Abstract
Objectives
Treatment failure is multifactorial. Despite the importance of host cell drug transporters and metabolizing enzymes in the accumulation, distribution and metabolism of drugs targeting intracellular pathogens, their impact on the efficacy of antileishmanials is unknown. We examined the contribution of pharmacologically relevant determinants in human macrophages in the antimony-mediated killing of intracellular Leishmania panamensis and its relationship with the outcome of treatment with meglumine antimoniate.
Methods
Patients with cutaneous leishmaniasis who failed (n = 8) or responded (n = 8) to treatment were recruited. Gene expression profiling of pharmacological determinants in primary macrophages was evaluated by quantitative RT–PCR and correlated to the drug-mediated intracellular parasite killing. Functional validation was conducted through short hairpin RNA gene knockdown.
Results
Survival of L. panamensis after exposure to antimonials was significantly higher in macrophages from patients who failed treatment. Sixteen macrophage drug-response genes were modulated by infection and exposure to meglumine antimoniate. Correlation analyses of gene expression and intracellular parasite survival revealed the involvement of host cell metallothionein-2A and ABCB6 in the survival of Leishmania during exposure to antimonials. ABCB6 was functionally validated as a transporter of antimonial compounds localized in both the cell and phagolysosomal membranes of macrophages, revealing a novel mechanism of host cell-mediated regulation of intracellular drug exposure and parasite survival within phagocytes.
Conclusions
These results provide insight into host cell mechanisms regulating the intracellular exposure of Leishmania to antimonials and variations among individuals that impact parasite survival. Understanding of host cell determinants of intracellular pharmacokinetics/pharmacodynamics opens new avenues to improved drug efficacy for intracellular pathogens.
Keywords: ABCB6, MT2A, host–pathogen interactions, leishmaniasis, phagolysosomes
Introduction
In the absence of a vaccine and sustainable strategies of vector management, control of leishmaniasis relies on drug therapy. Pentavalent antimonials [meglumine antimoniate (Glucantime) or sodium stibogluconate (Pentostam)] continue to be the first-line treatment in Latin America. However, the emergence of resistance to antimonial drugs and increased rates of treatment failure threaten this control measure.
Although treatment failure is commonly attributed to parasite drug resistance, the therapeutic response is multifactorial, involving intrinsic or acquired differences in the drug susceptibility of the infecting Leishmania strain, the host immune response, pharmacokinetic (PK) and pharmacodynamic characteristics of the drugs, and genetic, metabolic and physiological variations among individual hosts. The central role of immune functions in the effectiveness of antimonials has been supported by studies in murine models of visceral leishmaniasis and immunocompromised patients. In mice, deficiency in tumour necrosis factor-α, interferon-γ or interleukin-12 reduces the efficacy of antimonials,1–3 while in patients, down-regulation of these cytokines has been linked to mucosal and chronic cutaneous disease,4,5 and immunodeficiencies are conducive to higher rates of disease reactivation following treatment.6 Despite this, failure is also frequently observed in immunocompetent individuals.
Our group and others have demonstrated that antimonial treatment failure in patients with cutaneous leishmaniasis (CL) can occur in the absence of parasite drug resistance during infections with drug-susceptible Leishmania.7–9 Furthermore, evidence suggests that the PK of antimony (Sb) in plasma alone does not define treatment response, as we have shown minimal interindividual variation in the plasma PK of Sb in adult patients treated with meglumine antimoniate.10 Therefore, other factors, such as intracellular PK and pharmacodynamics, are likely to play a key role in the therapeutic response to antimonials. Despite this, understanding of the role of pharmacological determinants in the therapeutic response is largely unexplored.
Leishmania, an obligate intracellular parasite, resides preferentially in phagocytes within membrane-defined phagolysosomes; therefore, antileishmanial drugs must cross these host cell barriers to target the intracellular parasite. Mookerjee Basu et al.11 showed that monocytes from patients with visceral leishmaniasis that were unresponsive to pentavalent antimonials, overexpress the multidrug resistance protein-1 (MRP-1) and permeability glycoprotein (P-gp) at the plasma membrane and this phenotype correlated with lower accumulation of intracellular Sb. However, the molecules involved in the exposure of the intraphagosomal parasite to the drug and their influence on the drug-mediated intracellular parasite killing remain unknown.
Through the exploration of host–pathogen–drug interactions, we sought to identify pharmacological determinants of human macrophages that affect the Sb-mediated intracellular parasite killing. Our results show that infection of human macrophages with Leishmania and exposure to antimonial drugs modulate the expression of drug transporters and metabolizing enzymes in the host cell. These findings expand the scope of this highly dynamic host–pathogen interaction to the modulation of antileishmanial drug exposure and response. We identified human ABCB6 as a novel transporter of antimonial compounds in human macrophages, located at the cell membrane and surrounding the Leishmania-containing phagosome. Importantly, the expression of ABCB6 in human macrophages correlates with intracellular parasite survival, hence identifying this molecule as an important host cell regulator of the Sb-mediated parasite killing.
