Highlights
► Cowden Syndrome is a rare hereditary cancer syndrome, which confers an increased risk of breast, thyroid, endometrial and colon cancer. ► Atypical polypoid adenomyoma does not generally represent a premalignant lesion, but must be carefully screened for foci of malignancy. ► Cancer screening must be intensified for patients who meet the diagnostic criteria for Cowden Syndrome.
Keywords: Atypical polypoid adenocarcinoma, Cowden Syndrome, Endometrial cancer
Introduction
Cowden Syndrome is a rare multiple hamartoma syndrome that confers an increased risk of breast, thyroid, endometrial, and potentially colon cancer in affected individuals with lifetime risks estimated at 5–10% for endometrial cancer, 25–50% for breast cancer, 3–10% for thyroid cancer and 13% for colon cancer (Daley et al., 2011). Cowden Syndrome has an incidence of 1 in 200,000 (NCCN guidelines, reference 102–3), and is defined at the molecular level by a mutation in the phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase (PTEN) gene located at chromosome 10q23.3. PTEN is an intracellular signaling molecule involved in cellular proliferation and apoptosis (Daley et al., 2011). The clinical diagnosis of Cowden Syndrome can be difficult and is based on several clinical criteria. A personal history of Bannayan–Riley–Ruvalcaba Syndrome (BRRS), adult Lhermitte–Duclos disease (LDD), autism with macrocephaly, or ≥ 2 trichelemomma would warrant testing. Major criteria include breast cancer, mucocutaneous lesions, macrocephaly, endometrial cancer, non-medullary thyroid cancer, or multiple GI hamartomas or ganglioneuromas. Minor criteria include other thyroid lesions, mental retardation, autism spectrum disorder, single GI hamartomas or ganglioneuromas, fibrocystic breast disease, lipomas, fibromas, renal cell carcinoma, and uterine fibroids. Testing is warranted if: ≥ Three major criteria (only need two if macrocephaly is one of them), ≥ Two major plus one minor criteria, one major plus 3 minor criteria, or ≥ Four minor criteria (NCCN guidelines) (Nelen et al., 1996).
Atypical polypoid adenomyoma (APA) is an uncommon lesion of the uterus generally seen in premenopausal women. APA is defined pathologically by a proliferation of irregular endometrial glands with squamous metaplasia and morule formation embedded within a prominent cellular smooth-muscle stroma (Eng, 2000). APA usually presents with abnormal uterine bleeding in conjunction with a polypoid mass in the lower uterine segment (Mazur, 1981). These lesions are most commonly benign, but have been reported to co-exist with endometrioid endometrial carcinoma; however APA has not been previously reported as a manifestation of Cowden Syndrome in the English literature (Sugiyama et al., 1998).
The objective of this paper was to report a case of Cowden Syndrome with associated endometrial carcinoma and APA and to discuss the diagnosis, screening and risk reduction strategies for clinicians taking care of women with this significant gynecologic and breast cancer syndrome.
Case
A 33-year-old nulliparous woman with a past medical history significant for obesity, type 2 diabetes mellitus, hypertension, hyperlipidemia, and multinodular thyroid disease presented with abnormal vaginal bleeding and a large vaginal mass. Past gynecologic history is significant for menarche at age 10 with irregular periods, which had been previously managed with oral contraceptives. Four years prior to this presentation, a Pap test found atypical squamous cells (HPV negative) and endometrial cells but she had never had any endometrial evaluation.
She has a family history significant for Cowden Syndrome by way of positive molecular diagnostic testing in her father and she met clinical diagnosis criteria as she had three major criteria (macrocephaly, biopsy proven trichilemmoma and multiple GI hamartomas and ganglioneuromas), and several minor criteria (thyroid disease and lipomas). The patient had not received molecular diagnostic testing; however, she had undergone genetic counseling as part of her routine care but had never had been compliant with any additional screening or care recommendations based on the Cowden diagnosis.
Her abnormal bleeding was initially attributed to polycystic ovarian syndrome; however examination by referring physician revealed a large vaginal mass. Subsequent MRI of the pelvis revealed a large heterogeneous loosely packed uterine mass extending from the dome of the uterus to the introitus. The mass was seen to expand the endometrial and vaginal canals without evidence of myometrial or vaginal wall invasion (Fig. 1).
Fig. 1.
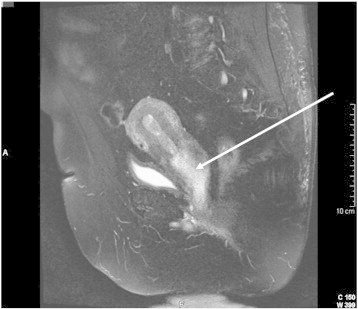
Large lower uterine segment mass.
