Abstract
Objective
Myocardial infarction (MI) is often preceded by severe chest pain. The use of inflammatory markers to distinguish between chest pain of cardiac and non cardiac origin are not well reported. The aim of the study was to distinguish the chest pain of non cardiac and cardiac origin by using reliable inflammatory markers.
Methods
The present study enrolled 80 subjects including chest pain which lead to myocardial infarction (n=40), non-cardiac chest pain (CP) patients (n=20) and healthy volunteers (N) (n=20). Leukotriene B4 (LTB4) and thromboxane B2 (TXB2) levels were analyzed along with hs-CRP.
Results
Receiver operating characteristic (ROC) curve analysis showed LTB4 and TXB2 to be a good discriminator between patients with chest pain of cardiac and non cardiac in origin. The area under the curve was found to be 0.988 and 0.925 for LTB4 and TXB2, respectively when compared with hs-CRP. The sensitivity and specificity of LTB4 and TXB2 were found to be 90, 85% and 95, 90%, respectively.
Conclusion
The measurement of LTB4 and TXB2 levels may therefore be useful to distinguish the chest pain leading to MI from that of non cardiac in origin and for the management of the disease.
Keywords: Myocardial infraction, Chest pain, Inflammation, Leukotriene B4, Thromboxane B2
1. Introduction
Myocardial infarction (MI) is one of the major causes of mortality and morbidity in the world.1 Inflammation plays a central role in the formation as well as rupturing of plaque leading to MI. The actual mechanism of plaque rupturing is still not known. High sensitivity C-reactive protein (hs-CRP), a biomarker for inflammation is viewed as a prominent partaker in endothelial dysfunction and atherosclerosis.2,3 Blake and Ridker have shown that elevated hs-CRP can predict risk of cardiovascular events (including death, acute myocardial infarction, and need for revascularization procedures) in patients with acute coronary syndromes (ACS).4
Studies of genetic polymorphisms have established significant associations for the leukotriene pathway with early signs of atherosclerosis5,6 as well as the development of stroke and myocardial infarction.7,8 Reports show that genetic variation in the gene ALOX5AP (arachidonic 5-lipoxygenase-activating protein) and LTA4H (leukotriene A4 hydrolase) contribute to the risk of myocardial infraction and stroke in Icelandic, Scottish and European-American population.9
Arachidonic acid (AA) is metabolized by cyclooxygenase (COX) and lipoxygenase (LOX) in the heart and coronary blood vessels, to numerous compounds with a variety of actions including vasodilation, vasoconstriction, enhanced myocardial injury and cardioprotection. Five-lipoxygenase (5-LOX), the rate-limiting enzyme in the biosynthesis of leukotrienes from AA, has been associated with the development of atherosclerosis in mouse models, and a 5-LOX promoter repeat polymorphism has been implicated in atherosclerosis in humans.10 5-LOX uses 5-lipoxygenase-activating protein (FLAP) to convert AA into leukotriene A4 (LTA4), an unstable epoxide. In cells equipped with LTA4H, such as neutrophils and monocytes, LTA4 is converted to leukotriene B4 (LTB4), which is a powerful chemoattractant for neutrophils acting at (leukotriene B4 receptor) BLT1 and BLT2 receptors on the plasma membrane of these cells.11
COX-1 and its AA metabolites have been long recognized to possess important roles in the myocardium during acute episodes of myocardial ischemia and reperfusion.12 COX converts AA to prostaglandins and thromboxane A2 (TXA2). TXA2 is very unstable and is hydrolyzed to stable thromboxane B2 (TXB2). Takase et al observed that the production of LTB4 and TXB2 in systemic artery blood was elevated during acute stage of AMI.13 So we hypothesized that levels of LTB4 and TXB2 may point out the biochemical differences between non cardiac chest pain and chest pain of cardiac origin leading to MI. We have selected young subjects with cholesterol level less than 250 mg/dl. Cholesterol has been considered as a risk factor for MI. We also wanted to study the correlation between cholesterol levels, inflammatory markers and occurrence of MI especially in young subjects. Hence the present study focused on the levels of LTB4 and TXB2 in young subjects (age below 45 yrs) whose cholesterol levels were below 250 mg/dl.
