Abstract
Background
Endovascular management using angiographic embolization (AE) has been widely used with success as non operative management (NOM) in blunt hepatic trauma. We, in a tertiary care hospital in North of India, assess our use of endovascular management in patients of blunt and post operative trauma with active hepatic vascular bleeding and unstable hemodynamics in controlling bleeding.
Methods
A retrospective review of inpatients from January 2006 to July 2012 requiring transarterial embolization/stenting for active hepatic vascular bleeding was done. All patients had evidence of ongoing hemorrhage as proved by clinical, laboratory and radiological findings in emergency settings. Angiographic intervention in an interventional suite with ongoing resuscitation was performed following which patients were monitored for morbidity and mortality benefits on intermediate follow up.
Results
10 adults and 3 children underwent AE with polyvinyl alcohol particle (PVA)/soft metal coil whereas 1 adult underwent revascularization with a covered stent for arterial bleeding. The mean age of case series was 36.18 ± 20.90 years with a mean liver injury computed tomography (CT) grade of 3.8 ± 0.83 in blunt trauma patients. The mean length of hospital stay was 9.62 ± 7.83 days and the mean follow up period of the group was 25.25 ± 21.02 months. All patients showed significant clinical improvement with prompt endovascular management resulting in no procedure related mortality.
Conclusion
Prompt endovascular management is the modality of choice in comparison to NOM without AE in both pediatric and adult patients with hemodynamically compromised inaccessible intra hepatic vascular trauma.
Keywords: Trauma, Angiographic embolization, Hepatic artery
1. Introduction
Hepatic injury with its immediate and late complications of hemorrhage is a significant cause of morbidity and mortality in both pediatric and adult population. There has been a paradigm shift in management of liver injuries globally towards non operative management (NOM) over the last two decades.1 Reduced time for imaging and diagnosis with the introduction of multi detector computed tomography (MDCT) scan and multi-disciplinary timely critical care has been mainly responsible for success of NOM and increased survival rates. The efficacy of transarterial embolization for vascular liver injury has been well established by authors worldwide with success rates ranging from 85 to 100%1–6; however, there is a debate over its use in management of hemodynamically unstable patients. There is also paucity of data regarding intermediate and long term follow up of this modality.
The adjunctive use of AE has become a mainstay in treating vascular hepatic injuries in institutions with well developed trauma services. At the same time, in a developing nation like ours, operative exploration is still the intervention of choice in hemodynamically unstable hepatic injury patients. We audited our experience with the use of endovascular management in a Catheterization laboratory for vascular hepatic injuries, with active bleeding by blunt and post operative trauma to address various important issues of survival benefit, morbidity, mortality on intermediate to long term follow up.
2. Patients and methods
A retrospective review of hospital record of inpatients from January 2006 to July 2012 was performed after approval from Ethics Committee, Dayanand Medical College and Hospital, a tertiary level care hospital in North of India. Data was collected on patients who underwent hepatic artery AE and included demographics, injury specific data, physical findings, CT grade of liver injury, hemodynamic parameters, operative intervention, laboratory findings, transfusion requirements, length of hospital stay (LOS), liver related morbidity and mortality, time of AE and final outcome at discharge.
The data was reviewed by an independent investigator not directly involved in patient care and a note was made of all injuries identified and diagnosed by focused assessment sonography for trauma (FAST) and MDCT scan. Blunt hepatic injuries were graded using the American Association for the Surgery of Trauma (AAST) liver injury scale. Post operative patients who got readmitted with hepatic injuries and underwent AE were not considered under AAST Liver Injury Scale. Indications to perform angiography were determined after evidence of active intra hepatic extravasation of contrast or pseudoaneurysm on MDCT scan along with hemodynamic status of the patient. Angiography/AE was performed after taking informed consent from patients' relatives as per the protocol.
