Abstract
Introduction
The paclitaxel-coated balloon catheter (DCB) based on the PACCOCATH® technology has yielded angiographic and clinical results superior to drug-eluting stents (DES) in situations like in-stent restenosis (ISR) and a trend towards superior results in small coronary vessels and side branches of coronary bifurcations. Using the DCB followed by cobalt–chromium stent (CoCr) deployment or with a reverse sequence may yield different outcomes in terms of late loss.
Methods
97 patients with de-novo coronary stenosis (55.6 ± 10.7 years, 79.4% male, ≥70%, length: ≤25 mm, vessel diameter: 2.5–4.0 mm) were randomly treated with the DCB (3 μg/mm²) followed by a CoCr-stent or stent first and DCB later. Six-month angiographic and one-year clinical follow-up intention-to-treat analyses were performed.
Results
Angiographic and demographic baseline data was comparable between the two groups. When comparing balloon first versus stent first technique, the primary outcome variables were not statistically different for mean in-segment (0.51 ± 0.56 mm vs. 0.36 ± 0.55 mm, p = 0.23) and in-stent (0.52 ± 0.55 mm vs. 0.46 ± 0.52 mm, p = 0.65) late lumen loss. The lesion related 12-month MACE rates were 5/49 (10.2%) and 2/48 (4.2%) (p = 0.44). Lesion related thrombotic events occurred in three patients in balloon first and in one patient in stent first group, two of which were associated with early discontinuation of continuous dual anti-platelet therapy, two with suboptimal PCI, and one each were performed in a thrombotic lesion and a bifurcation type 1.1.0.
Conclusion
Drug-coated balloon first followed by cobalt chromium stent deployment versus a reverse sequence is not associated with statistically significantly different 6-month angiographic or 12-month clinical outcomes.
Keywords: Drug coated balloon, Paclitaxel, Bail out stenting
1. Introduction
The paclitaxel-coated balloon catheter (DCB) based on the PACCOCATH® technology has yielded angiographic and clinical results superior to drug-eluting stents (DES) in in-stent restenosis (ISR) up to 5 years after the procedure1–4 and trended superior in small vessel coronary artery disease5 and in the side branches of coronary bifurcations.6 Pathophysiologically, these positive results may be explained by delivery of the antiproliferative agent along the entire treated segment as opposed to only 15% when deploying a DES.7,8 Hence, addressing recoil and neointimal growth as causes of restenosis by providing the mechanical stabilization through placing a new generation bare-metal stent and reducing neointimal proliferation by delivering the anti-neoplastic agent paclitaxel by means of a DCB catheter might combine the advantages of both devices. In bail-out situations of the PEPCAD I trial, however, the rate of major adverse cardiac events (MACE) was less favorable.5 Theoretically, the sequence in which the devices are used may affect the angiographic and clinical outcome. Consequently, it was investigated if either dilating the lesion with the DCB first followed by cobalt–chromium stent (CoCr) deployment or by reversing the sequence would be associated with different results in native coronary lesions. Six-month angiographic and one-year clinical follow-up is reported in this paper.
2. Methods
The study is a randomized, non-blinded, multi centric study conducted at 7 Indian cardiology centers. The study was sponsored by B. Braun Melsungen AG, Vascular Systems, Berlin, Germany, the manufacturer of the drug-coated balloon catheter. An independent Clinical Research Organization and core lab (Clinical Research Institute, Center of Cardiovascular Diseases, Rotenburg an der Fulda, Germany) took responsibility for the Quantitative coronary analysis and compilation of the data.
The study was performed according to the declaration of Helsinki, World Health Organization and ICMR guidelines. The protocol was approved by the ethics committees of all the participating centers. Patients provided written informed consent prior to enrolment.
Eligible patients were at least 18-year-old, had clinical evidence of stable (CCS class 1–3) or unstable angina (Braunwald class 1–2, A–C) or objective evidence of ischemia, and exhibited at least one stenosis in one native coronary artery or two in two distinct coronary arteries. Exclusion criteria comprised factors such as acute myocardial infarction within the preceding 48 hours; severe renal insufficiency (GFR <30 ml/min); hypersensitivity or contraindication to three months anti-platelet agents; or malignancies with a life expectancy of less than 3 years. Angiographic inclusion criteria encompassed lesions from 10 to 25 mm (inclusive) in length, vessel diameters from 2.5 to 4.0 mm (inclusive), and stenoses from 70% to less than 100% of the reference lumen diameter. Exclusion criteria encompassed unprotected left main stenosis, bifurcations, and lesions with an originating major side branch of more than 2 mm in diameter.
