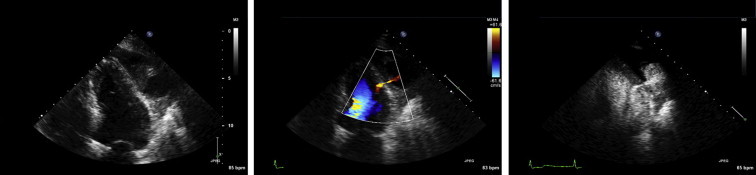
Standard apical 4-chamber view demonstrating an extra-cardiac collection at the level of the mid-anterolateral wall. Colour flow Doppler reveals a connection and flow between the LV cavity and the echo-free space. Contrast administration confirms the presence of a narrow neck communicating with an extra-cardiac cavity consistent with an LV pseudoaneurysm.
2.2. Off-label uses
2.2.1. Doppler signal enhancement
The peak tricuspid regurgitant (TR) jet velocity measured on continuous wave Doppler analysis is used in the estimation of the right ventricular systolic pressure. The degree of tricuspid regurgitation does not directly correlate with the degree of pulmonary hypertension. Trivial regurgitation can result in incomplete Doppler spectral signals and an underestimation of the true pressure gradient. Intravenous echo contrast can be used to augment the TR envelope (defined as a smooth parabolic well-defined signal) for more accurate assessment of pulmonary pressures.41 Similarly, on the left side of the heart, it can help improve the spectral Doppler assessment of the peak aortic jet velocity.
2.2.2. Myocardial contrast echocardiography (MCE)
As the contrast microbubbles remain entirely in the intravascular space and pass freely through the microcirculation they are potentially an ideal flow tracer. Wei et al first described the method for quantification of MBF using a continuous venous infusion of microbubble contrast, followed by destruction by exposure to high-intensity ultrasound pulses, and then measuring the rate of replenishment and intensity which is represented by a time–intensity curve.42 The rate of contrast reappearance calculated from the slope of the time–intensity curve is a measure of mean myocardial blood flow velocity (ß) while the peak plateau intensity (A) represents the cross-sectional area of the microvasculature such that A × ß equates to MBF.43 When there is hemodynamically significant epicardial coronary artery stenosis with resultant flow limitation that exceeds the distal compensatory microvascular vasodilatation (coronary flow reserve), the rate and intensity of contrast replenishment is reduced. Qualitative visual assessment, which is the more common approach in clinical applications of MCE, subjectively scores contrast reappearance as normal (homogenous enhancement within 5 s following flash microbubble destruction at rest or within 2 s at stress) or reduced.43,44 Detailed discussion of quantitative assessment of MBF is beyond the scope of the current article.
3. How to do it?
The 2008 American Society of Echocardiography consensus statement on contrast agents in echocardiography advocates a team approach for the successful introduction and implementation of contrast protocols into an echocardiography laboratory.2 The team would primarily consist of the sonographer, physician echocardiographer and where available, nursing staff who are competent and certified in obtaining intravenous access and administration of intravenous agents. Although there are no mandatory rules or legislations governing the use of microbubble contrast agents, it is recommended that the sonographers be appropriately qualified and credentialed in echocardiography and the responsible physicians be independent and competent echocardiographers (minimum of ASE level 2 or equivalent training) with skills in basic and advanced life support.45 This would form the foundation to then develop an understanding of contrast physics, indications for and contraindications to contrast administration, and contrast-specific ultrasound imaging techniques required to obtain optimal and diagnostic images. Team members must also be prepared and equipped to deal with patient adverse reactions associated with contrast use. Clinical training and practical experience is crucial for both sonographer and physician to become familiar with contrast echocardiography. In the absence of formal training programs, a suitable means of acquiring the necessary practical experience would be a preceptorship-type model that can be undertaken at an established institution with a high-volume of contrast studies and an adequate breadth of pathologies. Table 3 provides a summary of the basic elements and practical approach to setting up a contrast protocol.
Table 3.
Example of an echocardiography laboratory contrast setup and protocol for resting LVO.