Patients were defined as hemodynamically stable if they were euvolumic with normal clinical and laboratory parameters and hemodynamically unstable if they were in hypovolumic shock with worsening clinical and laboratory parameters. The second set of patients were further divided into two categories. The first category termed responsive shock patients responded to resuscitation with i.v fluids 1–1.5 L/hour, blood transfusions and amine administration while the second category termed non responsive shock patients did not benefit from resuscitation and were immediately taken up for laparotomy/re exploration (Fig. 1).
Fig. 1.
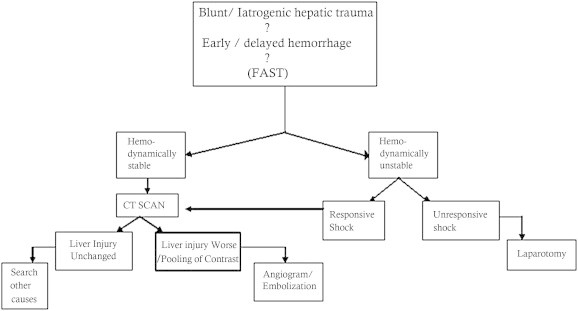
Flow chart of management of severe blunt/latrogenic hepatic trauma; FAST: Focused Assessment with Sonography for Trauma.
Angiography and embolization/stenting was performed in an angiographic suite with angiographic Philips Allura Integris System (Philips Medical Systems, Best, The Netherlands). Arterial access was obtained via the right femoral artery using the Seldinger technique. A 4 – 6 Fr. Cordis sheath was then placed to secure the arterial access and different diagnostic catheters (3 Fr Cook's microcatheter, 4 – 6 Fr Renal guide catheter, 4 – 6 Fr RCA Cordis, Cobra 1) and 0.014 PTCA guide wire of Abott were then used to access the culprit vessels and diagnostic arteriograms were performed. The arteriograms of culprit vessels either demonstrated active contrast extravasations, pseudo-aneursyms or arteriobiliary fistula and were selectively embolized/stented resulting in stasis of blood flow. Injured vessels were embolized as distally as possible to prevent hepatic necrosis and pseudo-aneurysms were embolized proximally as well as distally considering the so called “closing the front and back door” technique resulting in successful occlusion.
3. Results
During the 6 year period, 14 patients who were taken up for hepatic artery AE in the catheterization laboratory were evaluated. Demographics showed that 78% were men and 22% were women with mean age of 36.18 ± 20.90 (range, 4.5–70 years) (Table 1). Eight (57%) patients had post operative hepatic vascular injury and 6 (43%) patients had BHT out of which 3 (21%) were in the pediatric age group. The mean liver injury grade in BHT patients was 3.8 ± 0.83. Seven out of 14 (50%) patients were referred from other hospitals of our region to our institute with post operative/blunt hepatic trauma. Two patients with history of laparoscopic cholecystectomy from our hospital and 5 patients with BHT including 3 children were direct admissions. As per our institutional protocol, age and presenting symptoms patients were admitted in Pediatric, General Surgery and Gastroenterology departments for initial evaluation and management.
Table 1.
Patient demographics.