Methods
Ethics
This study was approved and monitored by the institutional review board for ethical conduct of research involving human subjects of the Centro Internacional de Entrenamiento e Investigaciones Médicas, in accordance with national (resolution 008430, República de Colombia, Ministry of Health, 1993) and international (Declaration of Helsinki and amendments, World Medical Association, Seoul, Korea, October 2008) guidelines. All individuals voluntarily participated in the study and written informed consent was obtained from each patient.
Study design and study subjects
In order to evaluate the role of host cell factors on the therapeutic outcome of CL, patients were included based on the therapeutic response to meglumine antimoniate, infection with a Leishmania strain that was susceptible to Sb, age of 18–65 years and no apparent immune deficiencies or negative HIV tests. This strategy minimized the likelihood of potentially confounding effects of treatment failure as a result of parasite drug resistance, variations in drug PK10 or altered immune functions. The drug susceptibility of the clinical strains isolated from the participating patients was determined as intracellular amastigotes as previously described.12 Gene expression profiling of human drug transporters and metabolizing enzymes of primary human macrophages derived from peripheral blood mononuclear cells (PBMCs) obtained from each patient was employed to identify host cell genes with putative roles in intracellular parasite survival during drug exposure. The human monocytic cell line THP-1 was used for functional validation assays.
Adult patients (18–65 years) with diagnosis of active CL or documented history of parasitologically confirmed CL were recruited for this study based on the following criteria: (i) time of evolution <6 months; (ii) availability of the corresponding Leishmania strain; (iii) lack of apparent immune deficiencies (negative HIV test, evidence of immunological disorder or treatment with medication having immunomodulating effects); and (iv) who received standard-of-care treatment with meglumine antimoniate (20 mg/kg/day for 20 days). Patients were included in two study groups: (i) those failing standard-of-care treatment with meglumine antimoniate (n = 8); and (ii) those who were cured (n = 8).
Treatment outcome was evaluated at week 13 after initiation of treatment. Cure was defined as complete re-epithelialization and absence of inflammatory signs for all lesions. Clinical failure was defined as: incomplete re-epithelialization and/or the presence of induration, raised borders or redness in any lesion; reactivation of the original lesion(s); or the appearance of new lesions. The characteristics of the study participants are summarized in Table 1.
Table 1.
Characteristics of patients and Leishmania strains
| Characteristics | Treatment response | Treatment failure | P valuea |
|---|---|---|---|
| Subjects | n = 8 | n = 8 | |
| age (years), mean (range) | 40.3 (24–60) | 38.2 (20–50) | 0.368 |
| male, n (%) | 5 (62.5) | 7 (87.5) | 0.56 |
| ethnic group, n (%) | 0.46 | ||
| Afro-Colombian | 8 (100) | 6 (75) | |
| Mestizo | 0 | 2 (25) | |
| lesions per subject, median (range) | 1.5 (1–7) | 1 (1–3) | 0.26 |
| duration of older lesion (months), median (range) | 1 (1–2) | 2 (1–4) | 0.136 |
| Lesion characteristics | n = 19 | n = 12 | |
| location of lesion, n (%) | 0.023 | ||
| upper limbs | 11 (57.9) | 7 (58.3) | |
| head and neck | 4 (21.1) | 1 (8.3) | |
| trunk | 0 | 4 (33.3) | |
| lower limbs | 4 (21.1) | 0 | |
| type of lesion, n (%) | 0.16 | ||
| ulcer | 19 (100) | 10 (83.3) | |
| plaque | 0 | 1 (8.3) | |
| other | 0 | 1 (8.3) | |
| lesion areab (mm2), median (range) | 26.6 (9.4–62.8) | 63.7 (20.5–111.4) | 0.0001 |
| Leishmania strains | n = 8 | n = 8 | |
| species identification, n (%) | 0.46 | ||
| Leishmania (Viannia) panamensis | 8 (100) | 7 (87.5) | |
| Leishmania (Viannia) guyanensis | 0 | 1 (12.5) | |
| susceptibility profile, n (%) | |||
| susceptible | 8 (100) | 8 (100) | |
| resistant | 0 | 0 |
aStatistical analyses for categorical variables were performed using Fisher's exact test and for continuous variables using the Mann–Whitney U-test. Significance was established as P < 0.05.
bLesions were not measured in two subjects, one in each group.