Basic equipment
|
3.1. Laboratory setup and equipment
The basic equipment required to set up a contrast injection should be readily available in majority of echocardiography laboratories. Essentially the same items used for an agitated saline contrast study are required – syringes, sterile normal saline for dilution and flushing, large guage needles for vial venting and drawing up, intravenous cannulae, alcohol based pads or swabs, gloves, three-way stopcock, gauze pads and medical tape.46,47 A refrigerator for storage of the contrast agents and the appropriate disposal containers for medical sharps as per standard universal precautions will also be necessary. Lastly, a fully-equipped resuscitation trolley with emergency airway equipment, drugs required for advanced life support, and an external defibrillator should be mandatory in the event of a severe adverse reaction resulting in significant cardio-respiratory compromise. This should already be available, especially in laboratories where stress echocardiograms are being performed.
3.2. Patient identification
The sonographer has a key central role in implementing the contrast protocol and maintaining an efficient workflow. They are at the first point of contact with the patient and therefore allow early identification of a technically difficult study where contrast enhancement will improve the diagnostic yield. Sonographers are then able to initiate some of the steps required leading up to the actual administration of the microbubble contrast agent.
The decision to proceed with contrast enhancement should remain at the discretion of the attending physician echocardiographer. The responsible physician may do this following review on a case-by-case basis or there may be pre-defined criteria agreed upon by medical staff and the laboratory director such that a timely and efficient sonographer-driven protocol can be implemented.48 As an example, the Mayo Clinic has a policy of starting with the apical views for 90–120 s to determine if endocardial border definition is adequate with standard harmonic imaging or, if contrast is required where the study is then undertaken using a contrast-specific imaging protocol.49 Early recognition of the need for contrast will streamline the whole process and can potentially allow the recommended 30 min monitoring requirement following contrast administration to occur while the remainder of the study is being performed.
3.3. Contrast administration
Once the indication has been established and screening for the absence of allergies or contraindications has been completed, informed consent from the patient must then be obtained. This may be in the form of verbal or written consent depending on the institutional policies.
Preparation of the different contrasts agent should be performed according to the product information inserts. Sonovue and Optison both require “activation” by hand agitation while the vials of Definity are agitated for 45 s in the VialMix® device. The vials are then “vented” with an 18–21 G needle and the contrast solution slowly drawn up with a second needle and syringe to minimize microbubble destruction. For resting transthoracic echocardiograms ideally a ≥20 G intravenous cannulae is inserted into a larger forearm or antecubital vein and a 3-way stopcock can be attached. Boluses of contrast agent can then be injected through the direct port in-line with the vein and saline flushes can then be given through the 90° sideport.50 Preferably, either no connector line or if needed, a short tubing can be used. An alternative site for cannulation will be required for treadmill exercise stress echocardiograms that will not interfere with the patient's movements.
Contrast can be administered via either bolus or infusion methods. The continuous infusion method offers the advantages of extending the duration of LVO, providing a more consistent or uniform contrast effect with reduction in duration of attenuation, and reducing the incidence of other artefacts (swirling or blooming).51,52 These benefits would be extremely useful for prolonged studies requiring multiple images, and during stress echocardiography and MCE with quantitative perfusion protocols.3 However, this approach requires the use of infusion pumps, a period of dose or rate titration to achieve the optimal contrast effect, and the constant need to manually agitate the contrast syringe. Given the time consuming nature (and cost implications) of such infusions the bolus approach is the most widely used for resting LVO and for improving EBD in rest and stress echocardiography.
The “diluted” bolus technique for LVO will be the focus of the remainder of this review.47,53 Essentially the contents of the vial of contrast (usually ∼1.3–1.5 mL) are withdrawn as previously described and diluted with sterile normal saline to 10 mL in total. The initial bolus should be in the order of 1–2 mL followed by a slow flush of 5–10 mL of normal saline to clear the line of any contrast agent. The flush is stopped when contrast appears in the right ventricular cavity during real-time imaging. Ideally, enough contrast solution should be injected to achieve a uniformly bright LV cavity with the darker appearing myocardium. Usually the contrast effect will persist for 15–30 s but this is dependent on the patient's heart rate and cardiac output. Repeat boluses are then administered in individualized titrated doses of 0.5–1.0 mL with adjusted flushes depending on the quality of contrast enhancement.
The bolus method is easy and practical and provides rapid LVO but is limited by the relatively short duration of contrast enhancement that requires repeated blousing. Therefore, some variation in contrast intensity and more frequent artefacts throughout the study would be not unexpected. However, these can be easily addressed with increasing experience and maintaining good communication between the injector and sonographer to optimize the timing of bolus delivery. This is especially critical during stress echocardiograms where pre-emptive contrast bolus injections are needed to minimize delay between optimal LVO and image acquisition at the various stages of stress.