| Patient no. | Gender | Age (yrs) | Grade | Mechanism of injury | Time of AE | CT scan finding | AE complication | Systemic presentations | Outcome after AE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 4.5 | III | BHT (delayed hemorrhage) | Early | Pseudoaneurysm left hepatic artery | None | Pain abdomen, GI bleed, shock | Alive |
| 2 | M | 9 | III | BHT (delayed hemorrhage) | Early | Extravasation in left hepatic artery | None | Pain abdomen, GI bleed, hemoperitoneum, shock | Alive |
| 3 | M | 35 | IV | BHT (immediate hemorrhage) | Early | Pseudoaneurysm left hepatic artery & extravasations right hepatic artery | Rebleeding | Right hemothorax, GI bleed, hemoperitoneum, shock | Alive |
| 4 | F | 28 | IV | BHT (immediate hemorrhage) | Early | Pseudoaneurysm left hepatic artery | Bilioma | Pain abdomen, GI bleed, shock | Alive |
| 5 | M | 32 | V | BHT/post hepatic resection (delayed hemorrhage) | Early | Pseudoaneurysm right hepatic artery | None | Pain abdomen, hemoperitoneum, shock | Alive |
| 6 | F | 28 | – | Post lap cholecystectomy post laparotomy | Early | Pseudoaneurysm left hepatic artery Arteriobiliary fistula |
None | Pain abdomen, hemobilia on T-tube drain | Alive |
| 7 | M | 60 | – | Post lap cholecystectomy blocked CBD stent hepatic SOL | Early | Pseudoaneurysm superior pancreatico duodenal artery | None | Pain abdomen, GI bleed | Alive |
| 8 | M | 53 | – | Periampullary CA; post Whipple's procedures | Early | Pseudoaneurysm common hepatic and gastroduodenal artery | None | GI bleed, shock | Alive |
| 9 | M | 67 | – | Post lap cholecystectomy | Early | Extravasation in right hepatic artery | None | Pain abdomen, hemobilia, shock | Alive |
| 10 | M | 70 | – | HCC with PHT, coagulopathy | Early | Pseudoaneurysm left hepatic artery | Biliary stricture | Pain abdomen, hemobilia with sub-hepatic hematoma, shock | Alive |
| 11 | M | 40 | – | Post lap cholecystectomy | Early | Pseudoaneurysm right hepatic artery | Hepatic necrosis | Pain abdomen, GI bleed, shock | Alive |
| 12 | M | 23 | – | Post lap & open cholecystectomy | Early | Pseudoaneurysm right hepatic artery | None | Hemobilia on T-tube drain, shock | Alive |
| 13 | F | 45 | – | Post lap cholecystectomy | Early | Pseudoaneurysm right hepatic artery | None | Pain abdomen, GI bleed, shock | Alive |
| 14 | M | 12 | III | BHT (delayed hemorrhage) | Early | Pseudoaneurysm left hepatic artery | None | Pain abdomen, UGI bleed, shock | Alive |
Mechanism of injury in blunt trauma was due to road traffic accidents in 2 and accidental fall in 3. One patient with history of gun shot injury, having undergone hepatic resection at an outside hospital one month ago presented with hematemesis and radiological finding suggestive of large pseudoaneurysm of right hepatic artery. Only 2 patients of BHT had immediate presentation of hepatic bleeding to the hospital (i.e. within 12 h of injury) while rest of the 12 patients presented with delayed hemorrhage (range, 7–45 days before admission). They included all patients of post operative trauma group and 4 patients of BHT. Hepatic arterial injuries were confirmed by MDCT scan in 100% of the series. All 14 patients underwent AE immediately after CT scan and were labeled as Early AE.7 All patients presented with hemodynamic instability, in responsive shock category and responded to blood/blood product transfusion ranging from 2 to 12 units along with i.v. fluid resuscitation/ionotropic support.
Patients who had underwent Laparoscopic cholecystectomy/Whipple's procedure earlier, presented to our institution with significant upper gastrointestinal bleed and shock. They were initially managed by an admitting surgeon and taken up for Angiography and embolization after evidence of intrahepatic inaccessible bleed on MDCT. Fig. 2 demonstrates the imaging studies of a post cholecystectomy female patient with right hepatic artery bleed, diagnosed on MDCT scan and eventually successful management with coil embolization. Twelve (85%) patients underwent AE in local anesthesia while 1 child and 1 adult (BHT group) had to be electively intubated under short general anesthesia during AE. Both were extubated after 24 h post AE. Fig. 3 shows successful PVA particle embolization of a 12 year old child with BHT who underwent AE after elective intubation.
Fig. 2.
