Abstract
Objectives
The imidazoquinoline family of drugs are Toll-like receptor 7/8 agonists that have previously been used in the treatment of cutaneous leishmaniasis. Because of the hydrophobic nature of imidazoquinolines, they are traditionally not administered systemically for the treatment of visceral leishmaniasis. We formulated liposomal resiquimod, an imidazoquinoline, for the systemic treatment of visceral leishmaniasis.
Methods
By using lipid film hydration with extrusion, we encapsulated resiquimod in liposomes. These liposomes were then injected intravenously to treat BALB/c mice infected with Leishmania donovani.
Results
Treatment with liposomal resiquimod significantly decreased the parasite load in the liver, spleen and bone marrow. In addition, resiquimod treatment increased interferon-γ and interleukin-10 production in an antigen recall assay. Resiquimod was shown to be non-toxic in histology and in vitro culture experiments.
Conclusions
FDA-approved resiquimod, in a liposomal formulation, displays promising results in treating visceral leishmaniasis.
Keywords: drug delivery, imidazoquinoline, visceral, therapy
Introduction
There are over 30 species of Leishmania, which are the causative agent of the zoonotic disease leishmaniasis.1 Approximately 12 million people are infected with leishmaniasis worldwide, which results in ∼50 000 deaths a year, a death toll for parasitic infection that is surpassed only by malaria.2
Depending on which subspecies infects the patient, the disease can develop as cutaneous (L. major, L. tropica and L. mexicana), mucosal (L. braziliensis) or visceral leishmaniasis (VL; L. donovani and L. infantum).3,4 The most deadly form of the disease is the visceral form. In VL (kala-azar), L. donovani are transmitted by phlebotomine sand flies.5 Upon introduction into the mammalian host, L. donovani is usually internalized by macrophages in the dermis, whereupon they lose their flagella and become amastigotes. L. donovani is capable of surviving in the macrophage's phagolysosome. It proliferates and eventually spreads through the vascular and lymphatic systems. A high proportion of the parasite will eventually reside in the liver, spleen and bone marrow. If left untreated, VL usually results in death.
For the last 70 years, the most common treatment for VL has been the systemic injection of antimonials such as sodium stibogluconate (SSG) or meglumine antimoniate formulations. Antimonials are highly toxic and have severe adverse side effects including pancreatitis and cardiac arrhythmia.6 In addition, some strains of L. donovani have become resistant to antimonial therapies, requiring the development of other chemotherapeutics for VL. These therapies include the intravenous injection of liposomal amphotericin B (AmBisome) or orally administered miltefosine (Impavido, Miltex). Amphotericin B is highly toxic, requiring liposomal encapsulation for its parenteral administration. Moreover, with the increase in use of amphotericin B, there has been an emergence of drug resistance in Leishmania.7 Miltefosine has been shown in a clinical trial to have an 82% cure rate.8 However, it is teratogenic, which prevents its use by pregnant or potentially pregnant women. In addition, similarly to amphotericin B, there are now miltefosine-resistant Leishmania strains.9 Due to increased drug resistance and the limited application of current therapies, there is a need to develop alternative treatments for VL.
One alternative to conventional therapies, which primarily target the pathogen, is to target the host–pathogen interface.10 By enhancing the host's immune response, the pathogen can be effectively cleared while decreasing the potential for the development of drug resistance. This strategy has been employed for treating not only typhoid fever,11 tuberculosis12 and fungal infections,13 but also leishmaniasis. Prior research has shown that polysaccharides,14 non-methylated DNA,15 lipid A16 and other immune adjuvants17 have been successful in treating Leishmania infections. In particular, imiquimod, an FDA-approved Toll-like receptor (TLR) 7/8 agonist has been successful in clinical trials for cutaneous leishmaniasis.18,19 In addition, resiquimod (Figure 1), an imiquimod derivative and an FDA-approved molecule for topical delivery, has also shown promise in treating cutaneous Leishmania infections.20 Both imidazoquinolines induce interferon-α (IFN-α), interleukin-1β (IL-1β), IL-6 and tumour necrosis factor-α (TNF-α) in macrophages and monocytes.20 Buates and Matlashewski21 have shown that resiquimod decreases the amount of intracellular Leishmania by inducing the production of nitric oxide yet has no direct effect on the parasite.
Figure 1.
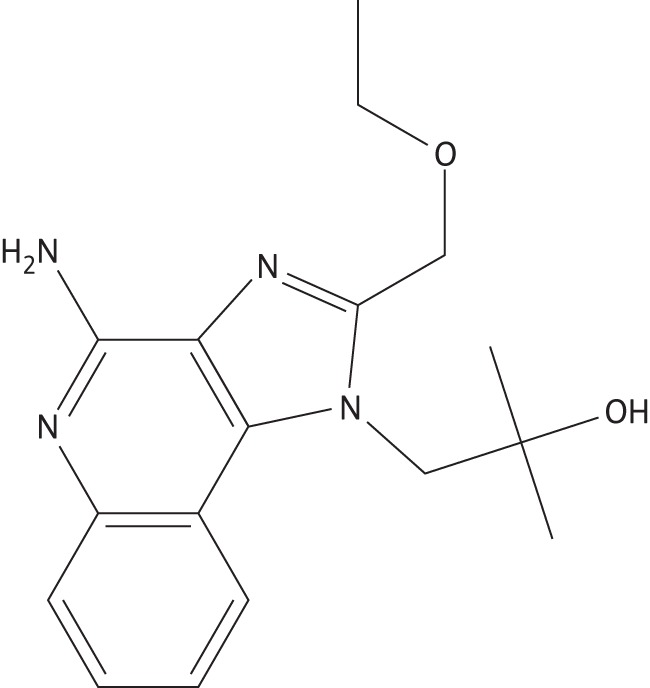
Chemical structure of resiquimod.