2.1. Study devices
The drug-coated coronary angioplasty balloon catheter (SeQuent® Please, B. Braun Melsungen AG, Vascular Systems, Berlin, Germany) is covered with 3 μg paclitaxel/mm2 of balloon surface area using iopromide as the hydrophilic spacer. The balloons used were 14 to 30 mm (inclusive) long with diameters ranging from 2.5 to 4.0 mm (inclusive). Drug release is more than 90% upon single balloon inflation.9
The cobalt–chromium L605 stent (Coroflex® Blue, B. Braun Melsungen AG, Vascular Systems, Berlin, Germany) is based on the SeQuent® Rapid PTCA Catheter Exchange Technology and features thin struts of 65 μm (0.0025"), a crossing profile of 0.84 mm (0.033"), and a balloon overhang of <0.5 mm (0.019") on each side of the stent.
2.2. Interventional procedure
Percutaneous coronary interventions were performed through the femoral access. Patients were administered 350 mg of aspirin, heparin as an initial bolus of 70–200 IU/kg body weight adjusted according to the activated clotting time with a target of 200 to 250 sec, and obtained a loading dose of 300 mg of clopidogrel the day prior to the procedure or 600 mg immediately before the intervention. Glycoprotein IIb/IIIa antagonists were administered at the operator's discretion. Intracoronary injection of 100 to 200 μg nitroglycerin preceded baseline angiography of the target vessel in at least two near-orthogonal views to avoid foreshortening and vessel overlap. After assessment of the angiographic in- and exclusion criteria, each eligible patient was randomly assigned by envelope to treatment of the target lesion with either of the two treatment sequences.
Before using either of the drug-eluting devices, pre-dilation of the target lesion was optional by means of a conventional balloon of any brand. The recommended inflation time for the DCB was ≥30 sec.10 Post procedure, the vascular sheaths were removed according to usual hospital practice.
2.3. Quantitative coronary angiography
Angiography was performed before and after all interventions, at 6 months, and when clinically indicated using identical projections. Quantitative analysis of the images was performed by an independent core laboratory (Clinical Research Institute, Rotenburg/ Fulda, Germany) by two operators. The CAAS II system (Pie Medical, The Netherlands) served for automated contour detection and quantification with manual adjustment in obvious cases of machine error. Measurements included the stented area from shoulder to shoulder (in-stent) and the total treated area plus 5 mm on either side (in-segment). Restenosis was defined as a diameter stenosis of ≥50%.
2.4. Follow-up and endpoints
All patients received ≥100 mg aspirin daily scheduled for life. Clopidogrel (75 mg/day) was administered for a minimum of three months. Patients were subject to clinical observation for 3 years following the index procedure. All endpoints and adverse events were adjudicated by an independent clinical events committee.
In-segment late lumen loss, the difference between the minimal lumen diameter after the procedure and at six months as evaluated by quantitative coronary angiography, was the primary endpoint. Secondary endpoints encompassed the rate of restenosis and the rate of the combined clinical events up to 3 years, including stent thrombosis, target lesion revascularization, myocardial infarction, and death. Stent thrombosis was defined according to ARC.11 Target-lesion revascularization was defined as percutaneous reintervention or coronary artery bypass grafting of the restenotic target lesion. The decision to perform revascularization was based on symptoms and angiographic findings at follow-up. Occurrence of myocardial infarction was assumed if at least two of the following five criteria were present: chest pain lasting longer than 30 minutes; electrocardiogram (ECG) diagnostic of acute myocardial infarction (ST-segment elevation of ≥0.1 mV in at least two adjacent ECG leads or the new occurrence of a complete left bundle-branch block); increase in the level of creatine kinase or its MB isoform of at least three times the upper normal limit; new, clinically significant Q-waves; and chest pain necessitating angiography up to 6 hours after onset of the pain with angiographic evidence of an occluded vessel. Serious adverse events were defined according to international (ICH) guidelines.11
2.5. Statistical analysis
Owing to the lack of even anecdotal predicate data no sample size estimation could be performed. In line with studies investigating new indications with this DCB, 125 patients were planned to have been enrolled in this pilot study during a period of 6 months starting August 2008.2,5,12,13 Since a definite endpoint of enrolment was set, only 97 patients could be enrolled till September 2011.