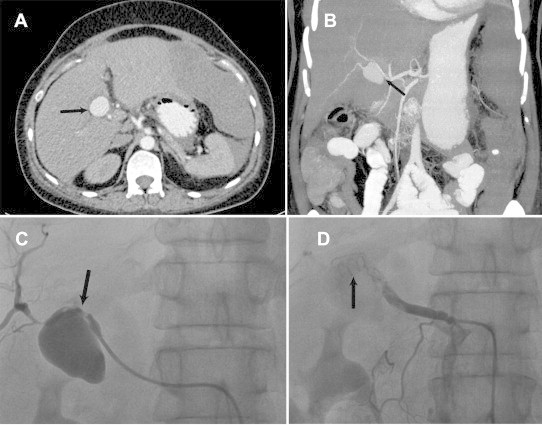
(A) CT scan of post cholecystectomy female patient with high-grade liver injury demonstrating active extravasation of contrast (black arrows). (B) CT Angiogram of same patient showing pseudoaneurysm of right hepatic artery (black arrow). (C) Angiogram of same patient demonstrating active extravasation (black arrow) and (D) successful embolization of right hepatic artery using ciols.
Fig. 3.
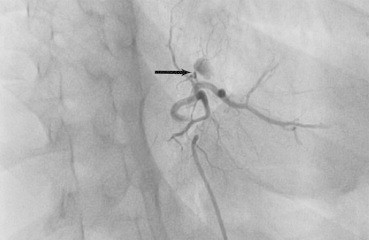
Angiogram of 12 year old child after successful PVA particle embolization of selected branch of left hepatic artery (black arrow).
Polyvinyl alcohol (PVA) particles ranging from 350 to 750μ and soft metal coils made from Teflon O35 wire were used for embolization. In one child, coronary hardware was used because of tortuosity of the vessel and embolization was done using “mother child technique”, (Fig. 4 showing angiogram of child with blunt hepatic trauma who underwent PVA particle embolization with coronary hardware). PRO 5 × 20 mm covered stent from Vascular Concept was used in revascularization in patient of gun shot injury (Fig. 5). All patients underwent AE with in 2 h of evidence of ongoing hemorrhage (MDCT scan) with an average time of 10 h between presentation to the hospital and successful AE. Seventy two hours post AE, hemodynamic stabilization was proved with clinical improvement (Systolic blood pressure > 90 mm Hg, Heart rate < 100/min) and no significant decrease in hemoglobin and hematocrit (%) values in all except 2 patients. Mean length of hospital stay was 9.62 ± 7.83 days (range 4–26 days).
Fig. 4.
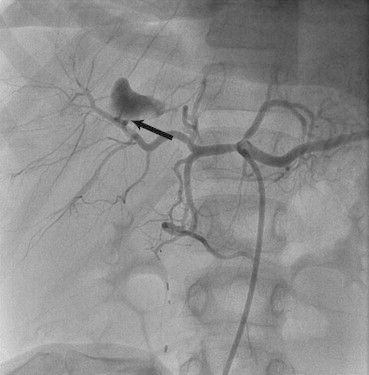
Angiogram of 9 year old child with BHT showing active extravasation of contrast in left hepatic artery (black arrow).
Fig. 5.
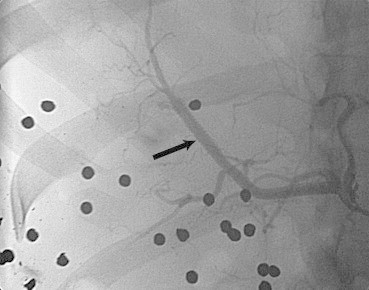
Angiogram of a 32 year male with history of gun shot injury after covered stent deployment (black arrow).
All patients underwent successful AE and there was no procedure related mortality in the group. There were no angiographic related local complications. One (7%) patient with bilateral hepatic artery bleed due to BHT had rebleeding following AE and went into hypovolemic shock. He was resuscitated and re-taken up for hepatic angiography resulting in complete hemostasis with PVA particles and coil embolization. One (7%) post surgical patient developed hepatic necrosis (HN) following proximal right hepatic artery AE (Fig. 6), which was diagnosed on MDCT scan. This patient underwent debridement and percutaneous drainage, required 10 blood transfusions and comparatively had a longer hospital stay. One (7%) blunt trauma adult had bilioma for which she underwent CT guided drainage where as 2 patients of cholecystectomy who were admitted with T tube in situ benefited from immediate hemostasis following AE. One (7%) post cholecystectomy patient with HCC developed biliary stricture and underwent common bile duct stenting. Gall bladder necrosis was not reported in children and adults with BHT.