Recently, our group, as a proof of principle, encapsulated resiquimod in a polymeric drug delivery vehicle because the hydrophobic nature of resiquimod limits its parenteral use in vivo.22 The data presented in this manuscript expand on this work. We encapsulated resiquimod in a liposomal carrier for the treatment of visceral L. donovani infection. The toxicity of the formulation was measured in a cell viability assay. In addition, the efficacy of liposomal resiquimod was tested in a murine model infected with L. donovani by quantifying the parasites in the infected organs, the cytokine responses and the liver enzyme levels.
Materials and methods
Materials
All reagents were purchased and used unmodified from Sigma-Aldrich (St Louis, MO, USA) unless otherwise noted. All lipid membranes used for liposome formulation were purchased from Avanti Polar Lipids (Alabaster, Alabama, USA). Water (H2O) was purified using a Millipore (Billerica, MA, USA) Milli-Q Integral water purification system. Fluorescence and absorbance measurements were obtained on a Molecular Devices FlexStation 3 (Sunnyvale, CA, USA), courtesy of the Department of Chemistry and Biochemistry of the Ohio State University, USA. All reagents used for cytokine detection by ELISA were purchased from BioLegend Inc. (San Diego, CA, USA).
Animals
BALB/c mice aged 6–8 weeks were purchased from Jackson Labs (Bar Harbor, ME, USA). Syrian Golden hamsters aged 6–8 weeks were purchased from Harlan Labs (Indianapolis, IN, USA). All animal studies were in accordance with and approved by the Institutional Animal Care and Use Committee of the Ohio State University, USA.
Preparation of empty or resiquimod-loaded liposomes
Liposomes were formulated by addition of chloroform and methanol (9 : 1 v/v) to a mixture of hydrogenated (soy)l-α-phosphatidylcholine, 1,2-distearoyl-sn-glycero-3-[phospho-rac-(1-glycerol)], cholesterol (ovine wool) and d-α-tocopherol (Acros Organics, Morris Plains, NJ, USA), either with or without resiquimod (Enzo Life Sciences, Farmingdale, NY, USA). The lipid solution was rotary-evaporated with a Buchi R-200 rotary evaporator (New Castle, DE, USA) and a Buchi B-490 water bath set at 60°C. Lipids were reconstituted in H2O for 30 min in a 60°C water bath. Liposomes were freeze-thawed three times, followed by extrusion through an Avanti Mini-Extruder/Heating Block with an 80 nm polycarbonate membrane and filter supports (Alabaster, AL, USA) 11 times and then passed through a disposable PD-10 column (GE Healthcare, Pataskala, OH, USA). Sucrose was added to the liposomes (150 wt %) followed by lyophilization.
Determining size and encapsulated resiquimod in liposomes
Liposome size was determined by dynamic light scatter (DLS). Liposomes were suspended in H2O and sizing was performed using a NiComp Submicron Particle Sizer Model 370 (Santa Barbara, CA, USA) courtesy of Dr Robert Lee, College of Pharmacy, Ohio State University, USA. Encapsulation of resiquimod was determined by dissolving three sets of empty and resiquimod-loaded liposomes in ethanol (1 mg/mL) and placing the solution into the wells of a solvent-resistant 96-well plate in triplicate. A calibration curve containing resiquimod dissolved in ethanol was added to the plate. The plate was read at excitation 260 nm and emission 360 nm. The fluorescence readings for empty liposomes were subtracted from those of resiquimod-loaded liposomes and encapsulation was determined by comparison of the fluorescence readout with the calibration curve.
Infection and treatment protocol
L. donovani (Lv82 strain) was grown and maintained in Syrian Golden hamsters. BALB/c mice were infected with 1 × 107 amastigotes by tail vein injection. Mice were treated 2 weeks post-infection with 100 μL of tail vein injections of empty liposomes, resiquimod-loaded liposomes (7.6 μg of resiquimod per mouse) or SSG (500 mg/kg) (Albert David Ltd, Kolkata, India). Mice were sacrificed and analysed 2 weeks post-injection.
Histology and L. donovani units (LDUs) in treated mice
Parasite loads in the spleen and liver were quantified as previously described.23 Briefly, the liver and spleen smears were stained using Giemsa (Thermo Fisher Scientific, Waltham, MA, USA) and parasite loads were quantified microscopically. LDUs were calculated as the number of amastigotes per 500 nuclei multiplied by the weight (mg) of the liver or spleen. Parasite load counts in bone marrow were performed as previously described.24 Briefly, bone marrow was removed from the femurs of BALB/c mice. Smears of the bone marrow were stained with Giemsa and bone marrow LDUs were quantified as for spleen and liver. Sections of the liver and spleen were fixed in 10% formalin for 24 h and submitted to the Histology Core at the Ohio State University for haematoxylin and eosin (H&E) staining and further histopathology. Tissue sections from the organs of L. donovani-infected mice were stained using H&E and subjected to histopathology by a pathologist. Liver granulomas were enumerated and scored as follows: (i) no reaction; (ii) developing; (iii) mature; and (iv) empty. Samples were imaged using an Olympus BX41 light microscope (Olympus, Center Valley, PA, USA).