Data were analyzed as per intention-to-treat. Normally distributed continuous variables are expressed as mean ± standard deviation. Categorical variables were compared with the Fisher's exact test, continuous variables with the two-sided Student's t-test or the Welch's test for unequal variances. Confidence intervals for the difference between proportions were calculated with a normal approximation of the binomial distribution with correction for continuity (PASW Statistics 18 and BiAS 9.06). Event-free survival was compared by Kaplan–Meier analysis with the Mantel–Cox log-rank test constructed by SPSS software, version 15.0. p-values of <0.05 were considered statistically significant.
3. Results
3.1. Patients
Ninety-seven patients (55.6 ± 10.7 years, 79.4% male) were enrolled in the study between August 2008 and March 2010. Forty-nine (50.5%) patients were randomly assigned to treatment with the DCB first, while in 48 (49.5%) subjects, the CoCr-stent was deployed prior to using the DCB. Demographics, angiographic and other baseline characteristics of the patients were comparable between the two groups (Table 1).
Table 1.
Baseline clinical and angiographic data (intention-to-treat analysis).a
| Balloon first (n = 49) | Stent first (n = 48) | p | |
|---|---|---|---|
| Coronary risk factors | |||
| Age | 54 ± 11.1 years | 57.3 ± 10.1 years | 0.14 |
| Male gender | 42 (85.7%) | 35 (72.9%) | 0.19 |
| Diabetes mellitus | 14 (28.6%) | 14 (29.2%) | 0.84 |
| Hyperlipidemia | 8 (16.3%) | 6 (12.5%) | 0.62 |
| Smoking | |||
| Current | 15 (30.6%) | 15 (23.1%) | 0.10 |
| Previous | 8 (16.3%) | 9 (18.8%) | |
| Never | 25 (51%) | 31 (64.6%) | |
| Hypertension | 30 (61.2%) | 26 (54.2%) | 0.64 |
| Body mass index | 25.35 ± 3.5 kg/m2 | 25.7 ± 4.8 kg/m2 | 0.67 |
| Family history of coronary artery disease | 11 (22.4%) | 9 (18.8%) | 0.8 |
| Unstable angina | 16 (32.7%) | 17 (35.4%) | 0.77 |
| Coronary artery disease | |||
| Single-vessel disease | 39 (79.6%) | 38 (79.2%) | 1.0 |
| Two-vessel disease | 9 (18.4%) | 9 (18.8%) | |
| Three-vessel disease | 1 (2%) | 1 (2.1%) | |
| Vessel with target lesion | |||
| LAD | 24 (41.4%) | 20 (35.7%) | 0.79 |
| LCx | 14 (24.1%) | 16 (28.6%) | |
| RCA | 20 (34.5%) | 20 (35.7%) | |
| Classification of 1st/2nd stenosis | 49/9 | 48/8 | 0.35/0.20 |
| A | 2 (4.1%)/0 | 5 (10.4%)/1 (12.5%) | |
| B1 | 29 (59.2%)/7 (77.8%) | 23 (47.9%)/3 (37.5%) | |
| B2 | 18 (36.7%)/2 (22.2%) | 20 (41.7%)/4 (50.0%) | |
| Classification of 1st and 2nd stenoses combinedb | 58 | 56 | 0.14 |
| A | 2 (3.4%) | 6 (10.7%) | |
| B1 | 36 (62.1%) | 26 (46.4%) | |
| B2 | 20 (32.8%) | 24 (42.9%) | |
All values are mean ± standard deviation or n(%). CAD, coronary artery disease; RCA, right coronary artery; LCx, left circumflex coronary artery; LAD, left anterior descending coronary artery.
ACC/AHA task force.
3.2. Angioplasty
The procedural success rates were 100% throughout the study with the DCB crossing all lesions in both groups. The inflation pressures of the Co-Cr stent were significantly higher (p = 0.02) in the balloon first (13.5 ± 2.1 bars) compared to the stent first (12.3 ± 3.1 bars) group after a longer balloon had been used for pre-dilation in the balloon first group (13.2 ± 2.3 mm vs 11.9 ± 2.2 mm, p = 0.047). The remaining procedural parameters were statistically not different between the two treatment sequences (Table 2).
Table 2.