2. Methods
2.1. Patient selection
Eighty subjects in the age group 35–45 years were enrolled in the study. They were categorized into three groups: the myocardial infarction (MI) group (n = 40) at presentation, non cardiac chest pain (CP) (n = 20) patients and healthy volunteers (N) group (n = 20). Patients admitted in the intensive care unit (ICU) of cardiology unit in PRS hospital, Trivandrum, India with clinical diagnosis of MI were included in the study. The inclusion criteria were patients with prolonged chest pain more than 30 min, ST segment elevation more than 0.1 mV on at least two adjacent ECG leads or positive biomarkers. These patients were the ones with first episode of chest pain and had neither past history of such clinical symptoms nor were on any known medications for the same. Cholesterol levels below 250 mg/dl and age below 45 years were also considered in the inclusion criteria. The exclusion criteria include those with chest pain less than 30 min or more than 12 h, no ST segment elevation, T inversion or ST depression in ECG, those with known renal or hepatic dysfunction, bleeding disorder or any malignancy. Patients or bystanders who were unable to give consent for the study were also excluded from the present study. The controls were healthy individuals with systolic blood pressure/diastolic blood pressure (SBP/DBP) = 135/85 mmHg or less, with no risk factors of CAD or clinical symptoms of any other organic disease. The CP patients were individuals with normal blood pressure and ECG, the chest pain was non cardiac in origin, Cholesterol levels was below 250 mg/dl and age below 45 years. As per the selection criteria in each group, subjects were recruited with their informed consent and a detailed data collection was done. The institutional ethics committee approved the study protocol.
2.2. Blood sample collection
The peripheral whole blood was collected in plain and sodium citrate (3.2%) vacutainer tubes. Subsequently, serum and plasma were separated by centrifugation at 1000 g for 10 min. For healthy individuals blood samples were collected randomly. From MI and CP patients, blood samples were collected at the time of admission to the ICCU before administration of any treatment.
2.3. Study protocol
Serum level of total cholesterol was measured by standard enzymatic method using kit (Agappe Diagnostics, India) and expressed as milligram per deciliter of serum. The level of hs-CRP in serum was estimated by using CRP-ultrasensitive latex turbidimetry kit according to the manufacturer's instruction (Spinreact, Spain) and expressed as milligram per deciliter.
Activities of 5-LOX and COX were evaluated in the monocytes. Monocytes were separated from whole blood by the method of Hutch (1996).14 The activity of 5-LOX was determined in monocytes by the method of Axelrod et al (1981)15 and activity of COX by the method of Shimazu et al (1981).16
LTB4 concentration were determined in the serum by using enzyme immunoassay kit as described by the manufacturer (R&D systems, USA). Similarly, TXB2 was measured as described by the manufacturer of an enzyme immunoassay kit (Cayman Chemicals, USA). The amount of LTB4 and TXB2 were expressed as picogram/milliliter of serum.
2.4. Statistical analysis
The data's were represented as mean ± standard deviation (SD). One way ANOVA was applied to determine the significance of biochemical parameters among the groups. Duncan's post-hoc multiple comparison tests of significant differences among groups were determined. Receiver operating characteristic (ROC) curve were established to evaluate the diagnostic value of LTB4 and TXB2 for differentiating between chest pain of cardiac and non cardiac in origin. A p < 0.05 was considered significant. All statistical analysis was performed using statistical software SPSS (version 17, Chicago, IL).
3. Results
The clinical characteristics of the CP and MI groups are presented in Table 1. There were no statistical differences between the CP subjects and the MI patients for any of the considered variables except for ESR which was slightly enhanced in MI patients, but was in the normal range. In addition, other biomarkers, such as SGPT and fibrinogen levels increased in MI patients when compared to that in CP subjects (Table 1). The level of total cholesterol in serum of MI patients showed no significant increase when compared to CP and N subjects (Table 1). The total cholesterol levels were within the normal range in all the subjects.
Table 1.
Clinical characteristics of the MI, CP and N patients.
