Highlights
► GISTs do not have a unique appearance on ultrasound examination. ► If a pelvic mass is detected, the possibility of a non-gynecological tumor like GISTs has to be considered.
Keywords: Gastrointestinal stromal tumor, Adnexal mass, Ultrasonography, Magnetic resonance imaging
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal (GI) tract. They are most often located in the stomach and proximal small intestine (Rubin et al., 2000; Miettinen and Lasota, 2001; Lucas, 2011). The gross appearance of GISTs is highly variable. Tumors range from nodules measured in millimeters to massive tumors measuring over 40 cm (median: 6–7 cm). Texture varies from fibrous to fleshy to gelatinous to hemorrhagic and some tumors show extensive cystic change or necrosis. Some form highly lobulated masses. Most bulge outward from the muscularis propria (Lucas, 2011). Clinical presentations of GISTs are depending on its size and location. When tumors become large, they begin to exert mass effect leading to symptoms such as pain, nausea, early satiety, and GI bleeding. Lesions located at narrow regions of the gastrointestinal (GI) tract, such as the gastroesophageal junction or pylorus, may cause luminal obstruction at a relatively modest size (Nilsson et al., 2005).
Contrast-enhanced CT is the imaging method of choice to characterize an abdominal mass, evaluate its extent, and the presence or absence of metastatic disease. Oral as well as IV contrast should be administered to define the bowel margins. Although MRI has a comparable diagnostic yield (Scarpa et al., 2008) and lacks radiation exposure, CT is a preferred initial imaging study for screening and staging, except, perhaps in a patient who cannot receive intravenous contrast. CT is better at global evaluation of the abdomen, especially the hollow viscera, than MRI. MRI may be preferred for GISTs at specific sites, such as rectum or liver. For these fixed structures, MRI may provide needed better anatomic definition, especially in evaluating for surgery.
In gynecologic practice, physicians need to determine the type of uterine, adnexal, gastrointestinal, and urologic masses. After hysterectomy and oophorectomy, in some patients an ovarian remnant can be found as a source of pelvic mass. We present a case of GIST originating from the jejunum, which was preoperatively misdiagnosed as a pelvic mass with MRI and ultrasound during gynecologic evaluation.
Case report
A 53-year-old woman presented with upper abdominal pain. In her medical history, she had subtotal hysterectomy and bilateral salpingo-oophorectomy operation due to myoma uteri, and as a prophylactic oophorectomy. During abdominal ultrasonography for ruling out cholelithiasis, a pelvic mass was found. On vaginal examination, cervical stump was seen as normal and with bimanual examination an ovoid shaped mass was found. It was 8 × 5 cm in size as a mobile mass. Despite abdominal palpation during ultrasound examination, it was possible to separate the mass from the uterine stump. It was found as a well-vascularized mass on color Doppler ultrasonography. Color Doppler imaging revealed multiple arterial and venous blood vessels at medial side of the mass. There was no free fluid in the pelvis or the abdomen. Tumor markers such as CA 125, CA 19‐9, and CA 15‐3 were in normal range.
On magnetic resonance imaging (MRI), a well-defined, lobulated solid tumor with 3.5 × 5 × 5.3 cm in size was seen in the right adnexa, neighboring small intestines. There were small cystic areas within the tumor. The tumor showed diffusion restriction; early enhancement and wash-out on postcontrast dynamic series; these MRI features suggested malignancy. The tumor displaced small intestines posteriorly but there was no evident invasion. We could not identify the ovarian vessels related to the mass. No lymphadenopathy was detected (Fig. 1).
Fig. 1.