Procedural data, angiographic findings at intervention and 6-month angiographic and clinical follow-up (intention-to-treat analysis).a
| Balloon first (n = 58 lesions) | Stent first (n = 56 lesions) | Difference (95% CI) | p | |
|---|---|---|---|---|
| Procedural data | ||||
| Drug-coated balloon | ||||
| Length | 22.5 ± 4.7 mm | 22.3 ± 5.4 mm | 0.19 (−1.71 to 2.08) | 0.85 |
| Diameter | 2.9 ± 0.38 mm | 3 ± 0.4 mm | 0.1 (−0.25 to 0.05) | 0.2 |
| Maximum inflation pressure | 9.67 ± 2.78 bar | 9.4 ± 2.7 bar | 0.26 (−0.78 to 1.29) | 0.62 |
| Duration of inflation | 35.6 ± 13.7 sec | 33.2 ± 11.7 sec | 2.41 (−2.55 to 7.36) | 0.34 |
| Cobalt-chromium stent | ||||
| Length | 18.5 ± 4.2 mm | 19.4 ± 4.9 mm | −0.95 (−2.69 to 0.77) | 0.28 |
| Diameter | 3 ± 0.4 mm | 3 ± 0.4 mm | −0.01 (−0.17 to 0.14) | 0.86 |
| Maximum inflation pressure | 13.5 ± 2.1 bar | 12.3 ± 3.1 bar | 1.18 (0.16 to 2.2) | 0.02 |
| Duration of inflation | 27 ± 18.1 sec | 23.4 ± 8.6 sec | 3.635 (−2.35 to 9.63) | 0.23 |
| Balloon for pre-dilation | ||||
| Length | 13.2 ± 3.2 mm | 11.9 ± 2.2 mm | 1.32 (0.02 to 2.62) | 0.047 |
| Diameter | 2.3 ± 0.5 mm | 2.3 ± 0.5 mm | 0.05 (−0.17 to 0.28) | 0.63 |
| Maximum inflation pressure | 10.5 ± 2.1 bar | 9.6 ± 3.1 bar | 0.89 (−0.44 to 2.2) | 0.19 |
| Duration of inflation | 23.2 ± 12.8 sec | 19.8 ± 7.8 sec | 0.34 (−2.8 to 9.7) | 0.27 |
| Additional stents | 1 (1.7%) | 2 (3.6%) | 0.02 (−0.10 to 0.06) | 0.98. |
| Lesion data | ||||
| Lesion length | 12 ± 4.3 mm | 12 ± 4.1 mm | −0.4 (−1.59 to 1.51) | 0.96 |
| Reference diameter | 2.8 ± 0.4 mm | 2.8 ± 0.4 mm | 0.02 (−0.14 to 0.17) | 0.84 |
| Diameter stenosis in-segment before intervention | 77.1 ± 9.4% | 75.2 ± 10.9% | 1.91 (−1.86 to 5.67) | 0.32 |
| Diameter stenosis in-segment post intervention | 16.2 ± 8.9% | 16.9 ± 8.5% | 1.63 (−3.96 to 2.49) | 0.65 |
| Minimal lumen diameter in-segment before intervention | 0.64 ± 0.29 mm | 0.7 ± 0.32 mm | −0.06 (−0.17 to 0.05) | 0.3 |
| Minimal lumen diameter in-segment post intervention | 2.41 ± 0.47 mm | 2.37 ± 0.46 mm | 0.04 (−1.13 to 0.21) | 0.63 |
| Angiographic 6-month follow-up | ||||
| 46 (79.3%) | 44 (78.6%) | 0.01 (−0.16 to 0.17) | 0.89 | |
| Time of angiographic follow-up | 6.8 ± 1.6 months | 6.6 ± 1.2 months | −0.17 (−0.54 to 0.88) | 0.64 |
| Minimal lumen diameter in-segment at follow-up | 1.93 ± 0.7 mm | 2.05 ± 0.6 mm | −0.12 (−0.41 to 0.16) | 0.39 |
| Diameter stenosis at follow-up | 32 ± 20% | 32.5 ± 16.8% | −0.43 (−7.63 to 8.48) | 0.92 |
| Late lumen loss | ||||
| In-segment | 0.51 ± 0.56 mm | 0.36 ± 0.56 mm | 0.15 (−0.1 to 0.39) | 0.23 |
| In-stent | 0.52 ± 0.55 mm | 0.46 ± 0.52 mm | 0.05 (−0.18 to −0.29) | 0.65 |
| Late lumen loss index | ||||
| In-segment | 0.29 ± 0.32 | 0.2 ± 0.38 | 0.09 (−0.06 to −0.25) | 0.22 |
| In-stent | 0.29 ± 0.31 | 0.27 ± 0.35 | 0.03 (−0.12 to −0.17) | 0.69 |
| Binary restenosis rate (including occlusion and thrombosis) | ||||
| In-segment | 8/58 (13.8%) | 6 (10.7%) | 0.03 (−0.11 to 0.17) | 0.78 |
| In-stent | 8 (13.8%) | 6 (10.7%) | 0.03 (−0.11 to 0.17 | 0.78 |
| Patterns of in-stent restenosisb | ||||
| I | 3/8 (37.5%) | 4/6 (66.7%) | 0.46 | |
| II | 1/8 (12.5%) | 0 (0%) | ||
| III | 0 (0%) | 0 (0%) | ||
| IV (including thrombus) | 4/8 (50.0%) | 2/6 (33.3%) | ||
All values are mean ± standard deviation or n (%). CI, confidence interval.