| Characteristics | MI patients (n = 40) | CP patients (n = 20) | N (n = 20) |
|---|---|---|---|
| Age (years) | 41.87 ± 5.82 | 40.85 ± 5.81 | 40.01 ± 5.78 |
| Male/female | 37/3 | 19/1 | 19/1 |
| RBS | 114.95 ± 15.42 | 110.2 ± 16.28 | 102.3 ± 13.75 |
| TC (mg/dl) | 222.95 ± 32.45 | 214.3 ± 29.87 | 211.5 ± 28.16 |
| HDL (mg/dl) | 47.8 ± 9.15 | 48 ± 10.02 | 55 ± 12.3 |
| LDL (mg/ml) | 98 ± 15.12 | 85 ± 13.24 | 82 ± 13.17 |
| Total count (cells/cumm) | 9330 ± 1811.80 | 7705 ± 1319.28 | 7915 ± 1108.54 |
| Platelet Count (lakhs/cumm) | 3.19 ± 0.65 | 2.67 ± 0.28 | 2.45 ± 0.25 |
| N (%) | 67.50 ± 9.57 | 63.15 ± 8.9 | 62.75 ± 8.5 |
| E (%) | 5.4 ± 1.63 | 4.6 ± 1.16 | 4.3 ± 1.03 |
| L (%) | 28.88 ± 6.65 | 25.60 ± 5.61 | 24.13 ± 5.20 |
| Hb (gm/dL) | 16.13 ± 2.79 | 14.71 ± 2.62 | 15.8 ± 2.68 |
| ESR (mm/hr) | 8.15 ± 2.38a | 3.47 ± 0.65 | 3.2 ± 0.43 |
| Urea (mg/dl) | 26.62 ± 6.35 | 24.50 ± 5.82 | 24.35 ± 5.21 |
| Creatinine (mg/dl) | 1.14 ± 0.32 | 0.97 ± 0.12 | 0.84 ± 0.10 |
| Na (mEq/L) | 140.1 ± 6.12 | 135.65 ± 4.22 | 124 ± 3.86 |
| K (mEq/L) | 4.09 ± 0.55 | 3.49 ± 0.46 | 3.19 ± 0.41 |
| SGPT (IU/L) | 58.6 ± 13.25a | 47.85 ± 10.52 | 44.25 ± 8.51 |
| Fibrinogen (mg/dl) | 480 ± 35.28a | 196 ± 22.84 | 175 ± 20.13 |
RBS: random blood sugar, TC: total cholesterol, HDL: high-density lipoprotein, LDL: low-density lipoprotein, N: neutrophil, E eosinophil, L: leukocytes, Hb: hemoglobin, ESR: erythrocyte sedimentation rate, Na: Sodium, K: potassium, SGPT: serum glutamate pyruvate transaminase. Values are expressed as mean ± SD.
p < 0.05.
The level of hs-CRP detected in serum of MI patients showed a significant increase when compared to other groups (Table 2). The marker enzymes of the arachidonic acid pathway such as 5-LOX and COX were studied in the three groups. The activities of both 5-LOX and COX were significantly elevated in MI subjects when compared to CP and N (p < 0.001). Similarly the level of TXB4 increased in the serum along with a nine fold increase in LTB4 level in MI patients when compared to CP and N subjects (p < 0.001) (Table 2).
Table 2.
Evaluation of hs-CRP, 5-LOX, COX,LTB4, TXB2 in MI, CP and N subjects.
| Parameters analyzed | N (n = 20) |
CP (n = 20) |
MI (n = 20) |
|---|---|---|---|
| hs-CRP (mg/L) | 0.82 ± 0.14a | 1.6 ± 0.28a | 5.4 ± 0.55b |
| 5-LOX (× 10−4 OD shift at 234 nm /min/mg protein) |
2. 97 ± 0.62a | 3.5 ± 0.84a | 6.47 ± 1.8b |
| COX (× 10−4 μ moles of MDA liberated/mg protein) | 3.44 ± 0.54a | 3.8 ± 0.64a | 5.77 ± 0.82b |
| LTB4 (pg/ml) | 242.24 ± 20.4a | 295 ± 22.5a | 2024.28 ± 77.5b |
| TXB2 (pg/ml) | UD | UD | 70.82 ± 8.2b |
UD – undetectable level (<10 pg/ml) in the serum with the specific EIA kit. Values are mean ± SD. p < 0.05. Different superscript (a, b) indicates the values are significantly different.
ROC curve analysis was done to demonstrate the usefulness of LTB4 and TXB2 as an individual risk determinant in subjects with chest pain of cardiac and non cardiac in origin. The area under curve (AUC) of LTB4 and TXB2 was 0.988 and 0.925, respectively when compared to hs-CRP (0.916) (Table 3) (Fig. 1). The sensitivity and specificity of hs-CRP, TXB2 and LTB4 were 75, 85, 90% and 80, 90, 95%, respectively.
Table 3.
Diagnostic usefulness of biomarkers.
| Sensitivity % | Specificity % | PPV | NPV | Accuracy | AUC | |
|---|---|---|---|---|---|---|
| hs-CRP | 75 | 80 | 88 | 62 | 77 | 0.916 |
| TXB2 | 85 | 90 | 94 | 75 | 87 | 0.925 |
| LTB4 | 90 | 95 | 97 | 82 | 92 | 0.988 |
PPV: positive predictive value, NPV: negative predictive value, AUC: area under the curve.
