Highlights
► Skin metastasis of ovarian cancer is rare, often nodular in appearance, and conveys a poor prognosis. ► This patient developed an unusual maculo-papular rash which was biopsy-proven to be metastatic endometrioid adenocarcinoma. ► Pruritic symptoms from skin metastases should be palliated; SSRIs, local radiation, and topical creams all may play a role.
Keywords: Ovarian cancer, Cutaneous metastases, Palliation
Introduction
Recurrent ovarian cancer most commonly presents with intra-peritoneal or intra-abdominal lymphatic metastases. Unusual sites of progressive or metastatic disease have been reported either in women who have been heavily pretreated or who recur after a prolonged disease free interval (Dauplat et al., 1987). The classic presentation with a “Sister Mary Joseph node” at the umbilicus from intra-abdominal extension via lymphatics is a well known example of skin metastases (Tourad et al., 2000). However, diffuse skin metastases in other locations are unusual. We describe a patient whose progressive ovarian cancer manifested as a pruritic rash and review the literature on this topic.
Case report
A 54 year old Caucasian woman presented to her oncologist complaining of a pruritic rash that had developed along her inguinal fold and the vulvo-vaginal area. At age 50 she was diagnosed with synchronous stage IIC high-grade endometrioid ovarian adenocarcinoma and a stage IIA low-grade endometrioid endometrial adenocarcinoma. After optimal cytoreductive surgery, she received six cycles of intravenous carboplatin and paclitaxel chemotherapy. Biopsy-proven disease recurrence was detected by CT scan 12 months after completing primary therapy, and she began treatment with weekly paclitaxel. Progressive lymphadenopathy along the left common iliac nodes was detected 9 months later and the patient switched to single-agent doxorubicin as part of a randomized clinical trial. Four months later, and 13 months after the initial cancer recurrence, a CT scan demonstrated increased retroperitoneal lymphadenopathy and the patient crossed-over to receive the experimental arm of the trial, receiving doxorubicin plus 3G3, a monoclonal antibody therapy (Shah et al., 2010). Disease progression led to Avastin with metronomic cyclophosphamide, on which she was maintained with good effect for another 10 months when progressive disease necessitated a switch to carboplatin and gemcitabine. Table 1 outlines the time line of her disease recurrences and the different types of therapy that she received.
Table 1.
Treatment timeline.
| Time since diagnosis (months) | Symptom | Intervention |
|---|---|---|
| 0 | Abdominal distention | Surgery; carboplatin/taxol × 6 cycles |
| 12 | Biopsy-proven nodal recurrence | Weekly paclitaxel |
| 21 | Progressive lymphadenopathy | Doxorubicin |
| 25 | Increased retroperitoneal lymphadenopathy | Doxorubicin + 3G3 |
| 27 | Progression | Avastin and metronomic cyclophosphamide |
| 37 | Peritoneal thickening and skin metastasis | Carboplatin and gemcitabine |
The patient presented to her oncologist complaining of a new intensely pruritic, erythematous, maculo-papular rash that had developed over the course of a few weeks along her left inner thigh, and extended along the inguinal fold to the left labia majora and mons pubis (Fig. 1). She had no personal history of dermatologic conditions, including psoriasis. The patient was referred to Dermatology after experiencing no relief from topical steroids and anti-fungal ointments and a punch biopsy of the skin showed malignant cells.
Fig. 1.
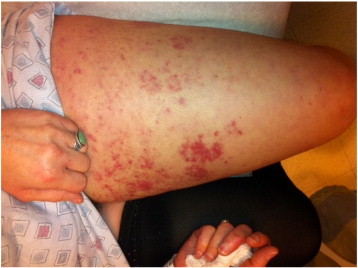
The erythematous rash began as seen here and extended to the mons pubis along the inguinal fold.
The patient had concurrent intraperitoneal disease progression at the time of her diagnosed skin lesion, and was offered conversion to chemotherapy with carboplatin and gemcitabine. The patient was referred to radiation oncology, where it was recommended she undergo local external beam radiation therapy to the site of skin metastasis for palliative treatment. She received 400 cGy fractions twice weekly for a total dose of 2400 cGy. On follow-up this appeared to have little impact on her local disease burden, which was noted still to be easily palpable 6 weeks after treatment. She was also treated with Paroxetine and Mirtazipine therapy for control of the pruritus. Over a gradual period of approximately 6 weeks she noted substantial improvement in her symptoms from the combination of medications and radiation. The patient's clinical course deteriorated with progression of her intra-abdominal disease, and she withdrew from curative therapy to enroll in a comprehensive palliative care program, of which these therapies were a part. She passed away from complications of her disease 10 months after her skin metastasis was diagnosed.
