Abstract
Ventilatory instability may play an important role in the pathogenesis of obstructive sleep apnea. We hypothesized that the influence of ventilatory instability in this disorder would vary depending on the underlying collapsibility of the upper airway. To test this hypothesis, we correlated loop gain with apnea–hypopnea index during supine, nonrapid eye movement sleep in three groups of patients with obstructive sleep apnea based on pharyngeal closing pressure: negative pressure group (pharyngeal closing pressure less than –1 cm H2O), atmospheric pressure group (between –1 and +1 cm H2O), and positive pressure group (greater than +1 cm H2O). Loop gain was measured by sequentially increasing proportional assist ventilation until periodic breathing developed, which occurred in 24 of 25 subjects. Mean loop gain for all three groups was 0.37 ± 0.11. A significant correlation was found between loop gain and apnea–hypopnea index in the atmospheric group only (r = 0.88, p = 0.0016). We conclude that loop gain has a substantial impact on apnea severity in certain patients with sleep apnea, particularly those with a pharyngeal closing pressure near atmospheric.
Keywords: control of breathing, loop gain, pharyngeal closing pressure, pharyngeal collapsibility, ventilatory stability
Obstructive sleep apnea (OSA) may be due to several factors, each of which contributes more or less to the disorder in a given patient. A major factor in most patients is a small pharyngeal airway (1–3). However, airway anatomy differs considerably amongst patients with OSA and fails to explain much of the variance in apnea–hypopnea index (AHI) (1, 4–7). One explanation for this may be the influence of ventilatory control instability. Reports have suggested that ventilatory control is less stable in OSA (8–10). This too, however, varies among patients, and many appear to have normal ventilatory control despite OSA. Thus, the purpose of this study was to determine whether ventilatory instability is an important feature in OSA, and if so in which patients this is the case.
We hypothesized that the contribution of ventilatory instability would vary between patients depending on the predisposition to upper airway collapse. Specifically, in patients with either favorable anatomy or markedly poor anatomy, ventilatory instability may be a less influential factor. We base this on the well-described interaction between airway caliber and ventilatory stability. In general, fluctuations in ventilation/respiratory drive (due to ventilatory instability) are associated with reciprocal fluctuations in airway resistance; the airway dilates during peak ventilation and narrows at the nadir (11–15). If adequate narrowing occurs, obstructive apnea or hypopnea may result. Hence, among other things, the determinants of pharyngeal obstruction include (1) the initial predisposition to upper airway collapse (anatomy), and (2) the magnitude of reduction in respiratory drive during unstable breathing (ventilatory instability) (16–19). Some patients may exhibit such a marked predisposition to collapse that ventilatory instability has little effect on upper airway obstruction. Likewise, obstruction may not occur in patients with a minimally collapsible airway until ventilatory control becomes highly unstable. Although previous studies examining this question have confirmed the causal relationship between ventilatory instability and upper airway obstruction, they were performed under conditions of marked ventilatory instability (hypoxia-induced periodic breathing) in predominantly normal subjects (13–15). This does not address whether less severe ventilatory instability is important in actual patients with OSA, or in whom such instability is likely to be a contributing factor (given that the risk for ventilatory instability-induced upper airway collapse may differ depending on the predisposition to airway obstruction). Thus, we asked two questions: Does the level of ventilatory instability we find in OSA have an association with apnea severity? Is this association different between different anatomic groups?
To answer these questions, we made three measurements during supine, nonrapid eye movement (NREM) sleep in a group of patients with OSA: AHI, loop gain (a measure of ventilatory instability; see below), and pharyngeal closing pressure (Pcrit, a measure of airway collapsibility). Some results from this study have been previously reported in abstract form (20).
METHODS
A more detailed account of Methods is provided in the online supplement.
Subjects
To achieve a range of pharyngeal collapsibilities, subjects with snoring were recruited from the community, and patients with known OSA were recruited from the clinical sleep laboratory at Brigham and Women's Hospital (Boston, MA). Forty-four subjects were enrolled in the study.
Baseline Polysomnography
A standard montage for sleep staging, arousals, and respiratory monitoring was used to classify the severity of disordered breathing. Apneas and hypopneas were scored according to more recently described research criteria (21), and AHI was calculated from supine, NREM sleep only.
Pharyngeal Closing Pressure
Flow, airway pressure, and polysomnography signals were recorded. Subjects breathed through a nasal mask connected via a bidirectional valve to a ventilator capable of delivering either continuous positive or negative pressure. Using a previously described technique (22), Pcrit was determined by periodically lowering continuous positive airway pressure (CPAP) for three breaths from an optimum level (holding pressure) to sequentially lower levels until zero flow occurred. Peak flow from the third breath after a pressure drop was plotted against mask pressure and fit using a linear regression equation. The x intercept of this equation (zero crossing) was taken as the Pcrit. Only flow-limited breaths were used to construct the linear regression plot, with flow limitation being defined as a characteristic peak–plateau (negative effort dependence) or obvious flattening in inspiratory airflow. These criteria were previously validated in 12 subjects with epiglottic pressure measurements (23) (see the online supplement for details of this validation procedure). Each subject was placed into one of three prespecified groups based on Pcrit: negative group (Pcrit less than –1 cm H2O), atmospheric group (Pcrit between –1 and +1 cm H2O), and positive group (Pcrit greater than +1 cm H2O).