Fig. 1.
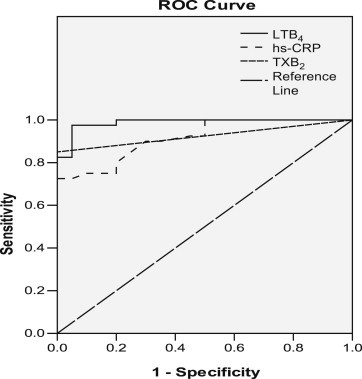
Receiver operating characteristic (ROC) curve of different markers.
4. Discussion and conclusions
Chest pain is an important symptom shown by most of the patients prior to a severe MI. But there also prevail chest pains of non cardiac in origin. The plaque which ruptures leading to MI is filled with cholesterol deposits, but studies have shown that only 35% of the patients with cholesterol levels greater than 200 mg/dl have developed MI.17 In the present study only patients with cholesterol levels below 250 mg/dl were included.
hs-CRP is considered as a biomarker of inflammation especially in MI patients. The level of hs-CRP in the MI subjects increased significantly when compared to CP and N (p < 0.05). Our observation that hs-CRP levels were enhanced in MI patients is in support of the report by Ridker et al18 which states that hs-CRP predicts not only incident myocardial infarction and cardiovascular death, but also the risk of ischemic. hs-CRP showed an AUC of 0.916 with 75% sensitivity, 80% specificity and 77% accuracy.
Our study was mainly focused on LTB4 and TXB2. Their production depends on the activities of the key enzymes 5-LOX and COX. Their activities were enhanced in the MI patients indicating the upregulation of AA pathway and production of LTB4 and TXB2. This is supported by the enhanced levels of LTB4 and TXB2 in MI subjects. Spanbroek et al,19 reported that 5-LOX is the main LOX expressed within atherosclerotic lesions. Polymorphisms in the 5-LOX gene promoter and certain 5-LOX-activating protein haplotypes have been linked to an increased risk of infarction and stroke.20 van der Net et al21 reported that genetic variation in the ALOX5AP gene contributes to CHD risk in patients with familial hypercholesterolemia. A study by Wong et al22 has reported the expression of COX in ischemic human myocardium and in dilated cardiomyopathy, but not in normal cardiomyocytes indicating a role of COX in cardiac disease.
The present study revealed a significant increase in the LTB4 levels in MI subjects. Takase et al,13 have observed that LTB4 levels are elevated in acute myocardial patients during acute stage. Sanchez Galan et al,11 characterized the expression of 5-LOX pathway and LTB4 receptors in blood and plaques from patients with carotid atherosclerosis. TXB4 level was increased in the serum of MI patients. Studies by Moscardo et al,23 have showed that residual-COX-1 activity and epinephrine enhance TXA2-dependent platelet function, which may reduce the clinical benefit of aspirin in patients with AMI. LTB4 showed an AUC of 0.988 with 90% sensitivity, 95% specificity and 92% accuracy, indicating its usefulness for the management of disease.
In conclusion, the increase in oxidative stress during MI might have upregulated the AA pathway which was evident by the significantly higher activities of 5-LOX and COX suggesting the strong involvement of AA pathway in the onset of MI. We found that the levels of LTB4 and TXB2 were raised in the serum of MI subjects and were undetectable in the serum of CP and N subjects. Due to the significant increases in the levels of LTB4 and TXB2, the chances of overlapping of results can be prevented. This is due to the high sensitivity and specificity of LTB4 and TXB2. The drawback of the present study is the small sample size. Investigations including a larger scale of patients need to be performed to confirm the use of LTB4 and TXB2 in ruling out the disease and aid in timely management if MI.
Conflicts of interest
All authors have none to declare.
Acknowledgment
I would like to acknowledge Integrated Tribal Development Project, India for their financial support.