Pathology
The punch biopsy showed a poorly differentiated adenocarcinoma present in the dermis with extensive lymphovascular invasion. Immunohistochemical stains revealed that the tumor is positive for CK7 and CA-125 and negative for CK20 (not shown), CDX-2, and WT-1 (not shown). The immunohistochemistry stains of the skin metastasis match the ascites drained at the time of the ovarian debulking, and the poorly differentiated morphology is consistent with metastasis from the patient's known endometrioid adenocarcinoma. While IHC stains were not initially done on each of the patient's two primary tumors, the morphology of the metastasis is much more similar to the ovarian tumor and is much more likely to be an ovarian metastasis, as opposed to a poorly-differentiated metastasis of the synchronous low-grade endometrial tumor (Fig. 2).
Fig. 2.
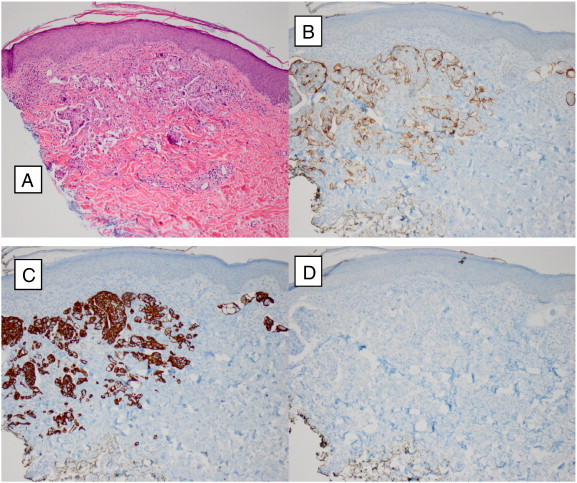
A: H&E 100 ×; B: CA-125 positive 100 ×; C: CK7 positive 100 ×; D: CDX 2 negative 100 × .
Discussion
Ovarian cancer remains the leading cause of death among gynecologic cancers (Howlader et al., 2011). Patterns of metastasis in ovarian carcinoma are well described (Dauplat et al., 1987). Intra-abdominal spread along the peritoneum and encasement of the intra-peritoneal structures is most commonly seen along with pelvic and para-aortic lymphatic involvement at advanced stages. The most common site of extra-peritoneal involvement is the lung, with right-sided pleural effusion being the most common clinical finding to qualify a patient as Stage IV disease (Dauplat et al., 1987).
Cutaneous disease, by contrast, is quite uncommon. In a case series of nine patients from over 200 treated at a single center in Italy, Cormio et al. detected a rate of 3.5% for metastasis to the skin (Cormio et al., 2003). Many of the reported cases present as nodular lesions, commonly on the abdomen or thorax (Cowan et al., 1995), and commonly at or around abdominal wall incisions, either laparotomy or laparoscopy scars (Cormio et al., 2003).
Because skin metastases commonly present in the setting of advanced-stage, metastatic disease, the prognosis is poor (Tourad et al., 2000; Cormio et al., 2003; Cowan et al., 1995). In the series of nine patients by Cormio, the median survival after presentation of skin metastasis was 4 months (range 2–65 months). The small numbers make comparison difficult between skin metastasis and other presentations of advanced ovarian cancer, but comparing to malignant small bowel obstruction, for example, with a median overall survival of 7 months (Sartori et al., 2010), skin metastasis could be seen as a marker of poor prognosis.
There are no published protocols for the treatment of isolated ovarian skin metastasis. In a case report from Turkey (Demirci et al., 2010), a 43-year-old patient with isolated abdominal wall metastases was treated with 37.5 Gy in 2.5 Gy/day fractions of external beam radiation therapy. In follow-up she was noted to have tumor regression and was still alive at 7 months of follow-up.
In the case series from Cormio, patients who presented with skin metastasis as manifestation of recurrence were treated with chemotherapy with or without surgical resection of the lesion. Patients with surgical resection of their disease had a median survival of 35 months (range 25–65) while patients treated with chemotherapy only live a median of 2 months (range 1–3) (Cormio et al., 2003). It appears that surgical resection may offer a survival advantage, but these data were not randomized and presumably there were clinical differences that precluded surgical resection in the patients treated with chemotherapy alone. Further study is warranted to determine the value of surgical resection for cutaneous metastasis of ovarian cancer.