Patterns of in-stent restenosis in patients with repeated restenosis at follow-up angiography according to the Mehran classification.
Pre-dilation was performed in 30/49 (61.2%) patients in the DCB first and in 29/48 (60.4%) patients of the stent first group. A second bare metal stent was required in 2/48 (4.2%) patients of the stent first group and in 1/49 (2.0%) patients of the balloon first group.
3.3. Angiographic follow-up
Follow-up angiography after 6.7 ± 1.4 months in 74/97 (76.2%) patients and 90/114 (78.9%) lesions showed no statistical difference in the angiographic results, in particular in the primary outcome parameters of mean late lumen loss in-segment of 0.51 ± 0.56 mm (balloon first) vs 0.36 ± 0.55 mm (stent first) (p = 0.23) and in-stent of 0.52 ± 0.55 mm (balloon first) vs 0.46 ± 0.52 mm (stent first) (p = 0.65). The QCA findings at baseline after the procedure and at 6 months are shown in Fig. 1.
Fig. 1.
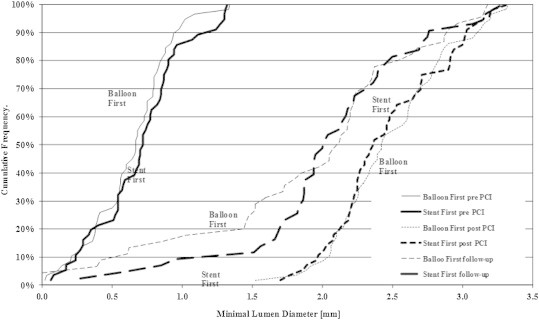
Angiographic patency: cumulative frequency distribution of in-segment minimal lumen diameters (MLD) determined by quantitative coronary angiography (n = 114 lesions in 97 patients). Balloon first vs stent first; pre-procedure (pre), post-procedure (post), and at six months (follow-up). Intention-to-treat analysis.
3.4. Clinical follow-up
After one year, for clinical follow-up 48/49 (98.0%) patients were available in the balloon first group and 44/48 (91.7%) in the stent first group (p = 0.2). In each group, the two deaths occurred within the first six months of follow-up. One patient in each group died of a non-cardiac cause. In the balloon first group, one patient died at home with no information available relative to the target lesion. The cardiac death was not related to the target lesion in the stent first group (Table 3).
Table 3.
One-year MACE rates.
| Balloon first | Stent first | p | |
|---|---|---|---|
| Count [n] | 49 (50.5%) | 48 (49.5%) | NA |
| Missing [n] | 1 (2.1%) | 4 (8.3%) | 0.20 |
| Deaths | |||
| Total | 2/49 (4.1%) | 2/48 (4.2%) | 1 |
| Cardiac | 1/49 (2.0%) | 1/48 (2.1%) | 1 |
| Lesion related | 0/49 (0%) | 0/48 (0%) | 1 |
| Non-lesion related | 0/49 (0%) | 1/48 (2.1%) | 1 |
| Unknown | 1/49 (2.0%) | 0/48 (0%) | 1 |
| Non-cardiac (no MACE) | 1/49 (2.0%) | 1/48 (2.1%) | 1 |
| Myocardial infarction | |||
| Total | 1/49 (2.0%) | 1/48 (2.1%) | 1 |
| CK-Elevation >3 times upper normal limit | 0/49 (0%) | 1/48 (2.1%) | 1 |
| MI other than target vessel | 1/49 (2.0%) | 0/48 (0%) | 1 |
| Stent thrombosis with MI target lesion related | 3/49 (6.1%) | 1/48 (2.1%) | 0.62 |
| PCI or CABG for in-segment stenosis >50% | 2/49 (4.1%)a | 1/48 (2.1%)a | 1 |
| Total MACE | 8/49 (16.3%) | 4/48 (8.4%) | 0.36 |
| Target lesion related MACE (cardiac death, myocardial infarction, revascularization) | 5/49 (10.2%) | 2/48 (4.2%) | 0.44 |
Each additional 4 patients with medical treatment for in-segment restenosis.