T cell proliferation and cytokine ELISA
T cell proliferation was performed as previously described.25 Briefly, 5 × 105 spleen cells were added in quadruplicate to the wells of sterile 96-well, flat-bottom tissue culture plates and stimulated with freeze-thawed L. donovani antigen (20 μg/mL). The proliferation responses were measured by an Alamar blue assay, as previously described.26 Supernatants were collected after 72 h of incubation at 37°C and analysed for the production of IFN-γ and IL-10.
Measurement of liver aminotransferase enzymes
Blood was collected in non-heparinized tubes and allowed to clot overnight at 4°C. Serum was obtained and submitted to the haematology laboratory of the Ohio State University Veterinary Hospital for analysis of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using the Cobas C501 serum analyser (Roche, Indianapolis, IN, USA).
Cell viability
Macrophages (RAW 264.7; ATCC, Manassas, VA, USA) were grown and maintained according to the manufacturer's guidelines. Dulbecco's Modified Eagle's Medium (500 mL; Fischer, Pittsburgh, PA) was supplemented with fetal bovine serum (50 mL; Hyclone, Pittsburgh, PA, USA) and penicillin–streptomycin (10 mL; Fischer, Pittsburgh, PA, USA). Macrophages were seeded in a 96-well plate at a concentration of 5 × 104 cells/well and allowed to become confluent. After the cells had adhered, the medium was removed and replaced with medium containing free resiquimod, liposomes loaded with resiquimod (0.312% wt/wt) or empty liposomes, or medium alone. Cell viability was determined after 24 h using an MTT assay. Briefly, medium in each well was replaced with fresh medium (150 μL) and MTT solution (20 μL of 5 mg/mL solution). The plate was incubated until purple formazan crystals had formed. These were then dissolved in isopropanol. The absorbance was measured at 560 nm and a background reading was measured at 670 nm. The viability of cells treated with resiquimod, liposomal resiquimod or empty liposomes was compared with that of untreated cells.
Results
Liposomal preparation
We used lipid film hydration with extrusion to form resiquimod liposomes. As measured by DLS, the resiquimod liposomes were found to be 75.0 ± 30.7 nm in diameter. After extrusion, liposomes were placed in a PD-10 size exclusion column to remove any non-encapsulated resiquimod. The liposomes were then lyophilized with a cryoprotectant (sucrose). The liposome yield was 60.4%, while the encapsulation efficiency of resiquimod was 7.0%, resulting in a weight loading of 1.1% drug per weight liposome.
Treatment of L. donovani infection
BALB/c mice were infected with L. donovani and treated 2 weeks post-infection with empty liposomes, resiquimod-loaded liposomes or SSG, as a positive control. Mice were sacrificed and analysed 2 weeks post-injection for L. donovani load. Figure 2(a) shows that the spleen LDUs of mice treated with liposomal resiquimod were lower than those of mice treated with empty liposomes (P ≤ 0.01). Mice treated with SSG, the current standard of treatment, had lower LDU counts than both empty liposomes (P ≤ 0.0005) and liposomes encapsulating resiquimod (P ≤ 0.003). In Figure 2(b), it can be noted that the liver LDUs of mice treated with liposomal resiquimod were lower than those of mice given empty liposomes (P ≤ 0.05). Mice treated with SSG had lower LDUs than mice treated with either empty liposomes (P ≤ 0.005) or resiquimod liposomes (P ≤ 0.03). In Figure 2(c), the bone marrow counts of amastigotes per 200 macrophages were lower in mice receiving liposomal resiquimod than those treated with empty liposomes (P ≤ 0.05), and bone marrow amastigote counts were significantly lower in mice treated with SSG compared with empty liposomes (P ≤ 0.005) or resiquimod liposomes (P ≤ 0.03).
Figure 2.
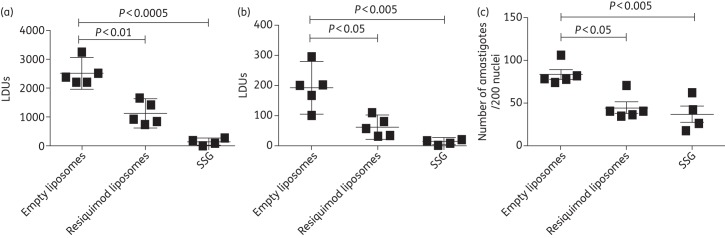
LDUs in the liver (a), spleen (b) and bone marrow (c) of BALB/c mice infected with L. donovani. Mice were treated 2 weeks post-infection with tail vein injections of empty liposomes, resiquimod (7.6 µg)-loaded liposomes or 500 mg/kg SSG. LDUs were determined 2 weeks post-injection using the number of amastigotes/500 nuclei. Data are presented as mean with 95% CI. n = 5 for empty or resiquimod-loaded liposomes and n = 4 for SSG.
T cell proliferation and cytokines
Splenocytes were isolated and cultured from sacrificed BALB/c mice infected with L. donovani. The cells were pulsed with L. donovani antigens and proliferation was measured after 72 h (Figure 3). Mice treated with encapsulated resiquimod showed a higher level of proliferation compared with those treated with empty liposomes (P ≤ 0.01), while those treated with the positive control SSG had a higher proliferation rate than mice treated with empty liposomes (P ≤ 0.0005) and were close to showing significantly higher proliferation when compared with mice treated with resiquimod liposomes (P ≤ 0.06).