Reagents and chemicals
Additive-free meglumine antimoniate (Walter Reed 214975AK; lot no. BLO918690-278-1A1W601) was kindly provided by the Walter Reed Army Institute, Silver Spring, MD, USA. Phorbol-12-myristate 13-acetate (PMA) was obtained from Sigma–Aldrich.
Clinical strains and drug susceptibility assays
Leishmania were isolated by needle aspiration of cutaneous lesions. Positive cultures were typed using monoclonal antibodies. The drug susceptibility of the intracellular parasites was estimated by evaluation of the percentage parasite survival in PMA-differentiated U-937 macrophages after exposure to meglumine antimoniate (32 μg/mL Sb; screening concentration), compared with the control without drug exposure. Leishmania strains were defined as Sb susceptible and Sb resistant when the percentage survival after drug exposure was <30% and >70%, respectively12 (Table S1, available as Supplementary data at JAC Online).
Cell culture and differentiation
Peripheral blood samples from healthy volunteers or patients with CL were obtained and processed to obtain PBMCs using a Ficoll-Hypaque (Sigma–Aldrich) gradient, following the manufacturer's instructions. Macrophages were differentiated from PBMCs by adherence to cell culture plasticware in serum-free RPMI for 2 h, followed by 7 days of incubation in RPMI supplemented with 20% fetal bovine serum (FBS) at 37°C and 5% CO2. The human monocytic cell line THP-1 was maintained at 1 × 106 cells/mL in RPMI 1640 (Gibco) supplemented with 10% heat-inactivated FBS, 100 μg/mL streptomycin and 100 U/mL penicillin at 37°C and 5% CO2. Cultured cells were differentiated to macrophages using 300 ng/mL PMA for 3 h, washed with PBS and allowed to adhere for 24 h.
Parasites, infection and intracellular survival assays
Sb-susceptible Leishmania panamensis MHOM/CO/2002/3594 promastigotes were stably transfected with the luciferase reporter gene (L.p-LUC 001) cloned into the pGL2-α-NEO-α vector as previously described.13 L. panamensis MHOM/CO/1995/1989/GFP012 was transfected with the green fluorescent protein (GFP) reporter gene cloned into the pGEM7zf-α-NEO-α vector14 (L.p-GFP). Promastigotes were kept at 25°C in RPMI supplemented with 10% heat-inactivated FBS, 5 mg/mL haemin and >120 μg/mL geneticin. Macrophages were infected with stationary-phase promastigotes at a 10 : 1 Leishmania : macrophage ratio for 2 h and washed twice with PBS, followed by a 24 h chase period at 34°C and 5% CO2. Non-phagocytized parasites were removed by washing with PBS, followed by the addition of meglumine antimoniate (8–32 μg/mL Sb). Intracellular parasite survival was measured by luciferase activity as previously described.13
Drug transporter and metabolizing enzyme PCR arrays and gene expression profiling
Total RNA was extracted from cultured cells with Trizol reagent (Invitrogen, USA) followed by RNA cleanup with RNeasy Mini Kit columns (Qiagen, USA). The expression of drug transporter and drug-metabolizing enzyme genes was evaluated by using commercially available PCR arrays (catalogue numbers PAHS-070Z and PAHS-002Z, respectively; SABiosciences-Qiagen) following the manufacturer's instructions. The data were analysed using the RT2 Profiler PCR Array Data Analysis online tool provided by the manufacturer. Selection of the candidate genes was based on up- or down-regulation (±2-fold) following Leishmania infection and exposure to meglumine antimoniate at 32 μg/mL Sb in two independent expression array replicas of infected and drug-treated human macrophages. Validation of the gene expression profiles was conducted by quantitative RT–PCR (qRT–PCR). Briefly, PBMC-derived macrophages from patients with CL were infected with L.p-LUC 001 as described above, followed by 24 h of exposure to meglumine antimoniate (32 μg/mL Sb). Cells were collected, RNA extracted and RNA reverse transcribed with a high-capacity cDNA reverse transcription kit (Applied Biosystems). Expression of genes abcb1 (P-gp, Hs01070641_g1), abcb6 (Hs01039213_m1), abcc1 (MRP-1, Hs01561510_m1), abcc2 (Hs00166123_m1), slc7a11 (Hs00204928_m1), slc5a4 (Hs00429526_m1), aqp-1 (Hs00166067_m1), aqp-9 (Hs01035887_m1), mt2a (Hs01591333_g1), abp1 (Hs00175631_m1), alox5 (Hs01095330_m1), nos3 (Hs01574659_m1), mgst2 (Hs00182064_m1), gsr (Hs00167317_m1), gstm3 (Hs00356079_m1), pkm2 (Hs00762869_s1) and gapdh (Hs99999905_m1) was evaluated using TaqMan Gene Expression Assays (Applied Biosystems) and a Bio-Rad CFX-96 real-time PCR detection platform. Ct values were normalized to GAPDH. Gene expression was calculated by the ΔΔCt method and expressed as 2−ΔΔCt.