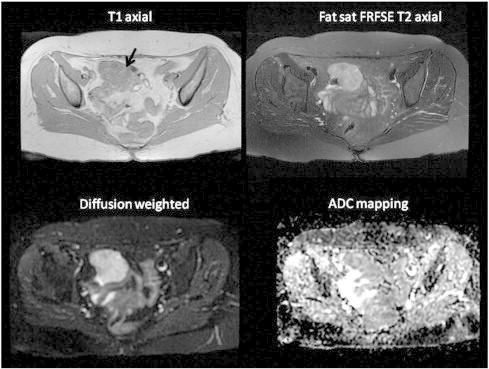
T1‐ and T2‐weighted axial MRI scans and diffusion weighted (B = 600), ADC mapping. A lobulated, well-defined tumor hypointense on T1‐weighted scan, hyperintense on T2‐weighted scan with diffusion restriction at the right side.
The patient underwent an exploratory laparotomy by gynecologists, there were no uterus and adnexa, and only cervical stump was there. After a thorough examination of the abdominal cavity, a subserosal tumor arising from the jejunum 100 cm away from the cecum was detected and resected with tumor free margins by a general surgeon (Fig. 2). The cervical stump was removed for to prevent cervical cancer formation.
Fig. 2.
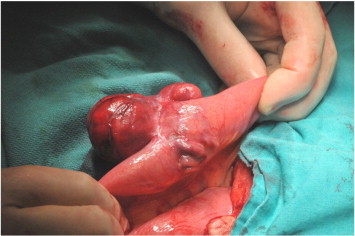
Representative image of tumoral mass arising from the wall of jejunum with increased vessel formation.
Frozen section was performed and histopathologic diagnosis was GIST. Macroscopically solid tumoral lesion measuring 8 × 5.5 × 4 cm attached to intestinal tissue measuring 2.2 × 1.8 cm was seen. Cross section of the tumor revealed focal hemorrhagic and cystic areas.
Microscopically tumor consisted of spindle cells forming sheets and fascicles. Mitotic activity was low (below 1/50 high power fields). Tumor cells showed diffuse positivity for CD117 and focal positivity for CD34 immunohistochemically. S-100 and smooth muscle actin were immunonegative (Fig. 3). The lesion was interpreted as being compatible with a gastrointestinal stromal tumor (GIST).
Fig. 3.
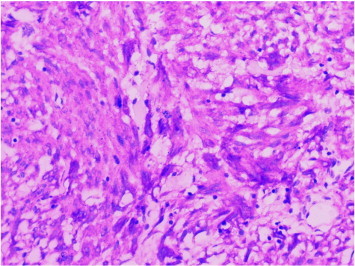
CD117 immunopositivity in tumor cells (× 400, CD117).
The patient made an uneventful postoperative recovery, being discharged from hospital 10 days after surgery and she was under the follow-up of our medical oncology service and administered with imitanib 400 mg/day.
Discussion
GISTs represent 0.1–1% of all gastrointestinal malignancies (Miettinen and Lasota, 2001). About two thirds of gastrointestinal stromal tumors occur in the stomach and about one fifth in the small intestine with few in the rectum, colon, and esophagus. Their cells are related to the interstitial cells of Cajal. They differ by site in terms of cell type and growth pattern. Benign and malignant tumors are separated based on their light microscopic appearances, size as measured by innumerable pathologists and assistants and mitotic counts (Appelman, 2011). GISTs may also be found outside the gastrointestinal tract in the omentum, mesentery, retroperitoneum, uterus and bladder, where they may present as a pelvic mass. Extra-abdominal locations are very rare (Pinto et al., 2007).
The preoperative diagnosis of GISTs is uncommon, due to their rarity and different modes of presentation as well as the lack of distinguishing characteristics on imaging studies (Hsu et al., 2006). In a review of literature by Pinto et al. (2007), only 10 patients with GISTs have been found in the gynecological literature. They reported that in five of these cases the tumor arose from the bowel, two from the stomach, a further two from the rectovaginal septum and in one case from the uterus. In no case was a correct preoperative diagnosis made of the type and origin of the tumor. Searching for the ‘sliding organ sign’ may be used to clarify the mobility of masses, distinguishing a possible mass confluent with the uterus or other pelvic organs or separate from it. Misdiagnoses may have significant therapeutic and prognostic implications because of the targeted imatinib-based therapy now available (Pinto et al., 2007).