Figure 3.
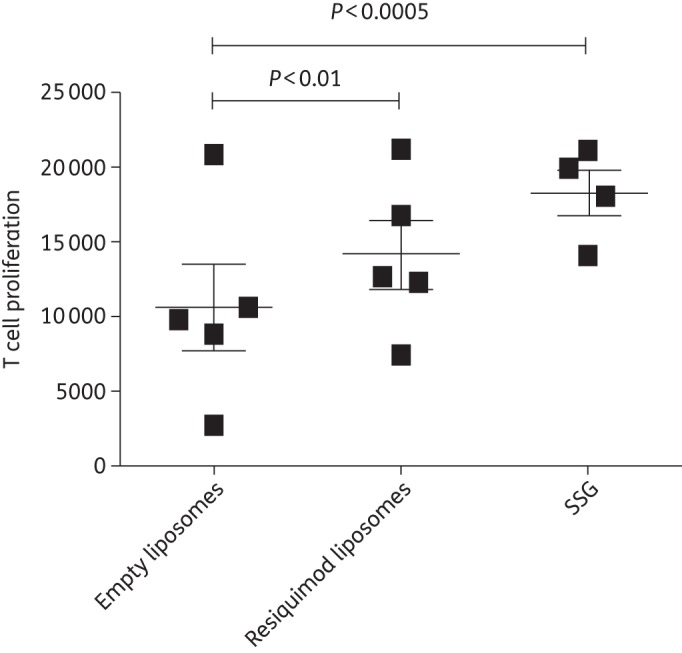
Proliferation of T cells isolated from the spleens of BALB/c mice infected with L. donovani and exposed to antigen. Mice were treated 2 weeks post-infection with empty liposomes, resiquimod (7.6 µg)-loaded liposomes or 500 mg/kg sodium SSG. Spleens were removed 2 weeks post-injection and pulsed with L. donovani antigen (20 µg/mL), and T cell proliferation was measured after 72 h. Data are presented as mean with 95% CI. n = 5 for empty or resiquimod-loaded liposomes and n = 4 for SSG.
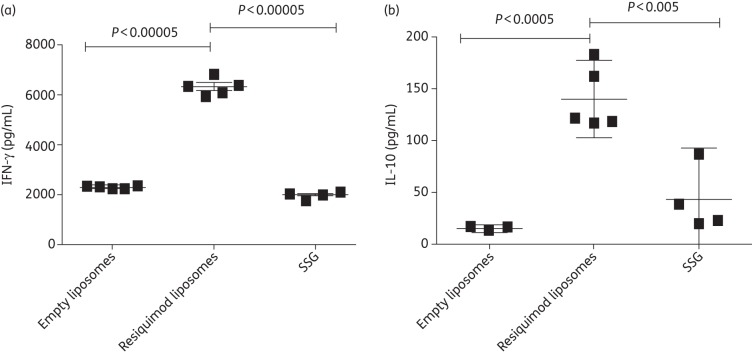
IFN-γ levels in mice treated with resiquimod liposomes were significantly higher than in mice treated with either empty liposomes (P ≤ 0.00005) or SSG (P ≤ 0.00005). Mice receiving resiquimod liposomes also had statistically significant increases in IL-10 production when compared with mice treated with empty liposomes (P ≤ 0.0005) or SSG (P ≤ 0.005) (Figure 4).
Figure 4.
Production of IFN-γ (a) or IL-10 (b) from splenic cells isolated from BALB/c mice infected with L. donovani. Mice were treated 2 weeks post-infection with empty liposomes, resiquimod (7.6 µg)-loaded liposomes or 500 mg/kg SSG. Spleens were removed 2 weeks post-injection and pulsed with L. donovani antigen (20 μg/mL), and cytokine production was measured after 72 h. Data are presented as mean with 95% CI. n = 5 for empty or resiquimod-loaded liposomes and n = 4 for SSG.
In vitro viability, histology and liver enzyme activity
RAW macrophages were cultured with various doses of resiquimod ranging from 0.04 to 5 μg/mL (Figure 5). The viability of macrophages cultured with free resiquimod or liposomal resiquimod was not significantly different. Free resiquimod at 5 μg/mL showed increased proliferation over empty liposomes (P ≤ 0.05). Macrophages cultured with liposomal resiquimod showed increased viability compared with empty liposomes at the two lowest concentrations, 0.07 and 0.03 μg/mL (P ≤ 0.05).
Figure 5.
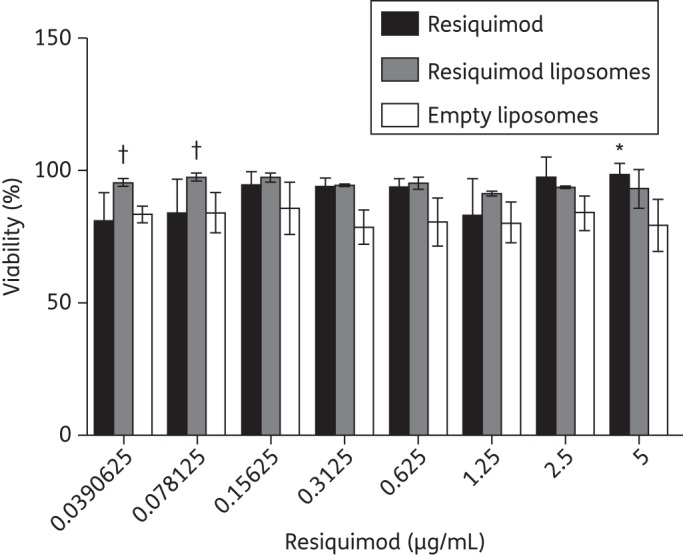
Macrophage viability after being cultured with free resiquimod, resiquimod-loaded liposomes or empty liposomes. Cells were seeded at 5 × 104 cells/well and treated for 24 h before viability was measured with respect to the untreated cells. Data are presented as mean ± SD. Statistical significance with respect to free resiquimod is presented as *P < 0.05 and significance with respect to empty liposomes is presented as †P < 0.05.