Loop Gain
Loop gain is an engineering term that describes the stability of a system (mechanical, electrical, physiological) controlled by negative feedback loops. In the case of respiration, loop gain represents the gain, or sensitivity, of the negative feedback loop that controls ventilation. Mathematically, it is defined as the ratio of a corrective response (e.g., hyperpnea) to a disturbance (e.g., apnea). If the corrective response is greater in magnitude than the disturbance (loop gain greater than 1), then small perturbations (e.g., noise entering the chemical feedback loop) have the potential to grow into self-sustaining oscillations (until saturating nonlinearities prevent further growth). A loop gain of less than 1 (e.g., 0.5), on the other hand, produces decaying oscillations, the magnitude and duration of which depend on the strength of the perturbation. Moreover, decaying oscillations may become sustained if reinforced by “destabilizing factors,” such as airway dilation at the peak of oscillation and/or pharyngeal occlusion at the nadir. Loop gain of the respiratory system can be measured during sleep in humans by using a proportional assist ventilator (PAV) (9, 24, 25).
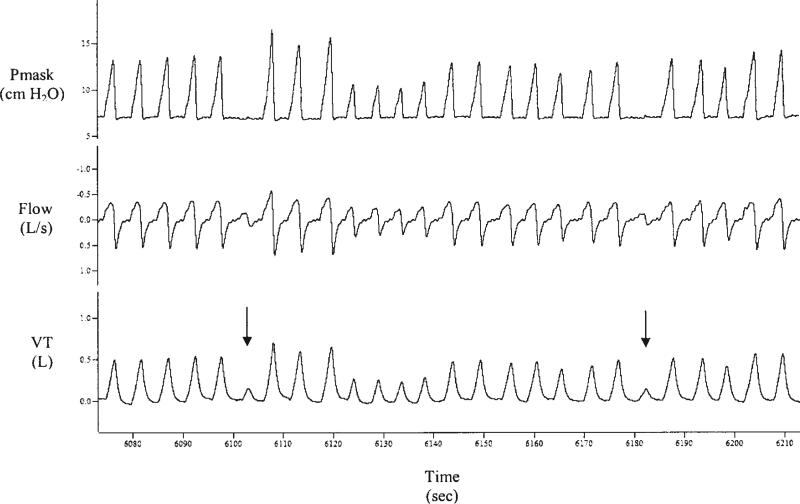
To measure loop gain, respiratory and polysomnography signals were recorded as described previously. Subjects breathed through a nasal mask connected to a PAV, which is capable of delivering ventilatory assistance in proportion to respiratory effort (26, 27). Loop gain was measured by the technique described by Younes and coworkers (9). Briefly, with subjects in supine NREM sleep, the percentage of PAV was increased to discrete levels for 3 minutes at a time in an effort to induce periodic breathing. At each level in which periodic breathing did not develop, the percent assistance was reduced to zero for one breath to determine the tidal volume (Vt) amplification factor (VTAF), which is the measure of how much PAV support is being provided at that level (Figure 1). Loop gain was calculated as the reciprocal of the amplification factor needed to induce periodic breathing. Periodic breathing was defined as four or more cycles of crescendo–decrescendo breathing (nadir tidal volume less than 50% of peak Vt) with a period of 20–90 seconds.
Figure 1.
Example of two tidal volume (Vt) amplification measurements (VTAFs) made at the level of proportional assist ventilation (PAV) immediately preceding periodic breathing. PAV is reduced to zero for one breath, yielding a single “unassisted breath” (arrows). The three breaths preceding PAV reduction are averaged for the “assisted Vt.” VTAF, which is the amount by which PAV increases the subject's intrinsic loop gain, is calculated as the ratio of assisted Vt to unassisted Vt. When PAV is increased 10% above the existing level (at time 6,310–100 seconds after the end of this recording), periodic breathing begins, indicating that the loop gain on PAV (LGpav) as shown is close to 1 (LGpav = 1). The subject's intrinsic loop gain, that is, loop gain in the absence of PAV (LGintrinsic), which is the variable of interest, is calculated as the reciprocal of VTAF based on the following relation: LGpav = 1 = LGintrinsic × VTAF. Here, the VTAF immediately preceding an LGpav of 1 (periodic breathing) is 3.24 (measured from the values shown), yielding an LGintrinsic of 0.31. Pmask = mask pressure (cm H2O); flow (L/second); Vt = tidal volume (L); time (seconds).
Statistical Methods
Mean values of measured variables were compared between the three groups using one-way analysis of variance followed by a Tukey test when appropriate. The relationships between loop gain versus AHI and Pcrit versus AHI were tested by Pearson product moment correlation.
RESULTS
Baseline Polysomnography
Full data sets were collected on 25 subjects. Mean duration of monitored supine, NREM sleep for AHI determination was 238 ± 83 minutes. All subjects enrolled from the community with a history of snoring had an AHI greater than 20 episodes/hour in the supine position, constituting polysomnographic evidence of OSA during supine sleep. For the entire group, AHI was 51.8 ± 31.8 episodes/hour. AHI was significantly different between the negative and positive Pcrit groups only (p < 0.05) (Table 1). There was no difference between groups in age, sex, BMI, or mean loop gain. Disordered breathing events in the negative Pcrit group consisted almost exclusively of hypopneas (98 ± 2.8% hypopneas), whereas 86 ± 22.5% of events were scored as hypopneas in the atmospheric Pcrit group, followed by 62 ± 37.7% in the positive Pcrit group. Virtually all events not classified as hypopneas were obstructive apneas.