A total of 4 thromboses (all angiographically proven) occurred during the one-year follow-up, three in the balloon first and one in the stent first group (Table 4).
Table 4.
Procedural and follow-up information of patients with thrombotic events.
| Treatment group | Time from PCI [days] | Thrombotic risk factors | Information on index PCI | Treatment of thrombosis | Follow-up |
|---|---|---|---|---|---|
| Balloon first | 274 | DAPT discontinued, smoking resumed (20 cigarettes/day) | Stent undersized and underexpanded | Abciximab and POBA | At one year follow the patient was asymptomatic |
| Balloon first | 15 | Arterial hypertension, current smoker, irregular intake of DAPT | Balloon and stent both too short relative to lesion | POBA | New in-stent thrombosis after 4 months. No PCI performed |
| Balloon first | 1 | None | Small hazy spot in proximal lesion suggestive of a small thrombus | Abciximab, POBA, and BMS | No angiographic restenosis after 6 months |
| Stent first | 6 | None | Bifurcation 110*. Balloon lead to dissection type B. Stent undersized | POBA + DCB + BMS | No angiographic restenosis after 4 months |
BMS = bare metal stent, DAPT = dual anti-platelet therapy, DCB = drug-coated balloon POBA = plain old balloon angioplasty∗.24
The Kaplan–Meier estimates of survival free from clinical events during the 1-year follow-up did not exhibit a statistical difference between the two treatment sequences (Fig. 2).
Fig. 2.
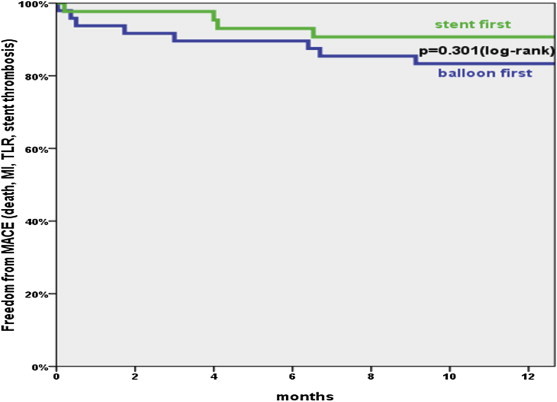
Freedom from stent thrombosis, target lesion revascularization, myocardial infarction, and death. Log rank (Mantel-Cox). Intention-to-treat analysis (n = 97 subjects with n = 114 lesions).
4. Discussion
The Paclitaxel DCB based on the PACCOCATH® technology has yielded angiographic and clinical results superior to DES in ISR up to five years after the procedure1–4 and trended superior in small vessel coronary artery disease5 and in the side branches of coronary bifurcations.6 Though centers on two continents with a large number of operators and a variety of indications participated in these studies the late lumen loss for DCB stand alone procedures was consistently below 0.2 mm, indicative of an easily handled device with reproducible and satisfactory outcomes.2,5,6,12,13 In some instances, however, a pronounced elastic recoil or vessel dissection, would necessitate deployment of a bare metal stent. In addition, theoretically, adding a bare metal stent might further reduce restenosis owing to the greater lumen gain post procedure.
The data of the current study seem to refute this hypothesis with the in-segment late lumen loss between 0.5 ± 0.56 mm (balloon first) and 0.36 ± 0.56 mm (stent first) (95% CI: 0.15 (−0.1 to 0.39), p = 0.23). Nonetheless, the difference of 0.14 mm raises concerns that with a larger number of subjects, the level of significance might have been reached. The difference for in-stent late lumen loss was much smaller (0.52 ± 0.55 mm vs 0.46 ± 0.52 mm, 95% CI 0.05 (−0.18 to −0.29), p = 0.65) suggesting that the weakest point may be the edges of the stent due to geographical mismatch.
The late lumen loss measured in both treatment sequences are within the range of those of paclitaxel-eluting stents.13–17 However, the in-segment late lumen losses in both treatment sequences are higher than all of those reported in DCB alone procedures. These are; however, considerably lower than in the bail-out situations of the PEPCAD I with 0.62 ± 0.73 mm.5 The difference in the late loss observed from the bailout situation may be due to the different pathology or an ethnic difference and is purely speculative.