Short hairpin RNA (shRNA)-mediated drug transporter gene silencing in host macrophages
A lentivirus-based system was used for shRNA-mediated gene silencing in THP-1 monocytes as previously described.15 Lentiviral particles for shRNA gene knockdown of ABCB6 were generated as follows: three independent sets of oligonucleotide pairs for gene knockdown of human abcb6, slc7a11 and aqp-9 (Table S2, available as Supplementary data at JAC Online) were synthesized and cloned into the pLKO.1-TCR cloning vector (Addgene, Cambridge, MA, USA).16 Lentiviral particles were generated by co-transfection of endotoxin-free hairpin-containing pLKO.1-TCR, psPAX2 and pMD2.G (Addgene) into HEK-293 T cells. FuGENE HD (Roche) was used as the transfection agent. Lentivirus-containing cell supernatant was collected 4 days after transfection and subsequently used to transduce THP-1 monocytes in medium containing 10 μg/mL polybrene. Transduced cells were selected under puromycin pressure (5 μg/mL) for a minimum of 5 days. Gene knockdown was confirmed by qRT–PCR.
Quantification of intracellular Sb
PBMC-derived primary macrophages from CL patients were infected with L. panamensis for 24 h, or left uninfected, followed by exposure to meglumine antimoniate at concentrations of 16 and 32 μg/mL Sb for an additional 24 h. Cells were washed three times with PBS to remove extracellular traces of Sb. Macrophage samples were resuspended in 100 μL of concentrated nitric acid and stored at −80°C until processing. Fifty microlitres of the concentrated sample was diluted into 7 mL of ultrapure water followed by filtration through a 0.22 μm filter. The intracellular Sb concentration was measured by inductively coupled plasma optical emission spectrometry in an iCAP 6300 platform (Thermo Scientific) according to the manufacturer''s instructions for water analysis. Sb concentrations were calculated based on a calibration curve with a linear range of 0.5–5 μg/mL. Sb concentrations were normalized to the total cell number in each sample preparation.
Confocal microscopy
Macrophages were plated on glass coverslips and infected with L.p-GFP for 2 h followed by a chase period of 24 h. Cells were washed with ice-cold PBS, fixed with 4% formaldehyde at 4°C and permeabilized for 5 min in PBS containing 1% BSA and 0.05% NP-40. After blocking in 5% non-fat evaporated milk in PBS, the coverslips were incubated with rabbit anti-human α-ABCB6 polyclonal antibody (H-300, Santa Cruz Biotechnology). The slides were washed with PBS and incubated with Alexa Fluor 568 α-rabbit antibody (Molecular Probes). After mounting, the cells were visualized by confocal microscopy with a Zeiss LSM 700 system.
Results
Survival of L. panamensis exposed to meglumine antimoniate is significantly higher in macrophages from patients who failed treatment
Sb-susceptible Leishmania strains were isolated from eight patients with CL who responded to standard-of-care treatment with meglumine antimoniate and from eight patients who failed treatment (Table 1), ruling out treatment failure as a consequence of parasite drug resistance. To understand the contribution of host cell factors in the response to treatment in patients infected with Sb-susceptible Leishmania, we evaluated the intracellular survival of L. panamensis in primary macrophages when exposed to meglumine antimoniate. Survival of the Sb-susceptible L. panamensis strain L.p-LUC 001 was significantly higher (P = 0.004) in macrophages from patients who failed treatment (n = 8) (Figure 1a), compared with macrophages from patients who responded to treatment (n = 8). Parasite loads following 24 h of infection in the absence of drug were similar in macrophages from the two study groups (Figure 1b), indicating that the difference in survival following drug exposure was not due to increased infection of macrophages from non-responding patients.
Figure 1.