TABLE 1.
PATIENT CHARACTERISTICS
| Negative Pcrit (n = 8) | Atmospheric Pcrit (n = 9) | Positive Pcrit (n = 8) | |
|---|---|---|---|
| Age, yr | 47 ± 6.6 | 44.9 ± 9.7 | 45.1 ± 11.5 |
| Sex | 4 M/4 F | 4 M/5 F | 6 M/2 F |
| BMI, kg/m2 | 33.8 ± 7.9 | 31.9 ± 7.4 | 34.1 ± 9.7 |
| AHI, episodes/h | 30.6 ± 8.8 | 52.3 ± 30.6 | 78.8 ± 31.5* |
| Percent hypopneas | 98 ± 2.8 | 86 ± 22.5 | 62 ± 37.7* |
| Pcrit, cm H2O | –2.62 ± 1.13 | 0.40 ± 0.30* | 2.13 ± 0.69*,† |
| Pressure-flow slope, ml/s/cm H2O | 69.9 ± 23.5 | 68.1 ± 15.6 | 67.5 ± 24.4 |
| Loop gain, dimensionless | 0.36 ± 0.11 | 0.33 ± 0.06 | 0.42 ± 0.13 |
| PetCO2, mm Hg | 41.8 ± 2.4 | 42.0 ± 2.1 | 39.4 ± 1.3† |
| Optimum CPAP, cm H2O | 8.9 ± 3.4 | 8.7 ± 1.7 | 13.4 ± 3.0*,† |
Definition of abbreviations: AHI = apnea-hypopnea index; BMI = body mass index; CPAP = continuous positive airway pressure; F = female; M = male; Pcrit = pharyngeal closing pressure; PetCO2 = end-tidal carbon dioxide.
Percent hypopneas was calculated as number of hypopneas divided by the total number of events (episodes per hour). All values represent means ± SD.
p < 0.05 compared with negative Pcrit group.
p < 0.05 compared with atmospheric Pcrit group.
Loop Gain
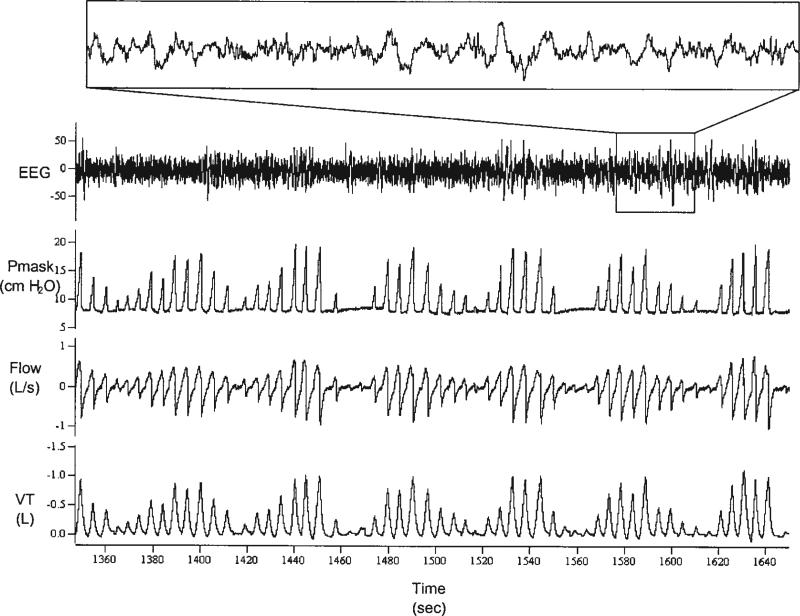
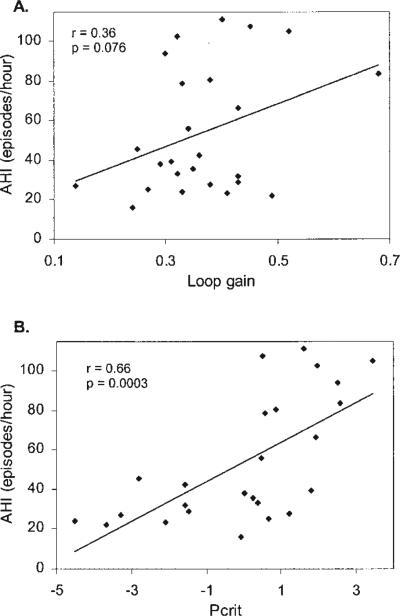
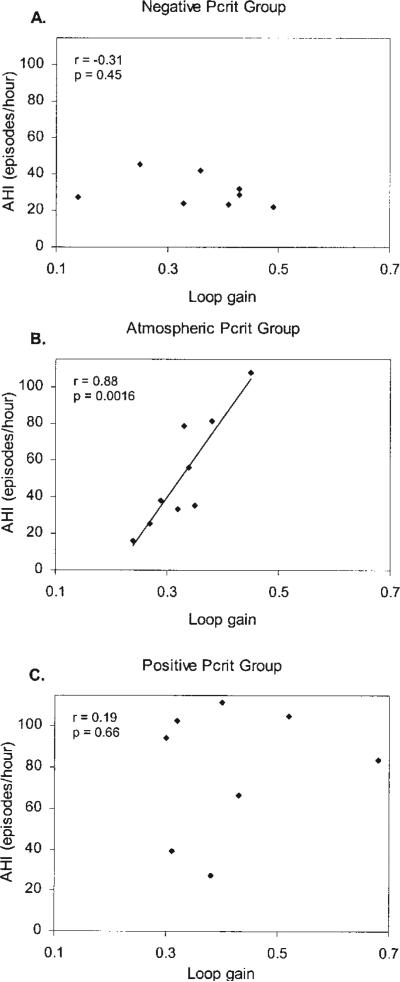
An example of PAV-induced periodic breathing is shown in Figure 2. Twenty-four of 25 subjects developed periodic breathing on PAV, yielding a mean loop gain for all three groups of 0.37 ± 0.11. Loop gain was not significantly different between any of the groups (p = 0.23) (Table 1). Loop gain correlated positively with AHI for the group as a whole (Figure 3A), but this relationship did not reach statistical significance (r = 0.36, p = 0.076). However, we found a strong correlation between loop gain and AHI in the atmospheric Pcrit group (r = 0.88, p = 0.0016) (Figure 4B), that was not evident in the other two groups (Figures 4A and 4C). End-tidal CO2 differed slightly between the positive and atmospheric Pcrit groups (Table 1), but did not correlate with the level of loop gain (r = 0.02, p = 0.90).