On ultrasound imaging, GISTs exhibit a variety of features that lack a typical pattern. These include hyperechoic central areas due to myxoid degeneration or the formation of microcysts within the mass (Pinto et al., 2007). Our case has a heterogenic texture as confirmed by histopathologic examination due to cystic degeneration and hemorrhagic areas.
Belics et al. (2003) reported that the presence of an uterine related pelvic mass should raise the suspicion of GIST, given that mature cystic teratomas are the only ovarian neoplasms that occur relatively frequently in this location.
The recognition of the origin of the tumor is a very difficult for abdominal masses like our case. MRI has high soft-tissue contrast and is useful for the characterization of the tumor component, such as a neurogenic component, hemorrhage and necrosis. Contrast-enhanced MRI following gadolinium injection for better visualization may also be of value in recording the mitotic index, which in turn may reflect the malignant potential of GISTs (Amano et al., 2006).
In conclusion, GISTs may mimic gynecological pelvic masses like ovarian malignancies and uterine leiomyomas. Although GISTs do not have a unique appearance on ultrasound examination, if a pelvic mass is detected on ultrasound examination, especially if related to unusual clinical signs, the possibility of a non-gynecological tumor like GISTs related to small bowels has to be considered.
Conflict of interest statement
No author has any potential conflict of interest.
References
- Amano M., Okuda T., Amano Y., Tajiri T., Kumazaki T. Magnetic resonance imaging of gastrointestinal stromal tumor in the abdomen and pelvis. Clin. Imaging. 2006;30(2):127–131. doi: 10.1016/j.clinimag.2005.09.025. Mar–Apr. [DOI] [PubMed] [Google Scholar]
- Appelman H.D. Morphology of gastrointestinal stromal tumors: historical perspectives. J. Surg. Oncol. 2011;104(8):874–881. doi: 10.1002/jso.21873. Dec. [DOI] [PubMed] [Google Scholar]
- Belics Z., Csapó Z., Szabó I., Pápay J., Szabó J., Papp Z. Large gastrointestinal stromal tumor presenting as an ovarian tumor. A case report. J. Reprod. Med. 2003;48(8):655–658. Aug. [PubMed] [Google Scholar]
- Hsu S., Chen S.S., Chen Y.Z. Gastrointestinal stromal tumors presenting as gynecological tumors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006;125(1):139–140. doi: 10.1016/j.ejogrb.2005.07.027. Mar 1. [DOI] [PubMed] [Google Scholar]
- Lucas D.R. Clinicopathology and molecular diagnostics of gastrointestinal stromal tumors. Curr. Probl. Cancer. 2011;35(5):233–244. doi: 10.1016/j.currproblcancer.2011.09.002. Sep. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lasota J. Gastrointestinal stromal tumors—definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438(1):1–12. doi: 10.1007/s004280000338. Jan. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Bümming P., Meis-Kindblom J.M. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—a population-based study in western Sweden. Cancer. 2005;103(4):821–829. doi: 10.1002/cncr.20862. Feb 15. [DOI] [PubMed] [Google Scholar]
- Pinto V., Ingravallo G., Cicinelli E. Gastrointestinal stromal tumors mimicking gynecological masses on ultrasound: a report of two cases. Ultrasound Obstet. Gynecol. 2007;30(3):359–361. doi: 10.1002/uog.4097. Sep. [DOI] [PubMed] [Google Scholar]
- Rubin B.P., Fletcher J.A., Fletcher C.D. Molecular insights into the histogenesis and pathogenesis of gastrointestinal stromal tumors. Int. J. Surg. Pathol. 2000;8(1):5–10. doi: 10.1177/106689690000800105. Jan. [DOI] [PubMed] [Google Scholar]
- Scarpa M., Bertin M., Ruffolo C., Polese L., D'Amico D.F., Angriman I. A systematic review on the clinical diagnosis of gastrointestinal stromal tumors. J. Surg. Oncol. 2008;98(5):384–392. doi: 10.1002/jso.21120. Oct 1. [DOI] [PubMed] [Google Scholar]