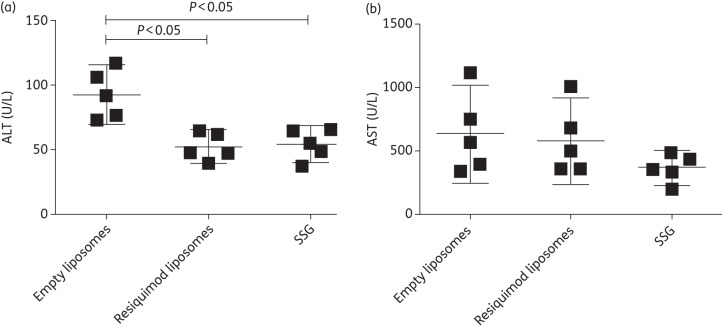
An analysis of the liver enzymes ALT (Figure 6a) and AST (Figure 6b) showed significantly lower levels of ALT in both the liposomal resiquimod (P ≤ 0.05) and SSG (P ≤ 0.05) groups; however, there was no significant difference in AST level between any of the groups. In addition, histology was performed on the liver and spleen and no visible differences in inflammation or toxicity were detected between mice treated with empty liposomes or liposomes encapsulating resiquimod (data not shown).
Figure 6.
Quantification of the liver enzymes ALT and AST in the serum of mice infected with L. donovani. Mice were treated 2 weeks post-infection with empty liposomes, resiquimod (7.6 µg)-loaded liposomes or 500 mg/kg SSG. Blood was collected 2 weeks post-injection, and serum levels are presented as mean with 95% CI. n = 5.
Discussion
Immunotherapy is a potential method that could be employed for the treatment of VL. Previous research has shown that TLR agonists such as CpG can be a possible treatment for VL infections.15 We propose the use of resiquimod, a TLR7/8 agonist, for the treatment of VL. Previous research has shown that a similar compound, imiquimod, was successful in treating cutaneous leishmaniasis.27,28
Unfortunately for their use via systemic administration, both imiquimod and resiquimod are highly insoluble in an aqueous environment. We hypothesized that liposomal resiquimod would allow for intravenous injection of the TLR 7/8 agonist for the treatment of VL. Liposomal encapsulation has been successful in the formulation of amphotericin B, a hydrophobic drug that is encapsulated in a liposome (AmBisome). Liposomal amphotericin B has shown decreased drug toxicity and facilitates the systemic delivery of the compound.29 We used a similar formulation to that used for AmBisome and encapsulated resiquimod in liposomes using lipid film hydration. The lipids were sized via extrusion to form particles approximately ∼80 nm in diameter, which is roughly the same size as those of AmBisome.30 This small liposomal size allows for a longer circulation time and substantial penetration into tissues such as the liver and spleen, which are sites where Leishmania resides. In addition, resiquimod-loaded liposomes would be likely to be approved for clinical use due to the similarity in formulation between our liposomes and those used in the FDA-approved AmBisome and also because of the FDA-approved status of resiquimod.
Our previous work of formulating resiquimod for in vivo applications used electrosprayed microparticles that were roughly 1 μm in diameter.22 Unfortunately, it would be difficult using electrospray technology or emulsion chemistry to make particles roughly the same size as those of AmBisome. Prior research has shown that liposomal formulations larger than 400 nm have selective uptake in the bone marrow,31 which can act as an important reservoir for the parasite32 and may contribute to relapses of VL after treatment has been administered. Future work will study the effect of the size of liposomal formulations on optimizing resiquimod concentrations in the liver, spleen, and bone marrow.
In this study, liposomal resiquimod was intravenously injected into mice 2 weeks post-infection. Our results indicate that encapsulated resiquimod treatment significantly decreased the number of L. donovani in the liver, spleen and bone marrow of infected mice compared with empty liposomes. As a positive control, we used a very high dose (500 mg/kg) of SSG (traditional dose <300 mg/kg). By developing an immunotherapy that targets the host interface, we can help to circumvent the formation of drug-resistant strains. This is significant, considering that in places such as Bihar State, India, it has been reported that over 60% of patients with VL do not respond to antimonial treatments. Sane et al.15 have shown that treating VL with both CpG and miltefosine led to a synergistic effect with respect to decreasing Leishmania load. Future experiments that co-deliver both amphotericin B and resiquimod, in liposomal forms, in the treatment of drug-resistant leishmaniasis might lead to an even a more significant reduction in parasite load.
To further characterize treatment with liposomal resiquimod, spleens from treated mice were cultured with Leishmania antigen and measured for cell proliferation and cytokine production. A slightly higher proliferation was observed when comparing the proliferation of splenocytes from resiquimod-treated mice and those given empty liposomes, but this difference was not seen with splenocytes from SSG-treated mice.