Fig. 6.
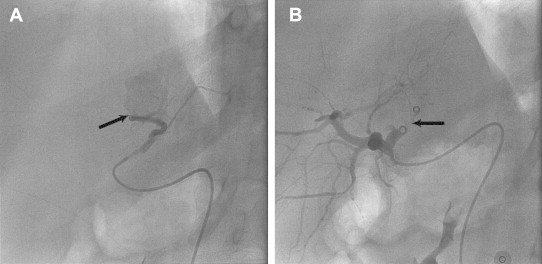
(A) Angiogram of a 40 year old post cholecystectomy patient showing active extravasation from right hepatic artery (black arrow). (B) Angiogram of same patient after successful proximal and distal coil embolization (black arrow).
4. Discussion
The management of hepatic injury depends on clinical and hemodynamic presentation of the patient. Non operative management (NOM) of hepatic injuries with AE is successful in 82–100% of population.2,8–11 At the same time, the management of hemodynamically unstable patients with higher grade liver injuries (III, IV, V) has been conflicting till date with only one recent study demonstrating a 93% success rate; with a multi-disciplinary approach stating a role for embolization in hemodynamically unstable patients.7
In acute as well delayed sequelae of trauma, hepatic artery bleeding can be due to delayed hematoma rupture, arterio-venous fistulae, pseudoaneurysm and arterio-biliary fistulae giving rise to clinical presentation of abdominal pain, anemia, jaundice, hematemesis or melena (due to arteriobiliary fistulae resulting in hemobilia) with or without shock. Historically, visceral aneurysms are rare (0.2%) out of which hepatic artery aneurysms account for 20%, with both true and pseudoaneurysms in equal percentage. They most commonly involve the common hepatic artery, followed by right and then left hepatic artery. They can bleed into biliary tract, portal vein or peritoneal cavity because of degeneration and weakness of the arterial wall; secondary to trauma (blunt/post operative) or an inflammation of the biliary tract.12–14
In a majority of patients of liver injury, who presented to our institution with non responsive shock; operative exploration to control bleeding was the gold standard management. Other subset of liver injury patients who presented in responsive shock category underwent initial resuscitation and confirmatory MDCT scan evaluation under the admitting surgeon/physician followed by AE in our catheterization laboratory. Overall, 100% angiographies resulted in subsequent therapeutic embolization. Although a small series, yet this finding corroborated with other published data which report rates of 70%–100% therapeutic embolization among all angiographies performed.4,15,16
Fifty-seven percent (8 of 14) of patients who had either undergone Cholecystectomy/Whipple's operation showed up with continued post operative intra hepatic hemorrhage on MDCT scan/angiography and required embolization. This high percentage of post surgical trauma which presented as delayed hemorrhage is an equally significant yet understated cause of hepatic artery bleed in comparison to available BHT data published globally. It suggests that despite improved surgical skills and techniques, intrahepatic arterial bleeding can occur in abdominal surgeries which is difficult to diagnose and potentially dangerous to manage surgically. The adjunctive use of AE and NOM had a favorable outcome in such cases. These patients had an average post operative morbidity of 25 days and in comparison, they got discharged within ten days following AE.
All 6 patients of BHT including 3 children benefited from Early AE. Delayed bleeding presentation was more common in this subset of patients too (4 out of 6), which suggests that subacute hepatic trauma leading onto hemodynamic instability is as significant a cause, as is acute hepatic bleed. We observed that patients with delayed presentation were usually screened for gastrointestinal bleed in other settings and diagnosis was missed. Our case series strongly puts forth the view that subacute hepatic injury is an important cause of concern for the clinicians which needs to be tackled with immaculate physiological, biochemical and radiological evaluation followed by a clinical endovascular management.