References
- 1.Kasap Secil, Gonenc Aymelek, Erten Sener Derya, Hisar Ismet. Serum cardiac markers in patients with acute myocardial infarction: oxidative stress, C-reactive protein and N-terminal probrain natriuretic peptide. J Clin Biochem Nutr. 2011;41:50–57. doi: 10.3164/jcbn.2007007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma S., Yeh E.T. C-reactive protein and atherothrombosis – beyond a biomarker: an actual partaker of lesion formation. Am J Physiol Regul Integr Comp Physiol. 2003;285:1253–1256. doi: 10.1152/ajpregu.00170.2003. [DOI] [PubMed] [Google Scholar]
- 3.Szmitko P.E., Wang C.H., Weisel R.D. New markers of inflammation and endothelial cell activation. Circulation. 2003;108:1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 4.Blake G.J., Ridker P.M. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol. 2003:4137–4142. doi: 10.1016/s0735-1097(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer J.H., Allayee H., Dwyer K.M. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2007;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 6.Iovannisci D.M., Lammer E.J., Steiner L. Association between a leukotriene C4 synthase gene promoter polymorphism and coronary artery calcium in young women: the Muscatine study. Arterioscler Thromb Vasc Biol. 2007;27:394–399. doi: 10.1161/01.ATV.0000252680.72734.10. [DOI] [PubMed] [Google Scholar]
- 7.Helgadottir A., Manolescu A., Thorleifsson G. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 8.Freiberg J.J., Tybjaerg Hansen A., Sillesen H., Jensen G.B., Nordestgaard B.G. Promotor polymorphisms in leukotriene C4 synthase and risk of ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol. 2008;28:990–996. doi: 10.1161/ATVBAHA.107.158873. [DOI] [PubMed] [Google Scholar]
- 9.Tsai A.K., Li N., Hanson N.Q., Tsai M.Y., Tang W. Associations of genetic polymorphisms of arachidonate 5-lipoxygenase-activating protein with risk of coronary artery disease in a European–American population. Atherosclerosis. 2009;207:487–491. doi: 10.1016/j.atherosclerosis.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Hannia C., Baylin A., Janna H., Hooman A. Dietary arachidonic acid, 5-lipoxygenase and myocardial infarction. Circulation. 2007;116:782. (abstract 3458) [Google Scholar]
- 11.Sanchez Galan E., Gomez Hernandez A., Vidal C., Luis Martin Ventura J., Miguel Blanco-Colio L. Leukotriene B4 enhances the activity of nuclear factor-kappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res. 2009;81:216–225. doi: 10.1093/cvr/cvn277. [DOI] [PubMed] [Google Scholar]
- 12.van Bilsen M., Engels W., van der Vusse G.J., Reneman R.S. Significance of myocardial eicosanoid production. Mol Cell Biochem. 1989;88:113–121. doi: 10.1007/BF00223432. [DOI] [PubMed] [Google Scholar]
- 13.Takase B., Maruyama T., Kurita A. Arachidonic acid metabolites in acute myocardial infarction. Angiology. 1996;47:649–661. doi: 10.1177/000331979604700703. [DOI] [PubMed] [Google Scholar]
- 14.Huch H.Y., Pearce S.F., Tersher I.M., Schindler I.L., Silverstain R.L. Blood. 1996:265–271. 8th. [Google Scholar]
- 15.Axelrod B., Cheesbrough T.M., Laakso S. Meth Enzymol. 1981;71:441–453. [Google Scholar]
- 16.Shimazu T., Kondo K., Hayaishi O. Role of prostaglandin endoperoxides in the serum thiobarbituric acid reaction. Arch Biochem Biophys. 1981;206:271–276. doi: 10.1016/0003-9861(81)90091-6. [DOI] [PubMed] [Google Scholar]
- 17.Castelli W.P. Lipids, risk factors and ischaemic heart disease. Atherosclerosis. 1996;124:1–9. doi: 10.1016/0021-9150(96)05851-0. [DOI] [PubMed] [Google Scholar]
- 18.Ridker P.M., Rifai N., Rose L., Buring J.E., Cook N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 19.Spanbroek R., Grabner R., Lotzer K. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L., Moos M.P., Grabner R. The 5-lipoxygenase pathway arachidonic acid metabolites in acute myocardial infarction. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 21.van der Net J.B., Versmissen J., Oosterveer D.M. Arachidonate 5-lipoxygenase-activating protein (ALOX5AP) gene and coronary heart disease risk in familial hypercholesterolemia. Atherosclerosis. 2009;203:472–478. doi: 10.1016/j.atherosclerosis.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Wong S.C., Fukuchi M., Melnyk P., Rodger I., Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-kappaB in myocardium of patients with congestive heart failure. Circulation. 1998;98:100–103. doi: 10.1161/01.cir.98.2.100. [DOI] [PubMed] [Google Scholar]
- 23.Moscardo A., Santos M.T., Fuset M.P., Ruano M., Valles J. Residual cyclooxygenase-1 activity and epinephrine reduce the antiplatelet effect of aspirin in patients with acute myocardial infarction. Thromb Haemost. 2011;105:663–669. doi: 10.1160/TH10-08-0550. [DOI] [PubMed] [Google Scholar]