Figure 2.
PAV-induced periodic breathing. Sleep state remained stable during cycling in this subject. The respiratory pattern is typical crescendo–decrescendo, indicative of a high loop gain state. Cycle length is 50 seconds. EEG = electroencephalography; Pmask = mask pressure (cm H2O); flow (L/second); Vt = tidal volume (L); time (seconds).
Figure 3.
(A) Correlation between loop gain and AHI (apnea–hypopnea index) for all subjects. (B) Correlation between Pcrit (pharyngeal closing pressure) and AHI for all subjects.
Figure 4.
Loop gain versus AHI for the three Pcrit groups: (A) negative Pcrit group (Pcrit less than –1 cm H2O); (B) atmospheric Pcrit group (Pcrit between –1 and +1 cm H2O); (C) positive Pcrit group (Pcrit greater than +1 cm H2O).
Pharyngeal Closing Pressure
Raw data used for Pcrit determination are provided in Figure 5, which demonstrates a peak–plateau flow pattern (characteristic of negative effort dependence) in the third breath after a reduction in mask pressure. Mean Pcrit values for the individual groups are shown in Table 1 and were significantly different between all three groups (p < 0.05). The slopes of the pressure–flow relationship, however, were almost identical between the groups (Table 1). Pcrit in all subjects combined was –0.01 ± 2.1 cm H2O and demonstrated a significant positive correlation with AHI (r = 0.66, p = 0.0003) (Figure 3B).
Figure 5.
Pharyngeal closing pressure was measured by dropping mask pressure (Pmask) abruptly for three breaths at a time. Negative effort dependence (peak–plateau flow pattern) is evidence that these drop-down breaths were flow limited (arrows). The pharynx is completely occluded (zero flow) at time 2,375 seconds, when mask pressure is 1 cm H2O (zero flow breaths were excluded from the Pcrit linear regression equation). EEG = electroencephalography; Vt = tidal volume (L); flow (L/second); Pmask = mask pressure (cm H2O); time (seconds).
DISCUSSION
The major purpose of this study was to examine the role of ventilatory instability (in the context of airway collapsibility) in patients with OSA. Specifically, we hypothesized that the correlation between ventilatory instability and apnea severity may vary in different anatomic groups due to differences in the risk for ventilatory instability-induced upper airway collapse. We found a strong correlation between loop gain and AHI in patients with a Pcrit near atmospheric pressure, suggesting that this group may be highly susceptible to changes in ventilatory instability. Outside this range, however, the existing levels of ventilatory instability were not associated with apnea severity, indicating that ventilatory control had relatively little influence on the number of apneas in these patients.
It is also worth noting that, consistent with several previous studies (8–10), we found that ventilatory instability alone in patients with OSA is not sufficient to produce periodic breathing. None of our patients cycled in the absence of upper airway obstruction (on CPAP alone). Therefore, our data do not address the question of how a highly unstable control system might affect OSA. It is possible that further destabilization in ventilatory control might worsen apnea severity in all three subgroups, but we did not find such high levels of instability in this study.
Relationship between Ventilatory Instability and OSA
Previous studies have shown that ventilatory control is less stable in patients with OSA, suggesting that this may be a pathophysiologic factor (8–10). However, there is considerable evidence that the effect of ventilatory instability in producing upper airway obstruction is highly dependent on the underlying predisposition to pharyngeal collapse (13–19). Thus, the extent to which ventilatory instability is pathophysiologically important in any given individual may relate to the anatomic properties of the airway. For instance, individuals with a less collapsible airway may require greater instability to produce obstruction, whereas relatively little instability (or none at all) may be needed if airway collapsibility is high. Our experiment is thus an extension of the previous studies (8–10) measuring ventilatory control in OSA in that we attempted to define certain anatomic groups highly susceptible to the influences of ventilatory instability.