Among 14 patients who underwent AE, 4 (29%) experienced liver related complications. This is consistent with previous reports.4 All patients including children underwent AE within 2 h of evidence of ongoing hemorrhage with improvement in their clinical profile and leading onto complete recovery. Embolization offers the benefit of minimally invasive treatment in unstable patients, allows selective and simultaneously, distal and proximal control of the hepatic artery bleed. Although hepatic ischemia/necrosis is uncommon after AE co-existing portal vein compression or stenosis may lead to the same. Proper skill and care should be taken to avoid embolization of non targeting tissues such as cystic arteries which can lead on to gall bladder infarction requiring cholecystectomy for the same. In our series none of the patients had this complication, however, it has been published elsewhere.4,7,17–20
The laboratory indices showed improvement in liver enzymes on serial assays with values returning to baseline within 4 weeks in all except 2 patients. Review Doppler study at 12 weeks following AE showed thrombosed hepatic vasculature at the intervened sites with no bleed. After the mean follow up of 25.25 ± 21.02 months, 12 out of 14 (86%) patients are doing well with no derangements of liver enzymes or jaundice. One patient of HCC and second post operative patient of periampullary carcinoma died due to multiorgan failure within one year following procedure, which were unrelated to AE.
5. Conclusion
High degree of suspicion and prompt embolization can not only save the patient but also avoid surgery which has a high mortality and morbidity in emergency settings. In brief, the comprehensive management of high grade vascular hepatic trauma with early or delayed settings in our angiographic suite involving a team approach of interventionist, anesthetist and intensive care physician adjudicates the use of embolization as the gold standard treatment in all age groups of hemodynamically unstable patients, on continuous resuscitation. The results with regular follow up till date point out to our strategy being thoroughly effective with a nil mortality rate due to angiography and intervention alone in management of such cases. To conclude, AE should be concommitantly used in all age groups following immediate or late hepatic vascular injury for a successful outcome.
Conflicts of interest
All authors have none to declare.
Contributor Information
Bishav Mohan, Email: bishav_68@yahoo.co.in.
Harpreet Singh Bhoday, Email: hsbhoday@yahoo.com.
Naved Aslam, Email: drnavedaslam@yahoo.com.
Harpreet Kaur, Email: drharpreet1207@yahoo.com.
Shibba Chhabra, Email: shibbachhabra@yahoo.com.
Naresh Sood, Email: nareshalkasood@yahoo.com.
Gurpreet Wander, Email: drgswander@yahoo.com.
References
- 1.David Richardson J., Franklin G.A., Lukan J.K. Evolution in the management of hepatic trauma: a 25-year perspective. Ann Surg. 2000;232:324–330. doi: 10.1097/00000658-200009000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciraulo D.L., Luk S., Palter M. Selective hepatic arterial embolization of grade IV and V blunt hepatic injuries: an extension of resuscitation in the nonoperative management of traumatic hepatic injuries. J Trauma. 1998;45:353–359. doi: 10.1097/00005373-199808000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Hagiwara A., Yukioka T., Ohta S. Nonsurgical management of patients with blunt hepatic injury: efficacy of transcatheter arterial embolization. AJR. 1997;169:1151–1156. doi: 10.2214/ajr.169.4.9308480. [DOI] [PubMed] [Google Scholar]