3.4. Instrumentation for contrast LVO and practical tips for common pitfalls
Most commercial vendors of ultrasound equipment have contrast-specific presets on their echocardiography machines that have greatly simplified the necessary system settings. However, fine adjustment may still be required to optimally reduce microbubble destruction, maximize persistence and reduce artefacts while maintaining the highest possible resolution.
The basic default settings for contrast LVO should include:
-
-
harmonic imaging (maximize frequency for best temporal resolution at the expense of penetration),
-
-
reduced transmit power or mechanical index (MI, starting at 0.2–0.4 but can be decreased further to minimize microbubble destruction), and
-
-
increase receiver gains to improve contrast visualization.50,54
The compression and dynamic range should be adjusted (usually decreased) to amplify the “contrast” difference between the LV myocardium and blood pool. The image depth should be reduced to “focus” on the LV and the focal zone moved down to the level of the mitral annulus. The pre-contrast image would appear as a dark or black LV cavity with a faintly visible myocardium.
The depth can be decreased further and the focal zone placed at the level of the apex for focused imaging, where indicated. Image orientation may become an issue as a result of this. The usual anatomical landmarks such as the atrio-ventricular valves are difficult to visualize. Use of the ventricular cavities, the left atrium, or aortic root may be more helpful in this instance or transiently returning to standard harmonic imaging mode as an alternative (especially when switching between imaging planes). Starting with the apical four-chamber window is always best. Parasternal views can then be attempted after the apical views have been acquired but the LV may be shadowed acoustically by contrast within the right ventricle in the near-field. Optimal delivery of contrast (volume and rate) is essential and the starting point has been outlined above. Images should be acquired at peak cavity opacification (the images during the first-pass are very useful to obtain as near uniform opacification is often achieved before far-field attenuation occurs).
The main artefacts related to real-time 2D LVO imaging are a) attenuation, b) swirling and c) rib artefacts.50,53,55
Attenuation occurs when there is a high concentration of accumulated microbubbles in the apex that results in a significant amount of near-field backscatter and acoustic shadowing of the far-field structures. Waiting for the contrast to dilute and the attenuation to clear is a simple solution or as an alternative, transiently increasing the MI to induce some degree of microbubble destruction, can be used if image acquisition is time critical. To avoid this problem a slower rate or smaller amount of contrast bolus should be used for subsequent cycles. Swirling is the “opposite” effect where there is inadequate opacification of the LV cavity with the contrast agent. The principal reasons for this are inadequate amount of contrast injected, often in the setting of poor LV systolic function, or excessive amount of microbubble destruction. Increasing the dose or rate of contrast administration and the saline flush and/or further reducing the MI will mitigate this effect. Increasing the overall gains or adjusting the vertical and horizontal time-gain compensations can also improve a “dim” contrast effect. The lateral artifact on the apical four-chamber view results from adjacent ribs obstructing the transmission of ultrasound from the transducer in the lateral scan planes and thereby obscuring the lateral segments. Contrast opacification does not occur as insonating sound waves are required to resonate the microbubbles and produce the returning harmonic signals. Moving the probe footprint to adjust the image orientation will usually compensate for this artefact.
Finally, when microbubble contrast agents are used for enhancement of spectral Doppler signals, significant “blooming” can occur from the strong acoustic backscatter. Only small concentrations of circulating contrast agent are required for this purpose and a similar approach of timely waiting for spontaneous dilution and dissipation or reducing the Doppler gain can help minimize this problem.
4. Safety and contraindications
There is no debate that the use of microbubble ultrasound contrast in echocardiography is safe and beneficial where indicated. Chronologically, post-marketing reports of four deaths and nearly two hundred other serious cardiopulmonary reactions during or within 30 min of the administration of Definity® contrast resulted in the revision of labelling and a black box warning issued by the Food and Drug Administration (FDA) released in October 2007.56 Perflutren-based agents were contraindicated for use in A) the setting of acute coronary syndromes, B) acute myocardial infarction and C) unstable heart failure.