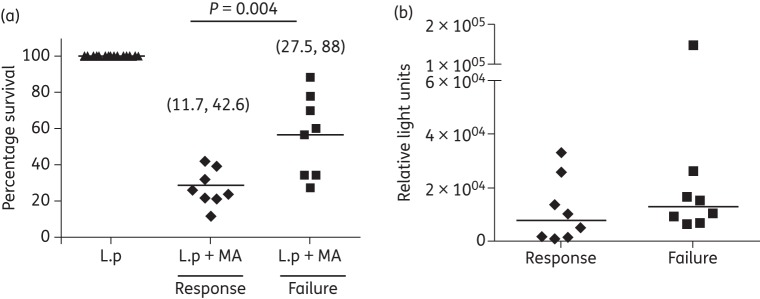
Survival of L. panamensis (L.p) is significantly higher in macrophages from patients who failed meglumine antimoniate (MA) treatment. (a) Intracellular survival of Sb-susceptible L. panamensis after in vitro exposure to meglumine antimoniate (32 μg/mL Sb) for 24 h in primary macrophages of patients with CL who failed (n = 8) or responded (n = 8) to meglumine antimoniate treatment. (b) Intracellular parasite loads in infected and untreated macrophages. Parasite survival was measured by luciferase activity. Data are shown as percentage survival compared with infected macrophages unexposed to meglumine antimoniate (a) and as absolute relative light units in infected macrophages unexposed to the drug (b). Statistical difference was determined by parametric or non-parametric analysis of variance tests, as appropriate.
Although Sb drug treatment failure in patients with visceral leishmaniasis was previously reported to be linked with reduced intracellular drug accumulation as a consequence of MRP-1 and P-gp overexpression in monocytes,11 neither MRP-1 and P-gp gene expression nor total intracellular Sb accumulation differed in either infected or uninfected macrophages from CL patients who failed or responded to meglumine antimoniate (Figure S1, available as Supplementary data at JAC Online). This suggests that other host cell mechanisms influence the antileishmanial efficacy of Sb and the intracellular parasite survival, despite intrinsic susceptibility of the parasite to antimonials.
Infection with L. panamensis and exposure to meglumine antimoniate modulate gene expression of macrophage transporters and metabolizing enzymes
To understand the mechanisms contributing to the increased survival of Leishmania in macrophages from patients with antimonial drug treatment failure, we sought to identify pharmacological determinants in macrophages having potential to modulate the exposure of the intraphagosomal parasite to the drug. The expression of 84 human drug transporter and 84 drug-metabolizing enzyme genes was assessed by qRT–PCR in infected and meglumine antimoniate-treated primary human macrophages from healthy donors using commercially available PCR arrays. A highly dynamic host–parasite and host–drug interaction was revealed, in which the expression of one-quarter of these genes was modulated (Table S3, available as Supplementary data at JAC Online), including macrophage ATP-binding cassette (ABC) transporters, solute liquid carriers (SLCs), aquaporins (AQPs) and phase II metabolizing enzymes. The magnitude of host cell genes modulated ±2-fold by infection or infection followed by drug exposure was similar (24 and 32 of 168 genes, respectively) (Table S3).
The consistency in the modulation of transporters of glutathione (GSH) and GSH conjugates (ABCB1, ABCC1 and ABCC2), enzymes involved in GSH metabolism [GSH reductase (GSR), microsomal GSH S-transferase 2 (MGST2), GSH S-transferase mu 3 (GSTM3) and amiloride-binding protein 1 (ABP1)] and heavy metal transporters and scavenger molecules [ABCB6 and AQP-9, and metallothionein-2A (MT2A), respectively] suggested their involvement in the transport and detoxification of antimonials in human macrophages. We therefore selected these genes for further validation by qRT–PCR in macrophages from patients with CL.
A heat map representation of the fold change in the gene expression of infected and drug-exposed macrophages compared with that in uninfected and untreated cells (Figure 2a) shows that exposure to meglumine antimoniate had a greater effect on host cell gene expression than L. panamensis infection. Meglumine antimoniate strongly induced the expression of abcb6, slc7a11, mt2a, gsr, gstm3 and to a lesser extent nos3 and abcc2, while down-regulating alox5 and p-gp (P < 0.05) (Figure 2b). Conversely, infection with L. panamensis down-regulated aqp-1, mgst2, nos3, abp1 and alox5 (P < 0.05) and induced mt2a and slc7a11 (P < 0.05). The effect on gene expression exerted by exposure to antimonials was unaffected in infected cells, with the exception of induction of nos3 and gstm3, which was abrogated in L. panamensis and meglumine antimoniate-treated cells (Figure 2b). The down-regulation of alox5 and induction of mt2a exerted by L. panamensis and meglumine antimoniate was significantly potentiated in infected and drug-treated macrophages. No significant differences were observed for mrp-1, aqp-9, slc5a4 and pkm2 gene expression in macrophages from CL patients (Figure 2b).
Figure 2.