There have been several studies demonstrating a direct cause-and-effect relationship between ventilatory instability and upper airway obstruction. The mechanism relates to an interaction between ventilatory drive/stability and upper airway patency. In general, stimulation of the respiratory system leads to pharyngeal muscle recruitment and dilation (16, 17, 28–42), whereas reduction leads to narrowing (16–19). Thus, ventilatory instability, which is associated with fluctuations in respiratory drive, is also associated with fluctuations in airway caliber (13–15). One difficulty in relating these previous studies to the mechanism of OSA is that they were performed under conditions of extreme ventilatory instability (hypoxia-induced periodic breathing) in patients without preexisting OSA, although a few did have obstructive hypopneas. Such a high degree of instability is generally not seen in OSA. Thus, they do not address the question of whether more modest increases in ventilatory instability are influential in actual OSA patients. Our findings suggest that ventilatory instability, at the levels we find in OSA, is associated with more severe apnea in a particular subgroup of patients.
Why Might Ventilatory Instability Correlate Better with Apnea Severity in Patients with an Atmospheric Pcrit?
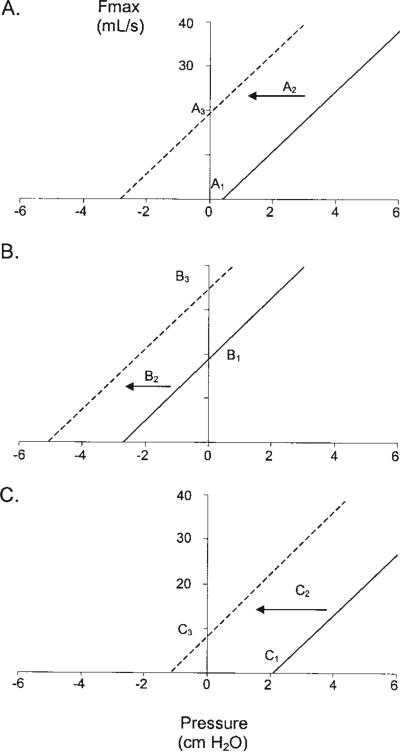
The pressure–flow relationships in Figure 6 provide a conceptual model for our explanation. The solid lines in each graph are the actual mean pressure–flow relationships (obtained during CPAP-induced pharyngeal muscle hypotonia) for each of the three Pcrit groups, whereas the dotted lines represent the theoretical shift due to pharyngeal muscle activation. A number of studies have shown that the predominant effect of muscle activation is a shift in closing pressure to more negative values, with little effect on airway stiffness (43–45). Consequently, the dashed lines are drawn with the same slope as the solid lines.
Figure 6.
Actual pressure–flow relationships (solid line) for each of the three Pcrit groups under hypotonic conditions. Dashed lines represent the theoretical effect of muscle activation. (A) Atmospheric Pcrit group. With sleep onset, airway closure occurs (A1), leading to a large build-up in chemical drive that activates pharyngeal muscles. Muscle activation exerts a dilating force on the airway (A2), which reestablishes airflow (A3). If loop gain is low, stable breathing results. If loop gain is increased, breathing may become unstable—recurrent cycling occurs. (B) Negative Pcrit group. Here, airflow persists after sleep onset (B1). If adequate ventilation cannot be maintained, the build-up in ventilatory drive recruits airway muscles and dilates the airway (B2, B3). Again, if loop gain is low, cycling with recurrent obstruction does not occur. If loop gain is high, fluctuations in breathing may occur. However, the risk of upper airway collapse for a given loop gain (or, for a given amount of fluctuation in ventilatory drive) is less, and it is likely that loop gain needs to be highly elevated before an association with AHI is seen. (C) Positive Pcrit group. The airway closes at sleep onset (C1) due to a net collapsing force on the pharynx. Increases in chemical drive, while producing a large dilating force (C2), are ineffective at opening the airway (C3), and arousal is necessary to reestablish flow. In this condition, ventilatory instability cannot be responsible for cycling, given that repeated airway closure and arousal are inevitable despite a high or low loop gain.
Figure 6A displays the pressure–flow relationship for the atmospheric Pcrit group. The solid line intersects the x axis at 0.40 cm H2O (A1), indicating that the pressure surrounding the pharyngeal lumen is slightly higher than atmospheric pressure. Insofar as the Pcrit depicted here is similar to that during the relative hypotonia of sleep, we can assume that the upper airway closes (or at least is near closure) after sleep onset in these patients. As a result, chemical stimuli begin increasing along with respiratory effort, which produce activation of the pharyngeal muscles and a leftward shift in the pressure–flow line (A2). In these patients, because the closing pressure is near zero, muscle activation leads to airway opening and the reestablishment of flow (A3). What happens subsequently depends in part on loop gain. If loop gain is low (i.e., ventilatory drive fluctuates minimally), stable breathing may result (e.g., snoring), provided that the new level of flow is sufficient to prevent arousal. If loop gain is elevated (i.e., ventilatory drive fluctuates widely), on the other hand, breathing may become unstable because of the substantial difference in airway patency and flow between the low drive condition (A1) and the high drive condition (A3). Thus, cyclic upper airway obstruction occurs.