Despite the temporal relationship, there was no clear causative effect established, and these fatalities were more likely related to the underlying disease states. Nonetheless, the recommendation was made to have patients undergo monitoring (vitals and electrocardiography) during and for 30 min after the contrast injection. This had significant time-efficiency implications in busy echocardiography laboratories. Following on from this, large retrospective studies and a large meta-analysis have repeatedly published the absence of any statistically significant difference in mortality between patients who underwent contrast-enhanced and non-contrast echocardiographic examinations despite the higher level of clinical acuity and co-morbidities in those patients requiring contrast administration.57–59 The same safety profile has been confirmed with stress echocardiography as well.60 In July 2008, the black box warning (for Definity® and Optison®) was revised and the 30 min monitoring requirement was limited to patients with pulmonary hypertension (no clear definition of severity provided) or unstable cardiopulmonary conditions.
Post-marketing surveillance of Definity® has estimated the risk of a serious adverse cardiopulmonary event to be in the order of 1 in 10,0000 – usually the result of an anaphylactoid reaction or a complement activation-related pseudoallergy.61 These can usually be managed with antihistamines, intravenous fluids, and/or intramuscular adrenaline (0.3 mg of 1:1000 dilution of adrenaline) depending on the severity of the reaction. Common, albeit infrequent (<2.1%) reported side effects from clinical trials have included headaches, flushing, back pain, nausea, vomiting, dizziness, chest pain, taste perversion and injection site reactions.16 These are often mild, self-limiting and may be managed symptomatically.
Current widely accepted contraindications are a known hypersensitivity to the contrast agent or any of its components (including blood products in the case of Optison®) and intra-cardiac shunts (right-to-left, bi-directional or transient right-to-left shunts) (Table 2). Other contraindications include pregnancy, lactation, severe pulmonary hypertension, and severe hepatic diseases. Intra-arterial or intra-coronary injections of contrast have been used during alcohol septal ablation procedures for hypertrophic cardiomyopathy but are not currently approved per se. Any adverse reaction should be documented in the study report for future reference and highlighted for serial studies.
Table 2.
Contraindications for echocardiographic contrast agents.
|
The US FDA 2008 revised labelling for microbubble contrast agents recommends a minimum 30-min period of monitoring post-contrast administration to patients with severe pulmonary hypertension or unstable cardiopulmonary conditions.
Intra-arterial injection remains listed as a contraindication but there are case reports of microbubble contrast agents being used to guide alcohol septal ablation procedures.
5. Cost-effectiveness
Owing to improved diagnostic power, accuracy and confidence, the use of microbubble contrast enhancement offers the potential of cost savings by reducing the need for further downstream testing and therefore also avoiding the risks associated with more invasive investigations. Shaw et al elegantly demonstrated the increased proportion of non-diagnostic studies in the non-contrast patients paralleled a 42% need for repeat confirmatory testing as compared with 12% when contrast was used (p < 0.0001).62 Although the upfront cost was higher with contrast use, there was a 17–70% reduction in further investigations, 2.7 fold higher diagnostic accuracy and a savings of $269 per patient. Along very similar lines, but in the stress echocardiography population, Thanigaraj and colleagues integrated the costs of additional nuclear testing for non-contrast non-diagnostic stress echocardiograms and found a further financial savings of $238 per patient (including the cost of the contrast agent).63 In the critically ill setting where patients are most likely to derive the greatest technical benefit from contrast use, the cost-effective ratio of contrast enhancement for the assessment of LVEF resulted in a $423 savings for every 1% increase in diagnostic accuracy per 100 patients.64
6. Conclusion
Microbubble contrast enhancement is an essential technique for any echocardiography laboratory in the modern era. Currently approved indications include LVO for delineation of LV structural abnormalities, improvement of EBD (during resting and stress echocardiography), and quantification of LVEF. Contrast enhancement has been demonstrated to improve the diagnostic accuracy, readers' confidence, and reproducibility for all of these categories. Contrast imaging protocols can be incorporated in a time-efficient manner into busy echocardiography laboratories and may have significant cost savings by reducing the number of non-diagnostic studies requiring further downstream testing. Microbubble contrast agents now have a proven safety track record and training in contrast-specific imaging techniques can be easily acquired by credentialed sonographers and accredited echocardiographers.
Conflicts of interest
All authors have none to declare.