Splenocytes from mice treated with resiquimod liposomes showed a significant increase in the production of IFN-γ compared with those treated with empty liposomes or SSG. Prior research has shown that systemic treatment with resiquimod enhances the production of IFN-γ, which prevented and treated a T-helper 2 (TH2) allergy response.33 Since liposomal resiquimod treatment induced a high level of IFN-γ, we hypothesize that this, in part, is how resiquimod decreased Leishmania load. IFN-γ has been shown to be essential in clearing VL infections since it has been reported that IFN-γ knockout mice are highly susceptible to infections.34 In addition, Scott et al.35 have shown that mouse strains resistant to VL infection make a substantial amount of IFN-γ compared with susceptible mouse strains. On the other hand, treatment with encapsulated resiquimod induced a high level of IL-10 in splenocytes isolated from treated mice. Prior research has shown that resiquimod can simultaneously induce both IFN-γ and IL-10 production,36–38 while Boghdadi et al.39 have shown that peripheral blood mononuclear cells from patients infected with Schistosoma mansoni make high levels of both IFN-γ and IL-10 when stimulated with resiquimod. IL-10 has been linked to increased pathogenesis of VL infection since IL-10 knockout mice of both C57BL/6 and BALB/c strains are highly resistant to infections.40 However, prior research has also shown that CpG up-regulates IL-10 production in monocytes, similar to resiquimod.41,42 Overall, it seems that even though treatment with resiquimod liposomes induced IL-10 in mice, the parasite load was still decreased, potentially due to the large up-regulation of IFN-γ.
Our prior research has shown that resiquimod is a highly effective adjuvant in stimulating immune cells, with saturating responses in vitro at ∼0.1 μg/mL.28 When cultured with liposomal resiquimod, RAW macrophages showed no difference in cell viability up to 5 μg/mL. We therefore conclude that, at the concentrations necessary to stimulate host cells, resiquimod does not affect the viability of macrophages. Schön et al.43 have shown that imiquimod, but not resiquimod, induces apoptosis by the activation of caspases and Bcl-2-dependent translocation of cytochrome C. Oral resiquimod has been used in a Phase IIa clinical trial for hepatitis C treatment; no toxicity was shown in the trial and only two patients left the trial due to flu-like symptoms.44
Our histology studies showed that there were no differences in inflammation and toxicity in mice when comparing animals treated with empty liposomes with animals treated with liposomal resiquimod. Elevated liver enzymes, such as ALT and AST, are indicative of disease and represent possible damage to the liver.45 Although increased levels of these enzymes may represent drug compounds acting on parasites in the liver, it is generally accepted that lowered liver enzyme levels in the serum are indicative of diminished disease.46 Our liposomal resiquimod formulation showed equal levels of ALT compared with mice treated with SSG and significantly lowered levels compared with mice treated with empty liposome. The ALT levels seen in mice treated with SSG and liposomal resiquimod were considered in the normal range for a mouse;47 however, all groups showed higher than normal AST levels47 and samples from mice treated with SSG or liposomal resiquimod did not differ from those of mice that received treatment with empty liposomes. Additional studies will be performed to determine whether the increased levels of AST seen in treated mice are indicative of a progression or a diminution of the disease. Interestingly, however, the liposomal resiquimod followed the same pattern of liver enzymes as the current standard for treatment, SSG.
Conclusions
Overall, we have shown liposomal resiquimod is a potential treatment for VL. Treatment with liposomal resiquimod lowered the parasite load of Leishmania in the liver, spleen and bone marrow of mice infected with L. donovani. Furthermore, resiquimod induced the production of IFN-γ and IL-10 in splenocytes isolated from infected mice, with antigen recall. In vitro testing reported no noticeable toxicity, and levels of the liver enzyme ALT were decreased in mice treated with both resiquimod liposomes and SSG compared with mice treated with empty liposomes. The enzyme levels show decreased liver damage, probably due to lowered parasite levels, and the similar levels seen in mice treated with resiquimod liposomes and SSG indicate that the liposomal resiquimod formulation has low in vivo liver toxicity. Future work will be performed to explore in depth the systemic cytokines that are released during liposomal resiquimod treatment.
Funding
This work was supported by the National Institutes of Health grants awarded to A. R. S. and DARPA W911NF-10-1-0264 awarded to K. M. A.
Transparency declarations
None to declare.
Acknowledgements
We would like to acknowledge Dr Robert Lee of the College of Pharmacy, Division of Pharmaceutics for the use of his NiComp Submicron Particle Sizer Model 370. We would also like to thank Dr Karl Werbovetz of the College of Pharmacy, Division of Medicinal Chemistry for the use of his Buchi R-200 rotary evaporator and Buchi B-490 water bath.