The situation may be different in patients with a less collapsible airway. In Figure 6B, the airway does not collapse until –2.62 cm H2O of suction pressure is applied. Thus, flow is likely to be maintained after sleep onset in these patients (B1), yielding less build-up in chemical drive and possibly less shift in the pressure–flow curve (B2). Again, what happens after the increase in flow (B3) may depend on loop gain. If loop gain is low, relatively few events may occur over the course of the night. If loop gain is high, unstable breathing may result. However, loop gain would likely need to be more elevated in this group (versus the atmospheric group) to have an effect on the incidence of pharyngeal obstructions, given that the airway is less susceptible to collapse when ventilatory drive fluctuates between high (B3) and low (B1) levels.
Last, in Figure 6C the airway is highly collapsible (Pcrit is above 2 cm H2O). In accordance with the same sequence described above, sleep onset leads to airway closure (C1) and a large build-up in chemical drive. Here, however, muscle recruitment (C2) may not produce an adequate mechanical dilation of the upper airway (or the dilation is minimal, C3) because of the high collapsing pressure. Arousal must occur (or occurs before airway dilation) for flow to resume. Subsequent sleep is again followed by airway collapse, and the cycle repeats itself. In this situation, OSA is inevitable regardless of the loop gain. Moreover, ventilatory instability may have little to do with the AHI, the latter being mostly a reflection of anatomic insufficiency and arousal responses.
Methodologic Limitations
There are a number of potential limitations in our methods. First, the veracity of loop gain measurement on the basis of PAV, and the effects of CPAP/PAV on ventilatory control variables, have been reviewed extensively (9) and are not repeated here.
Second, to facilitate sustained sleep, data on downstream (epiglottic/esophageal) pressure was not collected during Pcrit determination. Downstream pressure is commonly used to define flow-limited breaths (progressive increase in respiratory effort without increase in flow), which are necessary for accurate determination of Pcrit. We believe our methodology was acceptable for the following reasons: (1) a conservative definition of flow limitation was used that tends to under-call flow-limited breaths (46); (2) nasal pressure was reduced for only three breaths at a time, allowing us to collect a large number of pressure–flow points near Pcrit without arousal/awakening; and (3) our ability to accurately measure Pcrit in the absence of downstream pressure was validated in 12 subjects (23).
Third, the VTAF method, which is used for loop gain determination, may be inaccurate if tidal volume varies considerably. However, in NREM sleep, our subjects had a relatively stable breathing pattern with only occasional low-amplitude variations in tidal volume, which were more random than periodic. Even as PAV was increased, the cycle amplitude did not appear graded with the level of PAV (until the point at which periodic breathing occurred, in which case there was an obvious change in breathing pattern). We also measured at least 3–5 VTAFs for each level of assistance in which periodic breathing did not occur, and the three preceding breaths before a single breath reduction in PAV were averaged for the assisted tidal volume (numerator in the VTAF calculation, VTAF = assisted Vt/unassisted Vt). As a result, we believe the VTAF measurement was an accurate reflection of PAV amplification of loop gain. Moreover, with the investigator blinded to the previous loop gain value, we remeasured loop gain in four subjects on a separate night under the same experimental conditions and found similar results. In two of the subjects, loop gain changed from 0.33 to 0.36 and from 0.30 to 0.28 between nights, and it did not change at all in the other two. Thus, there is little between-night variability in loop gain.
Last, there are relatively few subjects in each group, which raises the possibility of a Type II error. As no previous data were available at the start of our study, the sample size was calculated after preliminary data were obtained in the atmospheric Pcrit group. We estimated a correlation coefficient of 0.85 between loop gain and AHI, because the initial coefficient from our data was high. To test the hypothesis of a correlation of 0.85 versus 0.0, a two-sided hypothesis test with α = 0.05 and power = 0.80 would require eight subjects in each group. Thus, eight subjects were studied in each Pcrit group (nine in the atmospheric group).
Conclusions
Our findings suggest that ventilatory instability has a greater effect on apnea severity in certain patients with OSA depending on the collapsibility of the airway. The most sensitive group appears to include those in whom the airway is susceptible to collapse, but not so collapsible that obstruction is inevitable. These data highlight the heterogeneous nature of OSA as well as the limitations of predicting apnea severity from anatomic or ventilatory instability measures alone. Identification of patients in whom ventilatory instability plays a role in OSA has potential therapeutic implications, as nonmechanical therapy may be useful in these patients.
Supplementary Material
Acknowledgment
The authors thank Mary MacDonald for help with scoring sleep studies.
Supported by NIH/NHLBI F32 HL072560-01, RO1 HL48531, P50 HL60292, NCRR GCRC MO1 RR02635, and NCRR GCRC RR01032. Dr. Jordan is the recipient of a TSANZ/Allen and Hanbury's respiratory research fellowship. Dr. Malhotra has received a Scientific Development Grant from the American Heart Association.