References
- 1.Yu E.H., Sloggett C.E., Iwanochko M. Feasibility and accuracy or left ventricular volumes and ejection fraction determination by fundamental, tissue harmonic, and intravenous contrast imaging in difficult-to-image patients. J Am Soc Echocardiogr. 2000;13:216–224. doi: 10.1067/mje.2000.103597. [DOI] [PubMed] [Google Scholar]
- 2.Mulvagh S.L., Rakowski H., Vannan M.A. American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr. 2008;21:1179–1201. doi: 10.1016/j.echo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Senior R., Becher H., Monaghan M. Contrast echocardiography: evidence-based recommendations by European Association of Echocardiography. Eur J Echocardiogr. 2009;10:194–212. doi: 10.1093/ejechocard/jep005. [DOI] [PubMed] [Google Scholar]
- 4.Douglas P.S., Garcia M.J., Haines D.E. ACCF/ASE/AHA/ASCN/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr. 2011;24:229–267. [Google Scholar]
- 5.McCulloch M., Gresser C., Moos S. Ultrasound contrast physics: a series on contrast echocardiography, article 3. J Am Soc Echocardiogr. 2000;13:959–967. doi: 10.1067/mje.2000.107004. [DOI] [PubMed] [Google Scholar]
- 6.Raisinghani A., DeMaria A.N. Physical principles of microbubble ultrasound contrast agents. Am J Cardiol. 2002;90(suppl):3J–7J. doi: 10.1016/s0002-9149(02)02858-8. [DOI] [PubMed] [Google Scholar]
- 7.Mulvagh S.L., DeMaria A.N., Feinstein S.B. Contrast echocardiography: current and future applications. J Am Soc Echocardiogr. 2000;13:331–342. doi: 10.1067/mje.2000.105462. [DOI] [PubMed] [Google Scholar]
- 8.Allen M.R., Pellikka P.A., Villarraga H.R. Harmonic imaging: echocardiographic enhanced contrast intensity and duration. Int J Cardiovasc Imaging. 1999;15:215–220. doi: 10.1023/a:1006140102056. [DOI] [PubMed] [Google Scholar]
- 9.Pellikka P.A., Nagueh S.F., Elhendy A.A. American Society of Echocardiography recommendations for performance, interpretation and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Bovelli D., Plataniotis G., Roila F. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO clinical practice guidelines. Ann Oncol. 2010;21:v277–v282. doi: 10.1093/annonc/mdq200. [DOI] [PubMed] [Google Scholar]
- 11.Hundley G., Kizilbash A.M., Afridi I. Administration of an intravenous perfluorocarbon contrast agent improves echocardiographic determination of left ventricular volumes and ejection fraction: comparison with cine magnetic resonance imaging. J Am Coll Cardiol. 1998;32:1426–1432. doi: 10.1016/s0735-1097(98)00409-4. [DOI] [PubMed] [Google Scholar]
- 12.Nahar T., Croft L., Shapiro R. Comparison of four echocardiographic techniques for measuring left ventricular ejection fraction. Am J Cardiol. 2000;86:1358–1362. doi: 10.1016/s0002-9149(00)01243-1. [DOI] [PubMed] [Google Scholar]
- 13.Dias B.F., Yu E.H., Sloggett C.E. Contrast-enhanced quantification of left ventricular ejection fraction: what is the best method? J Am Soc Echocardiogr. 2001;14:1183–1190. doi: 10.1067/mje.2001.115981. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann R., Von Bardeleben S., Cate F. Assessment of systolic left ventricular function: a multi–center comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhance echocardiography. Eur Heart J. 2005;26:607–616. doi: 10.1093/eurheartj/ehi083. [DOI] [PubMed] [Google Scholar]
- 15.Lim T.K., Burden L., Janardhanan R. Improved accuracy of low-power contrast echocardiography for the assessment of left ventricular remodelling compared with unenhanced harmonic echocardiography after acute myocardial infarction: comparison with cardiovascular magnetic resonance imaging. J Am Soc Echocardiogr. 2005;18:1203–1207. doi: 10.1016/j.echo.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia V.J., Senior R. Contrast echocardiography: evidence for clinical use. J Am Soc Echocardiogr. 2008;21:409–416. doi: 10.1016/j.echo.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Freeman A.P., Giles R.W., Walsh W.F. Regional left ventricular wall motion assessment: comparison of two-dimensional echocardiography and radionuclide angiography with contrast angiography in healed myocardial infarction. Am J Cardiol. 1985;56:8–12. doi: 10.1016/0002-9149(85)90556-9. [DOI] [PubMed] [Google Scholar]