Macrophage drug transporters and drug-metabolizing enzyme genes are modulated by L. panamensis (L.p) infection and exposure to meglumine antimoniate (MA). (a) Heat map representation of the overall macrophage gene expression profile and (b) fold change values of drug transporters and metabolizing enzymes of CL patients (eight responders and eight failures) induced by ex vivo infection with L. panamensis and exposure to meglumine antimoniate (32 μg/mL Sb). Green indicates down-regulation and red indicates up-regulation. Clustering and correlation analyses were performed using average linkage and uncentred correlation functions on Cluster3 software (a). Statistical differences were determined by parametric or non-parametric analysis of variance tests, as appropriate, and significance established at P<0.05. Mean or median values are represented according to distribution of data (b). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
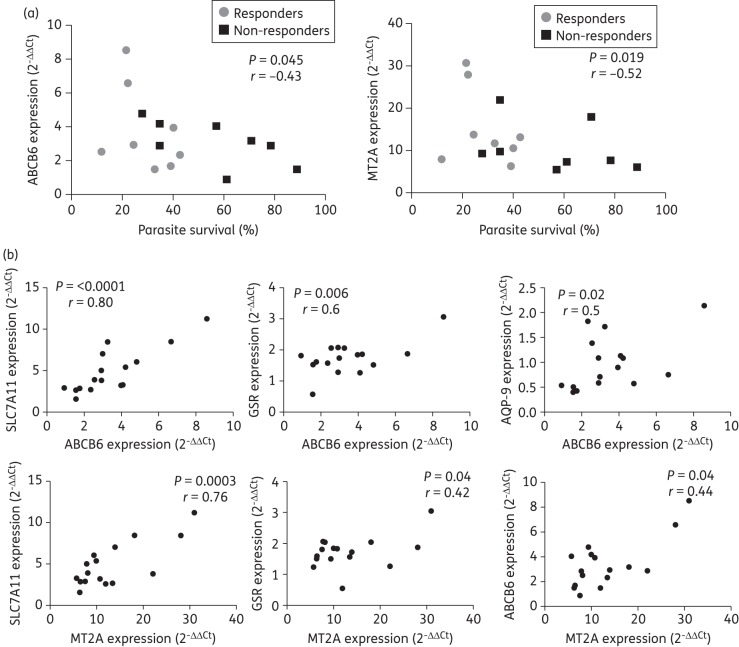
abcb6 and mt2a gene expression inversely correlates with intracellular survival of L. panamensis
To identify host cell transporter and metabolizing enzyme genes with putative contributions in the Sb-mediated parasite killing, we evaluated the correlation between host cell gene expression profiles and Leishmania survival upon exposure to meglumine antimoniate in macrophages from CL patients. Of the panel of 16 genes analysed, only expression of host cell abcb6 and mt2a were inversely correlated (P < 0.05) with the intracellular survival of L. panamensis (Figure 3a), indicating that the up-regulation of abcb6 and mt2a induced by meglumine antimoniate promotes intracellular parasite killing. Outlier identification analysis using the ROUT coefficient (Q = 1% and Q = 5%) did not identify any outlier data; thus, the statistical significance of these correlations was substantiated. The expression of abcb6 was positively correlated with mt2a, slc7a11, gsr and aqp-9 expression in infected and drug-treated cells, and mt2a was positively correlated with slc7a11, gsr and abcb6 expression (Figure 3b). This suggests possible coregulatory networks to be operating in response to antimonial drug exposure and Leishmania infection and putative functional implications for these gene products. No correlation between the expression of p-gp, mrp-1, abcc2, slc5a4, slc7a11, aqp-1, aqp-9, mgst2, gstm3, gsr, abp1, pkm2, nos3 and alox5 and the intracellular survival of L. panamensis was observed (data not shown).
Figure 3.
Intracellular survival of L. panamensis is correlated with expression of macrophage abcb6 and mt2a. (a) Correlation analysis of intracellular parasite survival versus gene expression of drug transporters and metabolizing enzymes and (b) correlation analysis of gene expression of pharmacological determinants in infected and drug-treated (meglumine antimoniate; 32 μg/mL Sb) macrophages from CL patients who failed (n = 8) or responded (n = 8) to meglumine antimoniate treatment. Statistical correlation was assessed by the Spearman correlation test and significance was established when P < 0.05.