Footnotes
Conflict of Interest Statement: A.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.S.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; R.B.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; E.S.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; K.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; J.K.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; D.P.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gleadhill I, Schwartz A, Wise R, Permutt S, Smith P. Upper airway collabsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 2.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 3.Schwab RJ. Upper airway imaging. Clin Chest Med. 1998;19:33–54. doi: 10.1016/s0272-5231(05)70430-5. [DOI] [PubMed] [Google Scholar]
- 4.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anesthesia and sleep. Lancet. 2002;359:1207–1209. doi: 10.1016/S0140-6736(02)08224-7. [DOI] [PubMed] [Google Scholar]
- 5.Abbey NC, Block AJ, Green D, Mancuso A, Hellard DW. Measurement of pharyngeal volume by digitized magnetic resonance imaging: effect of nasal continuous positive airway pressure. Am Rev Respir Dis. 1989;140:717–723. doi: 10.1164/ajrccm/140.3.717. [DOI] [PubMed] [Google Scholar]
- 6.Rivlin J, Hoffstein V, Kalbfleisch J, McNicholas W, Zamel N, Bryan AC. Upper airway morphology in patients with idiopathic obstructive sleep apnea. Am Rev Respir Dis. 1984;129:355–360. [Google Scholar]
- 7.Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome: clinical implications. Am J Respir Crit Care Med. 1999;159:149–157. doi: 10.1164/ajrccm.159.1.9804140. [DOI] [PubMed] [Google Scholar]
- 8.Hudgel DW, Gordon EA, Thanakitcharu S, Bruce EN. Instability of ventilatory control in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158:1142–1149. doi: 10.1164/ajrccm.158.4.9712105. [DOI] [PubMed] [Google Scholar]
- 9.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 10.Asyali M, Berry R, Khoo M. Assessment of closed-loop ventilatory stability in obstructive sleep apnea. IEEE Trans Biomed Eng. 2002;49:206– 216. doi: 10.1109/10.983454. [DOI] [PubMed] [Google Scholar]
- 11.Alex CG, Onal E, Lopata M. Upper airway occlusion during sleep in patients with Cheyne–Stokes respiration. Am Rev Respir Dis. 1986;133:42–45. doi: 10.1164/arrd.1986.133.1.42. [DOI] [PubMed] [Google Scholar]
- 12.Longobardo G, Gothe B, Goldman M, Cherniack N. Sleep apnea considered as a control system instability. Respir Physiol. 1982;50:311–333. doi: 10.1016/0034-5687(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 13.Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol. 1986;61:1438–1443. doi: 10.1152/jappl.1986.61.4.1438. [DOI] [PubMed] [Google Scholar]
- 14.Warner G, Skatrud JB, Dempsey JA. Effect of hypoxia-induced periodic breathing on upper airway obstruction during sleep. J Appl Physiol. 1987;62:2201–2211. doi: 10.1152/jappl.1987.62.6.2201. [DOI] [PubMed] [Google Scholar]
- 15.Hudgel DW, Chapman KR, Faulks C, Hendricks C. Changes in inspira-tory muscle electrical activity and upper airway resistance during peri odic breathing induced by hypoxia during sleep. Am Rev Respir Dis. 1987;135:899–906. doi: 10.1164/arrd.1987.135.4.899. [DOI] [PubMed] [Google Scholar]
- 16.Badr MS, Skatrud JB, Simon PM, Dempsey JA. Effect of hypercapnia on total pulmonary resistance during wakefulness and during NREM sleep. Am Rev Respir Dis. 1991;144:406–414. doi: 10.1164/ajrccm/144.2.406. [DOI] [PubMed] [Google Scholar]
- 17.Badr M, Skatrud J, Dempsey J. Effect of chemoreceptor stimulation and inhibition on total pulmonary resistance in humans during NREM sleep. J Appl Physiol. 1994;76:1682–1692. doi: 10.1152/jappl.1994.76.4.1682. [DOI] [PubMed] [Google Scholar]
- 18.Badr MS, Roiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/ occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–1815. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 19.Badr MS, Kawak A, Skatrud JB, Morrell MJ, Zahn BR, Babcock MA. Effect of induced hypocapnic hypopnea on upper airway patency in humans during NREM sleep. Respir Physiol. 1997;110:33–45. doi: 10.1016/s0034-5687(97)00072-8. [DOI] [PubMed] [Google Scholar]
- 20.Wellman A, Jordan AS, Malhotra A, Fogel RB, Edwards JK, Schory KE, White DP. Defining the role of ventilatory control instability in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:A791. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Sleep Medicine Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in adults. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 22.Boudewyns A, Punjabi N, Van de Heyning PH, De Backer WA, O'Don-nell CP, Schneider H, Smith PL, Schwartz AR. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118:1031–1041. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 23.Jordan AS, Wellman DA, Fogel RB, Pierce RJ, Edwards JK, Schory KE, Malhotra A, White DP. Pharyngeal critical closing pressure measurement without respiratory effort: a validation study. Am J Respir Crit Care Med. 2003;167:A600. [Google Scholar]