The one-year MACE rates were not statistically different (balloon first 10.2%, stent first 4.2%, p = 0.44) between the two treatment sequences. After six months, the lesions appeared clinically stable with only one event occurring in the stent first sequence. However, when the balloon was used first, three thrombotic events occurred, one each within the first hour after the procedure, on day 15 and day 274, while with stent first one such event was documented on day 6. All of these procedures were associated with thrombotic risk factors such as discontinuation of dual anti-platelet therapy or procedural issues including under sizing of the stent (Table 4). The variability in the brands of clopidogrel used as compared to the brand used in the other studies of the PEPCAD program1,5,6,12,13 could have been a factor. Relatively high frequency of smoking in our subjects with thrombosis could also have contributed even in presence of aspirin.18 Ethnic factors of clopidogrel resistance may also have played a causal role as well.19
Aside from these events, target lesion revascularization (4.1% vs 2.1%), lesion related myocardial infarction (0 vs 2.1%) or death (0 vs 2.1%) were low and within the range of the 8%–17.8% reported for paclitaxel-eluting stents.13–17 It is widely accepted that delivery of the antiproliferative agent along the entire treated segment as opposed to only 15% when deploying a drug-eluting stent is one of several theoretical pathophysiologic mechanisms that influence the incidence of restenosis.7,8 There is ample evidence that stents increase the post procedural vascular diameter, the metal scaffolds instigate negative mechanisms that ultimately may outweigh their benefits. Such effects encompass the inherent effects of any stent such as increased vascular damage by the force to the vascular wall20 and sustained mechanical irritation21 and the impairment of vascular mobility.22 It may be hypothesized that when the DCB precedes stent deployment, compression of the vascular wall by the stent struts may increase the concentration of the antiproliferative drug to supposedly toxic levels, thus triggering an inflammatory reaction that may enhance neointimal proliferation and endothelial dysfunction. Moreover, when using the DCB first, chances for geographic mismatch, identified as a predictor for restenosis may be higher as opposed to when using the stent first since in the former the reference point for stent or balloon placement is missing.5 When stent deployment precedes DCB dilation then the contact surface between the balloon and the vessel wall is reduced by around 15% owing to the surface of the stent struts.
A larger study would be more meaningful to substantiate the findings of this pilot study. Until studies with intravascular ultrasound and the routine careful assessment of the quality of platelet function are performed, the possible causes of the thrombotic events will remain at best speculative. It may thus be appropriate to continue dual anti-platelet therapy for 1 year when a combination of DEB and stent is used like in the case of a DES.
In conclusion, this study using the DCB based on the PACCOCATH® technology before a CoCr bare metal stent or with a reversed sequence of stent first did not show a statistical difference neither in the 6-month angiographic nor the 12-month clinical outcome parameters in the treatment of native coronary artery stenosis. Both procedural sequences “stent first” and “balloon first” appear as safe and effective treatment modalities for native coronary artery stenosis. These results match favorably with those of paclitaxel-eluting stents.13–17 The German Consensus Group for “DEB-only” recommends using this DCB based on the PACCOCATH® technology when stenting needs to be avoided.23 The current study lends further support to this recommendation since the option of adding a bare metal stent still remains as a safe and effective option. However, under sizing and under expansion of the bare metal stents seem to be important contributing factors to stent thrombosis and, therefore, must be avoided.
5. Study limitations
-
1.
Due to slow recruitment of cases, only 97 patients could be recruited in the study though the original plan was to include 125 patients.
-
2.
The angiographic follow-up was possible in only 78% cases though the clinical follow-up was available in 95% patients.
Conflicts of interest
All authors have none to declare.
Acknowledgments
The authors are indebted to the tremendous logistic contributions of Mr. Alok Sharma and Mr. Indranil Mukherjee who were instrumental to the success of this study.