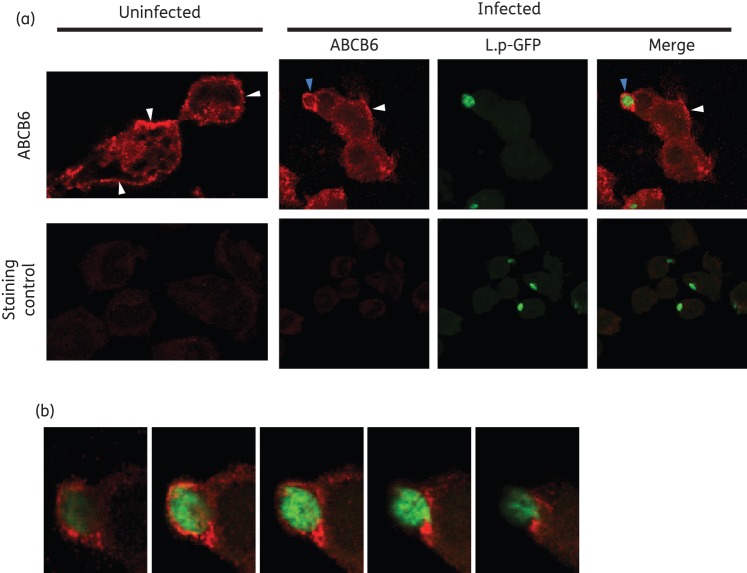
Macrophage ABCB6 is localized in the cell and phagolysosomal membranes in human macrophages
ABCB6 is a transporter of porphyrin17 and heavy metals including arsenic (As),18 a metalloid closely related to Sb. ABCB6 has been shown to localize to the mitochondrial and cell membranes of glioblastoma and erythroleukaemia cell lines19 and to late endosomal/lysosomal compartments of red blood cells and liver homogenates.17,20–22 However, its localization in myeloid cells was unknown. To further dissect the contribution of ABCB6 in the Sb-mediated parasite killing, we analysed its subcellular localization in human macrophages. Confocal microscopy of uninfected and L. panamensis-infected THP-1 macrophages showed that ABCB6 localizes to the cell membrane and in intracellular structures in uninfected macrophages (Figure 4a). In line with its previously shown subcellular localization at the cell membrane and endolysosomal compartments, ABCB6 was found in the plasma membrane of macrophages and in the phagosome surrounding L. panamensis amastigotes after 24 h of infection (Figure 4a and b).
Figure 4.
Subcellular localization of ABCB6 in uninfected and L. panamensis-infected macrophages. (a and b) ABCB6 is shown in red and intracellular Leishmania in green in THP-1 macrophages infected with GFP-transfected L. panamensis (L.p-GFP). ABCB6 is detected at the cell membrane both in infected and uninfected cells (white arrowheads) and surrounding the intracellular Leishmania (blue arrowheads). A Z-stack reconstitution of sequential images taken at a Z scaling value of 0.38 μm is shown (a). Zoomed sequential Z-stack images show the specific localization of ABCB6 at the periphery of the Leishmania-containing phagolysosome (b). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
ABCB6 expression is strongly induced by Sb and transports antimonial compounds in human macrophages
Having observed that abcb6 gene expression is strongly induced in human macrophages following exposure to meglumine antimoniate (Figure 2b) at concentrations as low as 8 μg/mL (data not shown) and that its expression inversely correlates with Leishmania survival after exposure to antimonials, we sought to functionally validate ABCB6 as an Sb transporter.
We first quantified the intracellular content of Sb in wild-type (WT) THP-1 cells and evaluated the dynamics of accumulation. The intracellular Sb concentration was linear (r2 = 0.99) over a range of exposure concentrations from 8 to 32 μg/mL Sb (Figure 5a). The average intracellular content of Sb was ∼50, 90 and 200 ng/106 cells when exposed for 24 h to Sb at 8, 16 and 32 μg/mL, respectively (Figure 5a). After single-dose exposure to meglumine antimoniate at either 16 or 32 μg/mL Sb, maximum intracellular Sb was detected at 24 h and ∼50% of the total intracellular Sb was retained after 72 h of exposure (Figure 5b). We created a human monocytic THP-1 ABCB6-knockdown (ABCB6KD) cell line, with stable gene knockdown ranging between 60% and 90%, as confirmed by qRT–PCR. A time-dependent Sb accumulation curve in ABCB6KD and WT macrophages showed a 12%–27% increase in intracellular Sb accumulation in ABCB6KD when exposed to 32 μg/mL Sb (WT AUC0–72 = 10.5 ± 2 μg/106 cells and ABCB6KD AUC0–72 = 11.8 ± 1.1 μg/106 cells, respectively) or 16 μg/mL Sb (WT AUC0–72 = 5.2 ± 0.1 μg/106 cells and ABCB6KD AUC0–72 = 6.62 ± 0.2 μg/106 cells, respectively), substantiating the function of ABCB6 as an Sb transporter.
Figure 5.