- 4.Mohr A.M., Lavery R.F., Barone A. Angiographic embolization for liver injuries: low mortality, high morbidity. J Trauma. 2003;55:1077–1081. doi: 10.1097/01.TA.0000100219.02085.AB. [DOI] [PubMed] [Google Scholar]
- 5.Wahl W.L., Ahrns K.S., Brandt M.M. The need for early angiographic embolization in blunt liver injuries. J Trauma. 2002;52:1097–1101. doi: 10.1097/00005373-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Asensio J.A., Roldan G., Petrone P. Operative management and outcomes in 103 AAST-OIS grades IV and V complex hepatic injuries: trauma surgeons still need to operate, but angioembolization helps. J Trauma. 2003;54:647–653. doi: 10.1097/01.TA.0000054647.59217.BB. [DOI] [PubMed] [Google Scholar]
- 7.Monnin v, Sengel C., Thony F. Place of arterial embolization in severe blunt hepatic Trauma: a multidisciplinary approach. Cardiovasc Intervent Radiol. 2008;31:875–882. doi: 10.1007/s00270-007-9277-1. [DOI] [PubMed] [Google Scholar]
- 8.Pachter H.L., Knudson M.M., Esrig B. Status of nonoperative management of blunt hepatic injuries in 1995: a multicenter experience with 404 patients. J Trauma. 1996;40:31–38. doi: 10.1097/00005373-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mulhim A.S., Mohammad H.A. Non-operative management of blunt hepatic injury in multiply injured adult patients. Surgeon. 2003;1:81–85. doi: 10.1016/s1479-666x(03)80120-8. [DOI] [PubMed] [Google Scholar]
- 10.Carrillo E.H., Platz A., Miller F.B. Non-operative management of blunt hepatic trauma. Br J Surg. 1998;85:461–468. doi: 10.1046/j.1365-2168.1998.00721.x. [DOI] [PubMed] [Google Scholar]
- 11.Kozar R.A., Moore J.B., Niles S.E. Complications of nonoperative management of high-grade blunt hepatic injuries. J Trauma. 2005;59:1066–1071. doi: 10.1097/01.ta.0000188937.75879.ab. [DOI] [PubMed] [Google Scholar]
- 12.Okuno A., Miyazaki M., Ito H. Nonsurgical management of ruptured pseudoaneurysm in patients with hepatobiliary pancreatic diseases. Am J Gastroenterol. 2001;96:1067–1071. doi: 10.1111/j.1572-0241.2001.03691.x. [DOI] [PubMed] [Google Scholar]
- 13.Pasha S.F., Gloviczki P., Stanson A.W. Splanchnic artery aneurysms. Mayo Clin Proc. 2007;82:472–479. doi: 10.4065/82.4.472. [DOI] [PubMed] [Google Scholar]
- 14.Green M.H., Duell R.M., Johnson C.D. Haemobilia. Br J Surg. 2001;88:773–786. doi: 10.1046/j.1365-2168.2001.01756.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J.W., Gracias V.H., Gupta R. Hepatic angiography in patients undergoing damage control laparotomy. J Trauma. 2002;52:1102–1106. doi: 10.1097/00005373-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie S., Kortbeck J.B., Mulloy R. Recent experiences with a multidisciplinary approach to complex hepatic trauma. Injury. 2004;35:869–877. doi: 10.1016/j.injury.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Nijhof H.W., Willemssen F.E., Jukema G.N. Transcatheter arterial embolization in a hemodynamically unstable patient with grade IV blunt liver injury: is nonsurgical management an option? Emerg Radiol. 2006;12:111–115. doi: 10.1007/s10140-005-0460-x. [DOI] [PubMed] [Google Scholar]
- 18.Gorich J., Rilinger N., Brado M. Non-operative management of arterial liver hemorrhages. Eur Radiol. 1999;9:85–88. doi: 10.1007/s003300050633. [DOI] [PubMed] [Google Scholar]
- 19.Goffette P.P., Laterre P.F. Traumatic injuries: imaging and intervention in post-traumatic complications (delayed intervention) Eur Radiol. 2002;12:994–1021. doi: 10.1007/s00330-002-1396-0. [DOI] [PubMed] [Google Scholar]
- 20.Takayasu K., Moriyama N., Muramatsu Y. Gallbladder infarction after hepatic artery embolization. AJR. 1985;144:135–138. doi: 10.2214/ajr.144.1.135. [DOI] [PubMed] [Google Scholar]