- 24.Meza S, Younes M. Ventilatory stability during sleep studied with proportional assist ventilation (PAV). Sleep. 1996;19(10 Suppl):S164–S166. doi: 10.1093/sleep/19.suppl_10.164. [DOI] [PubMed] [Google Scholar]
- 25.Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol. 1998;85:1929–1940. doi: 10.1152/jappl.1998.85.5.1929. [DOI] [PubMed] [Google Scholar]
- 26.Younes M. Proportional assist ventilation, a new approach to ventilatory support: theory. Am Rev Respir Dis. 1992;145:114–120. doi: 10.1164/ajrccm/145.1.114. [DOI] [PubMed] [Google Scholar]
- 27.Younes M. In: Proportional assist ventilation. Update in intensive care and emergency medicine. Mancebo J, Net A, Brochard L, editors. Springer-Verlag; New York: 2002. pp. 39–73. [Google Scholar]
- 28.Hudgel DW, Suratt PM. The human airway during sleep. In: Saunders NA, Sullivan CE, editors. Sleep and breathing: lung biology in health and disease. 2nd ed. Marcel Dekker; New York: 1994. pp. 605–648. [Google Scholar]
- 29.Stanchina M, Malhotra A, Fogel RB, Ayas NT, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical loading during NREM sleep. Am J Respir Crit Care Med. 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 30.Onal E, Lopata M, O'Connor T. Pathogenesis of apneas in hypersomnia– sleep apnea syndrome. Am Rev Respir Dis. 1982;125:167–174. doi: 10.1164/arrd.1982.125.2.167. [DOI] [PubMed] [Google Scholar]
- 31.Series F, Cormier Y, Desmeules M, La Forge J. Effects of respiratory drive on upper airways in sleep apnea patients and normal subjects. J Appl Physiol. 1989;67:973–979. doi: 10.1152/jappl.1989.67.3.973. [DOI] [PubMed] [Google Scholar]
- 32.Maltais F, Dinh L, Cormier Y, Series F. Changes in upper airway resistance during progressive normocapnic hypoxia in normal men. J Appl Physiol. 1991;70:548–553. doi: 10.1152/jappl.1991.70.2.548. [DOI] [PubMed] [Google Scholar]
- 33.Rowley J, Williams B, Smith P, Schwartz A. Neuromuscular activity and upper airway collapsibility: mechanisms of action in the decerebrate cat. Am J Respir Crit Care Med. 1997;156:515–521. doi: 10.1164/ajrccm.156.2.9607115. [DOI] [PubMed] [Google Scholar]
- 34.Megirian D, Hinrichsen CFL, Sherrey JH. Respiratory roles of genioglossus, sternothyroid, and sternohyoid muscles during sleep. Exp Neurol. 1985;90:118–128. doi: 10.1016/0014-4886(85)90045-7. [DOI] [PubMed] [Google Scholar]
- 35.van de Graaff WB, Gottfried SB, Mitra J, van Lunteren E, Cherniack NS, Strohl KP. Respiratory functions of hyoid muscles and hyoid arch. J Appl Physiol. 1984;57:197–204. doi: 10.1152/jappl.1984.57.1.197. [DOI] [PubMed] [Google Scholar]
- 36.Brouillette RT, Thach BT. Control of genioglossus muscle inspiratory activity. J Appl Physiol. 1980;49:801–808. doi: 10.1152/jappl.1980.49.5.801. [DOI] [PubMed] [Google Scholar]
- 37.Haxhiu MA, van Lunteren E, Mitra J, Salamone J, Bruce E, Cherniack NS. Response to chemical stimulation of upper airway muscles and the diaphragm in awake cats. J Appl Physiol. 1984;54:397–403. doi: 10.1152/jappl.1984.56.2.397. [DOI] [PubMed] [Google Scholar]
- 38.Seelagy MM, Schwartz AR, Russ DB, King ED, Wise RA, Smith PL. Reflex modulation of airflow dynamics through the upper airway. J Appl Physiol. 1994;76:2720–2725. doi: 10.1152/jappl.1994.76.6.2692. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz A, Thut D, Brower R, Gauda E, Roach D, Permutt S, Smith P. Modulation of maximal inspiratory airflow by neuromuscular activity: effect of CO2. J Appl Physiol. 1993;74:1597–1605. doi: 10.1152/jappl.1993.74.4.1597. [DOI] [PubMed] [Google Scholar]
- 40.Weiner D, Mitra J, Salamone J, Cherniack NS. Effect of chemical stimuli on nerves supplying upper airway muscles. J Appl Physiol. 1982;52:530–536. doi: 10.1152/jappl.1982.52.3.530. [DOI] [PubMed] [Google Scholar]
- 41.Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol. 1981;50:1052–1055. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- 42.Onal E, Lopata M, O'Connor T. Diaphragmatic and genioglossal electro-myogram responses to isocapnic hypoxia in humans. Am Rev Respir Dis. 1981;124:215–217. doi: 10.1164/arrd.1981.124.3.215. [DOI] [PubMed] [Google Scholar]
- 43.Rolfe I, Olson LG, Saunders NA. Pressure–volume properties of the upper airway in man. Respir Physiol. 1991;86:15–23. doi: 10.1016/0034-5687(91)90036-i. [DOI] [PubMed] [Google Scholar]
- 44.Fouke JM, Teeter JP, Strohl KP. Pressure–volume behavior of the upper airway. J Appl Physiol. 1986;61:912–918. doi: 10.1152/jappl.1986.61.3.912. [DOI] [PubMed] [Google Scholar]
- 45.Olson LG, Ulmer LG, Saunders NA. Pressure–volume properties of the upper airway of rabbits. J Appl Physiol. 1989;66:759–763. doi: 10.1152/jappl.1989.66.2.759. [DOI] [PubMed] [Google Scholar]
- 46.Clark S, Wilson C, Satoh M, Pegelow D, Dempsey J. Assessment of inspiratory flow limitation invasively and noninvasively during sleep. Am J Respir Crit Care Med. 1998;158:713–722. doi: 10.1164/ajrccm.158.3.9708056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.