T2 sagittal pelvic MRI with contrast demonstrating a large lower uterine segment mass, as indicated by arrow, prolapsing through the cervix and into the vagina.
She was taken to the operating room and transvaginal excision of mass and uterine curettage were performed. A 5.0 cm mass was prolapsing through the cervix with an endocervical vs. lower uterine segment origin and consisted of pink-brown soft tissue. Cut surfaces were white to tan and whorled in appearance. Microscopic view of hematoxylin and eosin stained slides demonstrated a complex mixture of smooth muscle and endometrial glands best described as atypical polypoid adenomyoma. Additionally, there was an endometrial tissue with a high degree of architectural complexity including cribriform and confluent glands consistent with endometrioid adenocarcinoma, FIGO Grade 1 with squamous metaplasia.
After discussion at Tumor Board and with the patient, she elected to undergo a robotic-assisted total laparoscopic hysterectomy. Frozen section revealed a grade one tumor with no evidence of myometrial invasion. Her ovaries were left in situ due to her desire to preserve ovarian function and possible future fertility. Her post-operative course was uneventful and she remains disease free.
Final pathologic analysis of the surgical specimen indicated a focal area of endometrioid adenocarcinoma, FIGO grade I, limited to the endometrium (Fig. 2). This well-differentiated lesion arose within the atypical polypoid adenomyoma and exhibited several areas of squamous differentiation.
Fig. 2.
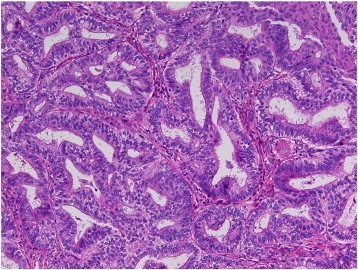
Focus of cancer in APA.
H&E stain at 40 × magnification of surgical specimen demonstrates a focus of FIGO grade 1 endometrioid adenocarcinoma arising in the APA. Sections show tightly packed/crowded endometrial glands with cribriforming.
Comment
The diagnosis of Cowden Syndrome can be difficult to establish partly due to the rarity of the diagnosis and the unfamiliarity of many physicians with the syndrome characteristics. This patient met the criteria for genetic testing for Cowden as she had three major criteria (biopsy proven trichilemmoma, endometrial cancer, multiple GI hamartomas and ganglioneuromas). In an individual with a relative diagnosed with Cowden Syndrome, only one major criterion or two minor criteria are necessary to justify testing. If there is a known familial PTEN mutation, then testing should be considered for the specific mutation. If there is no known mutation, then the affected family member with the highest likelihood of mutation should be tested. In this case, the patient's father had a known mutation. She declined testing. Therefore screening and counseling were provided as if she was positive.
Recommended cancer screening for women with Cowden Syndrome includes breast, colon, skin and thyroid evaluation (Table 1). NCCN recommends self-breast exam training and education starting at age 18 with a clinical breast exam annually at age 25 or 5–10 years before earliest known family breast cancer. Additionally annual mammography and breast MRI screening should begin at age 30–35 or 5–10 years before the earliest known family breast cancer. Endometrial cancer screening is based primarily on education. Patients should be educated about symptoms and there should be a low threshold for endometrial sampling. In addition to female specific screening exams, all patients with Cowden's syndrome should have comprehensive annual exam and dermatologic exam. They should also have a baseline thyroid ultrasound at age 18 with consideration for annual exam thereafter. Finally, colonoscopy at age 35 then every 5–10 years based on findings should be performed due to the association with early onset colon cancer (about 13%) as well as the numerous polyps. In addition to screening, patients should be counseled regarding risk reduction methods including mastectomy and hysterectomy; this should be discussed with patients on a case-by-case basis and patients should be counseled on degree of protection, extent of cancer risk, and reconstruction options.
Table 1.
Cowden Syndrome screening recommendations.
| Cancer | Screening recommendation |
|---|---|
| Breast | Annual clinical breast exam — starting at 25 or 5–10 years before earliest known breast cancer in family. Annual mammography and breast MRI — starting at 30–35 or 5–10 years before earliest known breast cancer in family. Discuss risk-reducing mastectomy including degree of protection, cancer risk, and reconstructive options. |
| Thyroid | Baseline thyroid ultrasound at age 18 with consideration for annual ultrasounds. |
| Endometrial | Patient education regarding symptoms along with screening methods and effectiveness. Discuss risk-reducing hysterectomy including degree of protection, cancer risk, and fertility options. |
| Colon | Consider colonoscopy at 35 then every 5–10 years based on findings. |
| Skin | Annual dermatologic exam |
Progression of atypical polypoid adenomyoma to endometrial adenocarcinoma is not generally seen as a risk in clinical practice. Exploration of the literature reveals that the molecular pathogenesis of Cowden Syndrome may be related to the development of APA and its progression to endometrial cancer. Inactivation of PTEN can cause activation of the PI3K–Akt pathway causing increased cellular levels of β-catenin (Longacre et al., 1996). β-catenin has been shown to activate gene expression leading to increased cellular proliferation and decreased apoptosis (Stambolic et al., 1998). Molecular analysis of APA specimens demonstrates accumulation of β-catenin in both the cytoplasm and nucleus without β-catenin gene (CTNNB-1) mutation. Additionally, CTNNB-1 mutation leading to increased cellular β-catenin is commonly seen in the transition from endometrial hyperplasia to endometrioid endometrial carcinoma (Peifer, 1997). Mutation in PTEN and its effects on down-stream pathways are also frequently seen in EEC (Ota et al., 2003). Therefore, a genetic background of Cowden Syndrome may have contributed to the development of APA and its progression to cancer in this patient.