- 18.Grayburn P.A., Mulvagh Crouse L. Left ventricular opacification at rest and during stress. Am J Cardiol. 2002;90(suppl):21J–27J. doi: 10.1016/s0002-9149(02)02862-x. [DOI] [PubMed] [Google Scholar]
- 19.Crouse L.J., Cheirif J., Hanly D.E. Opacification and border delineation improvement in patients with suboptimal endocardial border definition in routine echocardiography: results of the phase III Albunex multicenter trial. J Am Coll Cardiol. 1993;22:1494–1500. doi: 10.1016/0735-1097(93)90562-f. [DOI] [PubMed] [Google Scholar]
- 20.Lindner J.R., Dent J.M., Moos S.P. Enhancement of left ventricular cavity opacification by harmonic imaging after venous injection of Albunex. Am J Cardiol. 1997;79:1657–1662. doi: 10.1016/s0002-9149(97)00217-8. [DOI] [PubMed] [Google Scholar]
- 21.Daniel G.K., Chawla M.K., Sawada S.G. Echocardiographic imaging of technically difficult patients in the intensive care unit: use of Optison I combination with fundamental and harmonic imaging. J Am Soc Echocardiogr. 2001;14:917–920. doi: 10.1067/mje.2001.113003. [DOI] [PubMed] [Google Scholar]
- 22.Sorrell V.L., Ross W.D., Kumr S. Left ventricular endocardial and epicardial border length delineation with perflutren contrast during transthoracic echocardiography. Echocardiography. 2011;28:761–766. doi: 10.1111/j.1540-8175.2011.01420.x. [DOI] [PubMed] [Google Scholar]
- 23.Kitzman D.W., Goldman M.E., Gillam L.D. Efficacy and safety of the novel ultrasound contrast agent perflutren (Definity) in patients with suboptimal baseline left ventricular echocardiographic images. Am J Cardiol. 2000;86:669–674. doi: 10.1016/s0002-9149(00)01050-x. [DOI] [PubMed] [Google Scholar]
- 24.Reilly J.P., Tunick P.A., Timmermans R.J. Contrast echocardiography clarifies uninterpretable wall motion in intensive care unit patients. J Am Coll Cardiol. 2000;35:485–490. doi: 10.1016/s0735-1097(99)00558-6. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen T.T., Dhond M.R., Sabapathy R. Contrast microbubbles improve diagnostic yield in ICU patients with poor echocardiographic windows. Chest. 2001;120:1287–1292. doi: 10.1378/chest.120.4.1287. [DOI] [PubMed] [Google Scholar]
- 26.Costa J.M., Tsutsui J.M., Nozawa E. Contrast echocardiography can save nondiagnostic exams in mechanically ventilated patients. Echocardiography. 2005;22:389–394. doi: 10.1111/j.1540-8175.2005.03176.x. [DOI] [PubMed] [Google Scholar]
- 27.Marwick T.H., Nemec J.J., Pashkow F.J. Accuracy and limitations of exercise echocardiography in a routine clinical setting. J Am Coll Cardiol. 1992;19:74–81. doi: 10.1016/0735-1097(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann R., Lethen H., Marwick T.H. Analysis of interinstitutional observer agreement in interpretation of dobutamine stress echocardiograms. J Am Coll Cardiol. 1996;27:330–336. doi: 10.1016/0735-1097(95)00483-1. [DOI] [PubMed] [Google Scholar]
- 29.Porter T.R., Xie F., Kricsfeld A. Improved endocardial border resolution during dobutamine stress echocardiography with intravenous sonicated dextrose albumin. J Am Coll Cardiol. 1994;23:1440–1443. doi: 10.1016/0735-1097(94)90389-1. [DOI] [PubMed] [Google Scholar]
- 30.Falcone R.A., Marcovitz P.A., Perez J.E. Intravenous albunex during dobutamine stress echocardiography: enhanced localization of left ventricular endocardial borders. Am Heart J. 1995;130:254–258. doi: 10.1016/0002-8703(95)90437-9. [DOI] [PubMed] [Google Scholar]
- 31.Rainbird A.J., Mulvagh S.L., Oh J.K. Contrast dobutamine stress echocardiography: clinical practice assessment in 300 consecutive patients. J Am Soc Echocardiogr. 2001;14:378–385. doi: 10.1067/mje.2001.111264. [DOI] [PubMed] [Google Scholar]