References
- 1.Cummings HE, Tuladhar R, Satoskar AR. Cytokines and their STATs in cutaneous and visceral leishmaniasis. J Biomed Biotechnol. 2010;2010:294–389. doi: 10.1155/2010/294389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chappuis F, Sundar S, Hailu A, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–82. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 3.Alexander J, Bryson K. T helper (h)1/Th2 and Leishmania: paradox rather than paradigm. Immunol Lett. 2005;99:17–23. doi: 10.1016/j.imlet.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Kaye PM, Aebischer T. Visceral leishmaniasis: immunology and prospects for a vaccine. Clin Microbiol Infect. 2011;17:1462–70. doi: 10.1111/j.1469-0691.2011.03610.x. [DOI] [PubMed] [Google Scholar]
- 5.Elnaiem DE. Ecology and control of the sand fly vectors of Leishmania donovani in East Africa, with special emphasis on Phlebotomus orientalis. J Vector Ecol. 2011;36(Suppl 1):S23–31. doi: 10.1111/j.1948-7134.2011.00109.x. [DOI] [PubMed] [Google Scholar]
- 6.Seaman J, Mercer AJ, Sondorp HE, et al. Epidemic visceral leishmaniasis in southern Sudan: treatment of severely debilitated patients under wartime conditions and with limited resources. Ann Intern Med. 1996;124:664–72. doi: 10.7326/0003-4819-124-7-199604010-00007. [DOI] [PubMed] [Google Scholar]
- 7.Purkait B, Kumar A, Nandi N, et al. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother. 2012;56:1031–41. doi: 10.1128/AAC.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharya SK, Sinha PK, Sundar S, et al. Phase 4 trial of miltefosine for the treatment of Indian visceral leishmaniasis. J Infect Dis. 2007;196:591–8. doi: 10.1086/519690. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Victoria FJ, Castanys S, Gamarro F. Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob Agents Chemother. 2003;47:2397–403. doi: 10.1128/AAC.47.8.2397-2403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park B, Liu GY. Targeting the host-pathogen interface for treatment of Staphylococcus aureus infection. Semin Immunopathol. 2012;34:299–315. doi: 10.1007/s00281-011-0297-1. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Chen H, Wang Q, et al. IL-24 protects against Salmonella typhimurium infection by stimulating early neutrophil Th1 cytokine production, which in turn activates CD8+ T cells. Eur J Immunol. 2009;39:3357–68. doi: 10.1002/eji.200939678. [DOI] [PubMed] [Google Scholar]
- 12.Guo S, Zhao J. Immunotherapy for tuberculosis: what's the better choice? Front Biosci. 2012;17:2684–90. doi: 10.2741/4079. [DOI] [PubMed] [Google Scholar]
- 13.Parameswaran IG, Segal BH. Immunotherapy for fungal infections with special emphasis on central nervous system infections. Neurol India. 2007;55:260–6. doi: 10.4103/0028-3886.35687. [DOI] [PubMed] [Google Scholar]
- 14.Barroso PA, Marco JD, Calvopina M, et al. A trial of immunotherapy against Leishmania amazonensis infection in vitro and in vivo with Z-100, a polysaccharide obtained from Mycobacterium tuberculosis, alone or combined with meglumine antimoniate. J Antimicrob Chemother. 2007;59:1123–9. doi: 10.1093/jac/dkm079. [DOI] [PubMed] [Google Scholar]
- 15.Sane SA, Shakya N, Haq W, et al. CpG oligodeoxynucleotide augments the antileishmanial activity of miltefosine against experimental visceral leishmaniasis. J Antimicrob Chemother. 2010;65:1448–54. doi: 10.1093/jac/dkq164. [DOI] [PubMed] [Google Scholar]
- 16.Khan MA, Maruno M, Khaskhely NM, et al. Inhibition of intracellular proliferation of Leishmania parasites in vitro and suppression of skin lesion development in BALB/c mice by a novel lipid A analog (ONO-4007) Am J Trop Med Hyg. 2002;67:184–90. doi: 10.4269/ajtmh.2002.67.184. [DOI] [PubMed] [Google Scholar]
- 17.Hervas JA, Martin-Santiago A, Hervas D, et al. Old world Leishmania infantum cutaneous leishmaniasis unresponsive to liposomal amphotericin B treated with topical imiquimod. Pediatr Infect Dis J. 2012;31:97–100. doi: 10.1097/INF.0b013e31822dfbf7. [DOI] [PubMed] [Google Scholar]
- 18.Miranda-Verastegui C, Llanos-Cuentas A, Arevalo I, et al. Randomized, double-blind clinical trial of topical imiquimod 5% with parenteral meglumine antimoniate in the treatment of cutaneous leishmaniasis in Peru. Clin Infect Dis. 2005;40:1395–403. doi: 10.1086/429238. [DOI] [PubMed] [Google Scholar]
- 19.Arevalo I, Ward B, Miller R, et al. Successful treatment of drug-resistant cutaneous leishmaniasis in humans by use of imiquimod, an immunomodulator. Clin Infect Dis. 2001;33:1847–51. doi: 10.1086/324161. [DOI] [PubMed] [Google Scholar]
- 20.Dockrell DH, Kinghorn GR. Imiquimod and resiquimod as novel immunomodulators. J Antimicrob Chemother. 2001;48:751–5. doi: 10.1093/jac/48.6.751. [DOI] [PubMed] [Google Scholar]
- 21.Buates S, Matlashewski G. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. J Infect Dis. 1999;179:1485–94. doi: 10.1086/314782. [DOI] [PubMed] [Google Scholar]