Treatment of this patient's severe localized pruritis also proved difficult. Pruritis has been reported as a presenting complaint of many solid tumors, including scrotal itch (prostate cancer), itchy nares (brain tumors), vulvar itch (cervical cancer) and peri-anal itch (rectal or sigmoid colon cancer) (Twycross et al., 2003). In the case of itch caused by malignant cell invasion of tissue, the physiologic mechanism is speculated to be release of irritating chemokines including histamine, IL-2, and papain. These substances trigger stimulation of a subset of nerves called C-fibers, which transmit the signal from the pruritogen to the central nervous system, triggering awareness of the sensation. Some data exist for the use of paroxetine therapy to decrease pruritis symptoms however this appears to be a short-term benefit (Krajnik and Zylicz, 2001). Other localized therapy, including moisturizers to prevent dry skin, and treatment such as external beam radiation therapy to reduce the presence of disease at the site of pruritus is indicated as well. Of note, 2 months after our patient underwent radiation therapy; her pruritus had diminished significantly despite a persisting palpable disease burden. The mechanism for this improvement is unclear.
Summary
Cutaneous metastasis of ovarian epithelial adenocarcinoma is rare. Treatment strategies depend on the associated disease burden, but for treatment of the skin lesion itself, roles have been suggested for multi-modal therapy including surgical resection of nodular disease, systemic chemotherapy, and external beam radiation therapy to the site of metastasis. Overall, the prognosis remains poor. Attention should be paid to palliation of symptoms caused by the lesions, including pruritus. Local topical treatments, local radiation therapy, and orally-administered anti-serotonergic therapy also have been reported to have some success.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Cormio G., Capotort M., Di Vagno G., Cazzolla A., Carriero C., Carriero C., Selvaggi l. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol. Oncol. 2003;90(3):682–685. doi: 10.1016/s0090-8258(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Cowan L.J., Roller J.I., Connelly P.J., Nahhas W.A. Extraovarian stage IV peritoneal serous papillary carcinoma presenting as an asymptomatic skin lesion – a case report and literature review. Gynecol. Oncol. 1995;57(3):433–435. doi: 10.1006/gyno.1995.1169. [DOI] [PubMed] [Google Scholar]
- Dauplat J., Hacker N.F., Nieberg R.K., Berek J.S., Rose T.P., Sagae S. Distant metastases in epithelial ovarian carcinoma. Cancer. 1987;60:1561–1566. doi: 10.1002/1097-0142(19871001)60:7<1561::aid-cncr2820600725>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Demirci S., Yavas F., Ozsaran Z., Ozsaran A., Dikmen Y., Zekioglu O., Karabulut B., Aras A.B. Palliative radiotherapy for the skin metastasis of ovarian cancer: a case report and review of the literature. Med. Oncol. 2010;27(3):628–631. doi: 10.1007/s12032-009-9259-z. [DOI] [PubMed] [Google Scholar]
- Howlader N., Noone A.M., Krapcho M., Neyman N., Aminou R., Waldron W., Altekruse S.F., Kosary C.L., Ruhl J., Tatalovich Z., Cho H., Mariotto A., Eisner M.P., Lewis D.R., Chen H.S., Feuer E.J., Cronin K.A., Edwards B.K., editors. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: 2011. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site. [Google Scholar]
- Krajnik M., Zylicz Z. Understanding pruritus in systemic disease. J. Pain Symptom Manage. 2001;21:151–168. doi: 10.1016/s0885-3924(00)00256-6. [DOI] [PubMed] [Google Scholar]
- Sartori E., Chiudinelli F., Pasinetti B., Sostegni B., Maggino T. Possible role of palliative surgery for bowel obstruction in advanced ovarian cancer patients. Eur. J. Gynaecol. Oncol. 2010;21(1):31–36. [PubMed] [Google Scholar]
- Shah G.D., Loizos N., Youssoufian H., Schwartz J.D., Rowinsky E.K. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer. 2010;116(4 supplement):1018–1026. doi: 10.1002/cncr.24788. [DOI] [PubMed] [Google Scholar]
- Tourad J.P., Lentz N., Dutronc Y., Mercier E., Sagot P., Lambert D. Umbilical cutaneous metastasis (or Sister Mary Joseph's nodule) disclosing an ovarian adenocarcinoma. Gynecol. Obstet. Fertil. 2000;28(10):719–721. doi: 10.1016/s1297-9589(00)00009-6. [DOI] [PubMed] [Google Scholar]
- Twycross R., Greaves M.W., Handwerker H., Jones E.A., Libretto S.E., Szepietowski J.C., Zylicz Z. Itch: scratching more than the surface. Q. J. Med. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]