- 32.Dolan M.S., Riad K., El-Shafei A. Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients. Am Heart J. 2001;142:908–915. doi: 10.1067/mhj.2001.117608. [DOI] [PubMed] [Google Scholar]
- 33.Senior R., Dwivedi G., Hayat S. Clinical benefits of contrast-enhanced echocardiography during rest and stress examination. Eur J Echocardiogr. 2005;6:S6–S13. doi: 10.1016/s1525-2167(05)80723-1. [DOI] [PubMed] [Google Scholar]
- 34.Thanigaraj S., Schechtman K.B., Perez J.E. Improved echocardiographic delineation of left ventricular thrombus with the use of intravenous second-generation contrast image enhancement. J Am Soc Echocardiogr. 1999;12:1022–1026. doi: 10.1016/s0894-7317(99)70097-0. [DOI] [PubMed] [Google Scholar]
- 35.Soman P., Swinburn J., Callister M. Apical hypertrophic cardiomyopathy: bedside diagnosis by intravenous contrast echocardiography. J Am Soc Echocardiogr. 2001;14:311–313. doi: 10.1067/mje.2001.108475. [DOI] [PubMed] [Google Scholar]
- 36.Thanigaraj S., Perez J.E. Apical hypertrophic cardiomyopathy: echocardiographic diagnosis with the use of intravenous contrast image enhancement. J Am Soc Echocardiogr. 2000;3:146–149. doi: 10.1016/s0894-7317(00)90026-9. [DOI] [PubMed] [Google Scholar]
- 37.Gersch B.J., Maron B.J., Bonow R.O. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in collaboration with the American Association for Thoracic Surgery, American Society of echocardiography, American Society of nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212–e260. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Thanigaraj S., Perez J.E. Diagnosis of cardiac rupture with the use of contrast-enhanced echocardiography. J Am Soc Echocardiogr. 2000;13:862–865. doi: 10.1067/mje.2000.107005. [DOI] [PubMed] [Google Scholar]
- 39.Moreno R., Zamorano L., Rodrigo J.L. Usefulness of contrast agents in the diagnosis of left ventricular pseudoaneurysm after acute myocardial infarction. Eur J Echocardiogr. 2002;3:111–116. doi: 10.1053/euje.2001.0130. [DOI] [PubMed] [Google Scholar]
- 40.Mittle S., Makaryus A.N., Mangion J. Role of contrast echocardiography in the assessment of myocardial rupture. Echocardiography. 2003;20:77–81. doi: 10.1046/j.1540-8175.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 41.Byrd B.F., O’Kelly B.F., Schiller N.B. Contrast echocardiography enhances tricuspid but not mitral regurgitation. Clin Cardiol. 1991;14:v10–v14. doi: 10.1002/clc.4960141703. [DOI] [PubMed] [Google Scholar]
- 42.Wei K., Jayaweera A.R., Firoozan S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 43.Dijkmans P.A., Senior R., Becher H. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid and safe assessment of myocardial perfusion – the evidence so far. J Am Coll Cardiol. 2006;48:2168–2177. doi: 10.1016/j.jacc.2006.05.079. [DOI] [PubMed] [Google Scholar]
- 44.Senior R., Monaghan M., Main M.L. Detection of coronary artery disease with perfusion stress echocardiography using a novel ultrasound imaging agent: two phase 3 international trials in comparison with radionuclide imaging. Eur J Echocardiogr. 2009;10:26–35. doi: 10.1093/ejechocard/jen321. [DOI] [PubMed] [Google Scholar]
- 45.Waggoner A.D., Ehler D., Adams D. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: recommendations of the American Society of Echocardiography Council on Cardiac Sonography. J Am Soc Echocardiogr. 2001;14:417–420. doi: 10.1067/mje.2001.113817. [DOI] [PubMed] [Google Scholar]
- 46.Moos S., Odabashian J., Jasper S. Incorporating ultrasound contrast in the laboratory: a series on contrast echocardiography, article 1. J Am Soc Echocardiogr. 2000;13:240–247. doi: 10.1067/mje.2000.103599. [DOI] [PubMed] [Google Scholar]
- 47.Burgess P., Moore V., Bednorz J. Performing an echocardiographic examination with a contrast agent: a series on contrast echocardiography. Article 2. J Am Soc Echocardiogr. 2000;13:629–636. doi: 10.1067/mje.2000.105089. [DOI] [PubMed] [Google Scholar]