- 22.Duong AD, Sharma S, Peine KJ, et al. Electrospray encapsulation of toll-like receptor agonist resiquimod in polymer microparticles for the treatment of visceral leishmaniasis. Mol Pharm. 2013;10:1045–55. doi: 10.1021/mp3005098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray HW. Mononuclear cell recruitment, granuloma assembly, and response to treatment in experimental visceral leishmaniasis: intracellular adhesion molecule 1-dependent and -independent regulation. Infect Immun. 2000;68:6294–9. doi: 10.1128/iai.68.11.6294-6299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhlencord A, Maniera T, Eibl H, et al. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob Agents Chemother. 1992;36:1630–4. doi: 10.1128/aac.36.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosas LE, Satoskar AA, Roth KM, et al. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to Leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168:158–69. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed SA, Gogal RM, Jr, Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods. 1994;170:211–24. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 27.Firooz A, Khamesipour A, Ghoorchi MH, et al. Imiquimod in combination with meglumine antimoniate for cutaneous leishmaniasis: a randomized assessor-blind controlled trial. Arch Dermatol. 2006;142:1575–9. doi: 10.1001/archderm.142.12.1575. [DOI] [PubMed] [Google Scholar]
- 28.Shamsi Meymandi S, Javadi A, Dabiri S, et al. Comparative histological and immunohistochemical changes of dry type cutaneous leishmaniasis after administration of meglumine antimoniate, imiquimod or combination therapy. Arch Iran Med. 2011;14:238–43. [PubMed] [Google Scholar]
- 29.Sundar S, Chakravarty J, Agarwal D, et al. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362:504–12. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 30.Barratt G, Bretagne S. Optimizing efficacy of amphotericin B through nanomodification. Int J Nanomedicine. 2007;2:301–13. [PMC free article] [PubMed] [Google Scholar]
- 31.Nagayasu A, Uchiyama K, Nishida T, et al. Is control of distribution of liposomes between tumors and bone marrow possible? Biochim Biophys Acta. 1996;1278:29–34. doi: 10.1016/0005-2736(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 32.Tropia de Abreu R, Carvalho M, Carneiro CM, et al. Influence of clinical status and parasite load on erythropoiesis and leucopoiesis in dogs naturally infected with Leishmania (Leishmania) chagasi. PLoS One. 2011;6:e18873. doi: 10.1371/journal.pone.0018873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xirakia C, Koltsida O, Stavropoulos A, et al. Toll-like receptor 7-triggered immune response in the lung mediates acute and long-lasting suppression of experimental asthma. Am J Respir Crit Care Med. 2010;181:1207–16. doi: 10.1164/rccm.200908-1255OC. [DOI] [PubMed] [Google Scholar]
- 34.Wang ZE, Reiner SL, Zheng S, et al. CD4+ effector cells default to the Th2 pathway in interferon γ-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–71. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott P, Natovitz P, Coffman RL, et al. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–84. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferret-Bernard S, Remot A, Lacroix-Lamande S, et al. Cellular and molecular mechanisms underlying the strong neonatal IL-12 response of lamb mesenteric lymph node cells to R-848. PLoS One. 2010;5:e13705. doi: 10.1371/journal.pone.0013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lombardi V, Van Overtvelt L, Horiot S, et al. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-γ, and IL-17A by naive CD4+ T cells. J Immunol. 2009;182:3372–9. doi: 10.4049/jimmunol.0801969. [DOI] [PubMed] [Google Scholar]
- 38.Sel S, Wegmann M, Bauer S, et al. Immunomodulatory effects of viral TLR ligands on experimental asthma depend on the additive effects of IL-12 and IL-10. J Immunol. 2007;178:7805–13. doi: 10.4049/jimmunol.178.12.7805. [DOI] [PubMed] [Google Scholar]
- 39.Boghdadi G, Khalik DA, Wahab SA, et al. Immunomodulatory effect of R848 on cytokine production associated with Schistosoma mansoni infection. Parasitol Res. 2013;112:135–40. doi: 10.1007/s00436-012-3116-2. [DOI] [PubMed] [Google Scholar]
- 40.Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28:378–84. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Waibler Z, Anzaghe M, Konur A, et al. Excessive CpG 1668 stimulation triggers IL-10 production by cDC that inhibits IFN-α responses by pDC. Eur J Immunol. 2008;38:3127–37. doi: 10.1002/eji.200838184. [DOI] [PubMed] [Google Scholar]
- 42.Samarasinghe R, Tailor P, Tamura T, et al. Induction of an anti-inflammatory cytokine, IL-10, in dendritic cells after toll-like receptor signaling. J Interferon Cytokine Res. 2006;26:893–900. doi: 10.1089/jir.2006.26.893. [DOI] [PubMed] [Google Scholar]
- 43.Schön M, Bong AB, Drewniok C, et al. Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod. J Natl Cancer Inst. 2003;95:1138–49. doi: 10.1093/jnci/djg016. [DOI] [PubMed] [Google Scholar]
- 44.Pockros PJ, Guyader D, Patton H, et al. Oral resiquimod in chronic HCV infection: safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. J Hepatol. 2007;47:174–82. doi: 10.1016/j.jhep.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 45.Sharma M, Sehgal R, Kaur S. Evaluation of nephroprotective and immunomodulatory activities of antioxidants in combination with cisplatin against murine visceral leishmaniasis. PLoS Negl Trop Dis. 2012;b:e1629. doi: 10.1371/journal.pntd.0001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundar S, Jha TK, Thakur CP, et al. Injectable paromomycin for visceral leishmaniasis in India. N Engl J Med. 2007;356:2571–81. doi: 10.1056/NEJMoa066536. [DOI] [PubMed] [Google Scholar]
- 47.Wege AK, Florian C, Ernst W, et al. Leishmania major infection in humanized mice induces systemic infection and provokes a nonprotective human immune response. PLoS Negl Trop Dis. 2012;6:e1741. doi: 10.1371/journal.pntd.0001741. [DOI] [PMC free article] [PubMed] [Google Scholar]