References
- 1.Scheller B., Clever Y.P., Kelsch B. Long-term follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. JACC Cardiovasc Interv. 2012;5:323–330. doi: 10.1016/j.jcin.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Scheller B., Hehrlein C., Bocksch W. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. NEJM. 2006;355:2113–2124. doi: 10.1056/NEJMoa061254. [DOI] [PubMed] [Google Scholar]
- 3.Scheller B., Hehrlein C., Bocksch W. Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2008;97:773–781. doi: 10.1007/s00392-008-0682-5. [DOI] [PubMed] [Google Scholar]
- 4.Unverdorben M., Degenhardt R., Vallbracht C. Paclitaxelcoated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis e the three-year clinical follow-up of the PEPCAD II ISR study. Euro Interv. 2013 Accepted for publication. [Google Scholar]
- 5.Unverdorben M., Kleber F.X., Heuer H. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2010;99:165–174. doi: 10.1007/s00392-009-0101-6. [DOI] [PubMed] [Google Scholar]
- 6.Mathey D. Treatment of bifurcation lesions with a drug-eluting balloon: the PEPCAD V (Paclitaxel Eluting PTCA Balloon in Coronary Artery Disease) trial. Eurointervention. 2011;7(suppl K):K61–K65. doi: 10.4244/EIJV7SKA11. [DOI] [PubMed] [Google Scholar]
- 7.Hwang C.W., Wu D., Edelman E.R. Physiological transport forces govern drug distribution for stent-based delivery. Circulation. 2001;104:600–605. doi: 10.1161/hc3101.092214. [DOI] [PubMed] [Google Scholar]
- 8.Scheller B., Speck U., Bohm M. Prevention of restenosis: is angioplasty the answer? Heart. 2007;93:539–541. doi: 10.1136/hrt.2007.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheller B. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation. 2004;110:810–814. doi: 10.1161/01.CIR.0000138929.71660.E0. [DOI] [PubMed] [Google Scholar]
- 10.Cremers B., Speck U., Kaufels N. Drug-eluting balloon: very short-term exposure and overlapping. Thromb Haemost. 2009;101:201–206. [PubMed] [Google Scholar]
- 11.Cutlip D., Windecker S., Mehran R. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 12.Ali R.M., Degenhardt R., Zambahari R. Paclitaxel-eluting balloon angioplasty and cobalt-chromium stents versus conventional angioplasty and paclitaxel-eluting stents in the treatment of native coronary artery stenoses in patients with diabetes mellitus. EuroIntervention. 2011;7(suppl K):K83–K92. doi: 10.4244/EIJV7SKA15. [DOI] [PubMed] [Google Scholar]
- 13.Unverdorben M., Vallbracht C., Cremers B. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. 2009;119:2986–2994. doi: 10.1161/CIRCULATIONAHA.108.839282. [DOI] [PubMed] [Google Scholar]
- 14.Stone G., Ellis S., Cox D. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. NEJM. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 15.Windecker S., Remondino A., Eberli F.R. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. NEJM. 2005;353:653–662. doi: 10.1056/NEJMoa051175. [DOI] [PubMed] [Google Scholar]
- 16.Unverdorben M., Degenhardt R., Vallbracht C. The paclitaxel-eluting Coroflex Please stent pilot study (PECOPS I): acute and 6-month clinical and angiographic follow-up. Catheter Cardiovasc Interv. 2006;67:703–710. doi: 10.1002/ccd.20731. [DOI] [PubMed] [Google Scholar]
- 17.Wiemer M., Degenhardt R., Vallbracht C. The paclitaxel-eluting Coroflex please stent study (PECOPS II): acute and 6-month clinical and angiographic follow-up. 1-year clinical follow-up. J Interven Cariol. 2010;23:160–166. doi: 10.1111/j.1540-8183.2010.00529.x. [DOI] [PubMed] [Google Scholar]
- 18.Hung J., Lam J.Y., Lacoste L., Letchacovski G. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation. 1995;92:2432–2436. doi: 10.1161/01.cir.92.9.2432. [DOI] [PubMed] [Google Scholar]
- 19.Leschke M., Nhan V.T., Waliszewski M. ‘All comer’ Coroflex Please drug-eluting stent registry in Europe and Asia – an overall and transcontinental assessment of the 10-month major adverse cardiac events. IHJ. 2012;64:453–461. doi: 10.1016/j.ihj.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunn J., Arnold N., Chan K.H., Shepherd L., Cumberland D.C., Crossman D.C. Coronary artery stretch versus deep injury in the development of in- stent neointima. Heart. 2002;88:401–405. doi: 10.1136/heart.88.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heublein B. Catheter-based cardiovascular therapy – the impact of stenting in coronary artery disease. Prog Biomed Res. 1999;4:1–5. [Google Scholar]
- 22.Plass C.A., Sabdyusheva-Litschauer I., Bernhart A. Time course of endothelium-dependent and -independent coronary vasomotor response to coronary balloons and stents: comparison of plain and drug-eluting balloons and stents. JACC Cardiovasc Interv. 2012;5:741–751. doi: 10.1016/j.jcin.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Kleber F.X., Mathey D.G., Rittger H., Scheller B. How to use the drug-eluting balloon: recommendations by the German consensus group. EuroIntervention. 2011;7(suppl K):K125–K128. doi: 10.4244/EIJV7SKA21. [DOI] [PubMed] [Google Scholar]
- 24.Louvard Y., Thomas M., Dzavik V. Classification of coronary artery bifurcation lesions and treatments: time for a consensus! Catheter Cardiovasc Interv. 2008;71:175–183. doi: 10.1002/ccd.21314. [DOI] [PubMed] [Google Scholar]