APA does not have a standard treatment regimen in clinical practice. Patients who desire preserved fertility are often treated with polypectomy or dilation and curettage. Successful pregnancies have been documented after these procedures (Bussaglia et al., 2000). When fertility is no longer desired, simple hysterectomy serves as a definitive treatment. In a pre-menopausal woman with no other indication for salpingo-oophorectomy, the ovaries can be left in situ in order to prevent surgical menopause.
Development of endometrioid endometrial carcinoma in APA warrants definitive treatment. The most appropriate treatment is hysterectomy with staging. In the setting of Cowden Syndrome, full pathologic evaluation of all APA specimens in search of an occult carcinoma is especially important because of the possible molecular contribution to progression and the implications on clinical treatment. Even though this patient demonstrated malignant transformation, no surveillance above that required for any patient with Cowden Syndrome is required.
Limited knowledge of both endometrioid endometrial carcinoma in Cowden Syndrome and APA indicates that further studies are necessary in order to elucidate the molecular pathogenesis of these conditions. The unusual progression of APA to cancer in this genetic background highlights the role of both PTEN and its downstream activators on carcinogenesis, especially endometrial carcinogenesis. Additionally, clinical screening, prevention, and treatment guidelines need to be developed based on both the molecular and clinical knowledge of these conditions.
Conflict of interest statement
None of the aforementioned authors have any conflicts of interest to declare in relation to this publication.
Contributor Information
James M. Edwards, Email: james.edwards@duke.edu.
Susan C. Modesitt, Email: scm6h@virginia.edu.
References
- Bussaglia E., Del Rio E., Matias-Guiu X., Prat J. PTEN mutations in endometrial carcinomas: a molecular and clinicopathologic analysis of 38 cases. Hum. Pathol. 2000;31:312–317. doi: 10.1016/s0046-8177(00)80244-0. [DOI] [PubMed] [Google Scholar]
- Daley M.B., Allen J., Axilbund J.D., Buys S., Crawford B., Farrell C.D. NCCN Guidelines Version 1. 2011. Genetic/Familial High-risk Assessment: Breast and Ovarian. [Google Scholar]
- Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J. Med. Genet. 2000;37:828–830. doi: 10.1136/jmg.37.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longacre T.A., Chung M.H., Rouse R.V., Hendrickson M.R. Atypical polypoid adenomyofibroma (atypical polypoid adenomyomas) of the uterus. Am. J. Surg. Pathol. 1996;20:1–20. doi: 10.1097/00000478-199601000-00001. [DOI] [PubMed] [Google Scholar]
- Mazur M.T. Atypical polypoid adenomyoma of the endometrium. Am. J. Surg. Pathol. 1981;5:473–482. doi: 10.1097/00000478-198107000-00006. [DOI] [PubMed] [Google Scholar]
- Nelen M.R., Padberg G.W., Peeters E.A.J., Lin A.Y., van den Helm B., Frants R.R. Localization of the gene for Cowden disease to 10q22–23. Nat. Genet. 1996;13:114–116. doi: 10.1038/ng0596-114. [DOI] [PubMed] [Google Scholar]
- Ota S., Catasus L., Matias-Guiu X., Bussaglia E., Lagarda H., Pons Molecular pathology of atypical polypoid adenomyoma of the uterus. Hum. Pathol. 2003;34:784–788. doi: 10.1016/s0046-8177(03)00246-6. [DOI] [PubMed] [Google Scholar]
- Peifer M. β-catenin as oncogene. Science. 1997;275:1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- Stambolic V., Suzuki A., de la Pompa J.L., Brothers G.M., Mirtsos C., Sasaki T. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Ota S., Nishida T., Okura N., Tanabe K., Yakushiji M. Two cases of endometrial adenocarcinoma arising from atypical polypoid adenomyoma. Gynecol. Oncol. 1998;71:141–144. doi: 10.1006/gyno.1998.5137. [DOI] [PubMed] [Google Scholar]