- 48.Castillo R., Bella J.N., Rover A. Efficacy and time-efficiency of a “sonographer-driven” contrast echocardiography protocol in a high-volume echocardiography laboratory. Am Heart J. 2003;145:535–541. doi: 10.1067/mhj.2003.164. [DOI] [PubMed] [Google Scholar]
- 49.Lester S.J., Miller F.A., Khandesi B.K. Contrast echocardiography: beyond a black box warning? J Am Soc Echocardiogr. 2008;21:417–418. doi: 10.1016/j.echo.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Witt S.A., McCulloch M., Sisk E. Achieving a diagnostic contrast-enhanced echocardiogram: a series on contrast echocardiography, article 4. J Am Soc Echocardiogr. 2001;14:327–334. doi: 10.1067/mje.2001.112242. [DOI] [PubMed] [Google Scholar]
- 51.Weismann N.J., Cohen M.C., Hack T.C. Infusion versus bolus contrast echocardiography: a multicenter, open-label, crossover trial. Am Heart J. 2000;139:399–404. doi: 10.1016/s0002-8703(00)90082-6. [DOI] [PubMed] [Google Scholar]
- 52.Kerstin B., Nahar T., Vannan M.A. Methods of contrast administration for myocardial perfusion imaging: continuous infusion versus bolus injection. Am J Cardiol. 2002;90(suppl):35J–37J. doi: 10.1016/s0002-9149(02)02946-6. [DOI] [PubMed] [Google Scholar]
- 53.Witt S. Implementing microbubble contrast in the echocardiography laboratory: a monographer’s perspective. Am J Cardiol. 2002;90:15J–16J. doi: 10.1016/s0002-9149(02)02860-6. [DOI] [PubMed] [Google Scholar]
- 54.St Michaels' Hospital DEFINITY® LVO protocol. 4 easy steps for usage.
- 55.Stewart M.J. Contrast echocardiography. Heart. 2003;89:342–348. doi: 10.1136/heart.89.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Main M.L., Goldman J.H., Grayburn P.A. Thinking outside the “box” – the ultrasound contrast controversy. J Am Coll Cardiol. 2007;50:2434–2437. doi: 10.1016/j.jacc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Kusnetzky L.L., Khalid A., Khumri T.M. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast. J Am Coll Cardiol. 2008;51:1702–1706. doi: 10.1016/j.jacc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Wei K., Mulvagh S.L., Carson L. Safety of Definity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr. 2008;21:1202–1206. doi: 10.1016/j.echo.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 59.Khajawa O.A., Shaikh K.A., Al-Mallah M.H. Meta-analysis of adverse cardiovascular events associated with echocardiographic contrast agents. Am J Cardiol. 2010;106:742–747. doi: 10.1016/j.amjcard.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 60.Timperley J., Mitchell A.R., Thibault H. Safety of contrast dobutamine stress echocardiography: a single center experience. J Am Soc Echocardiogr. 2005;18:163–167. doi: 10.1016/j.echo.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Douglas P.S., Weyman A.E., Lindner J.T. Contrast echocardiography: past, present, and future? J Am Coll Cardiol Img. 2008;1:107–110. doi: 10.1016/j.jcmg.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Shaw L.J., Gillam L., Feinstein S. Use of an intravenous contrast agent (Optison) to enhance echocardiography: efficacy and cost implications (Optison Multicenter Study Group) Am J Manag Care. 1998;4:SP169–SP176. [PubMed] [Google Scholar]
- 63.Thanigaraj S., Nease R., Schechtman K.B. Use of contrast for image enhancement during stress echocardiography is cost-effective and reduces additional diagnostic testing. Am J Cardiol. 2001;87:1430–1432. doi: 10.1016/s0002-9149(01)01573-9. [DOI] [PubMed] [Google Scholar]
- 64.Yong Y., Wu D., Fernandes V. Diagnostic accuracy and cost-effectiveness of contrast echocardiography on evaluation of cardiac function in technically very difficult patients in the intensive care unit. Am J Cardiol. 2002;89:711–718. doi: 10.1016/s0002-9149(01)02344-x. [DOI] [PubMed] [Google Scholar]