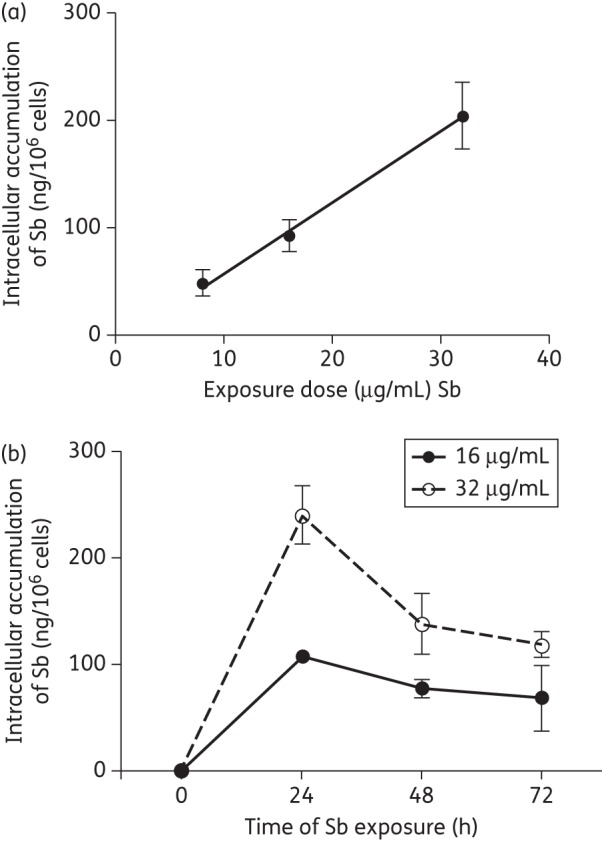
Characterization of intracellular uptake of Sb in THP-1 cells. (a) Intracellular Sb content in WT THP-1 monocytes is linear over a range of exposure to meglumine antimoniate (8–32 μg/mL Sb) for 24 h. (b) Sb accumulation curve constructed based on intracellular Sb concentrations after exposure of WT THP-1 macrophages to meglumine antimoniate at concentrations of 16 and 32 μg/mL Sb during a time course of 24–72 h. Graphs represent means ± SEM of experiments performed in triplicate.
ABCB6 promotes Sb-mediated killing of intracellular Leishmania
To validate the role of host cell ABCB6 in the drug-mediated intracellular parasite killing, we evaluated the survival of L. panamensis in ABCB6KD macrophages exposed to meglumine antimoniate. A dose-dependent effect of the drug was observed in parasite killing both in WT and knockdown macrophage cell lines (Figure 6a). Intracellular parasite survival was significantly higher in ABCB6KD cells, with an increase in parasite survival of 24% and 11% after exposure at drug concentrations of 16 and 32 μg/mL Sb, respectively, in knockdown cells compared with WT (Figure 6a). Enhanced survival of L. panamensis in ABCB6KD cells was not the result of intrinsic permissiveness of these cells to infection or intracellular parasite replication, as Leishmania survival in the absence of drug was similar in WT and knockdown cells up to 96 h post-infection (Figure 6b). The antimicrobial effect of antimonials was not solely dependent on ABCB6 function; a trend of increased parasite survival, although not statistically significant, was also observed in both AQP-9KD (6%–19% increased survival) and SLC7A11KD (10%–14% increased survival) cells compared with WT macrophages (Figure S2, available as Supplementary data at JAC Online).
Figure 6.

Knockdown of ABCB6 promotes intracellular survival of L. panamensis upon exposure to meglumine antimoniate (MA). (a) Parasite survival in WT and ABCB6KD macrophages infected for 24 h with L.p-LUC 001 and exposed to serially increasing concentrations of meglumine antimoniate for an additional 24 h, expressed as percentage survival over infected and untreated controls. (b) Parasite survival in WT and ABCB6KD over a 96 h time course in the absence of meglumine antimoniate expressed as absolute relative light units. Data are presented as means ± SEM of experiments performed in triplicate. Statistical significance was considered as P < 0.05 when applying unpaired t-test analysis.
Collectively, these findings support the function of ABCB6 as an Sb transporter located both at the cell and phagolysosomal membranes and potentially modulating the exposure of intraphagosomal Leishmania to Sb-containing drugs.
Discussion
Investigations of Leishmania–macrophage interactions have largely focused on the capacity of this parasite to modulate the host immune response.23 Our findings expand the scope of host cell functions modulated by Leishmania parasites and portray a very dynamic interaction at the interface of infection and subsequent antimicrobial drug exposure.
In the present study, we have explored the role of human macrophages in the antileishmanial effect of antimonials in the context of clinical infections with Sb-susceptible Leishmania strains. Upon exposure to meglumine antimoniate, the survival of Sb-susceptible L. panamensis was significantly higher in macrophages from patients who failed treatment with antimonials, compared with those from patients who responded. This finding substantiates the participation of host cell factors in the antimicrobial efficacy of antileishmanials and underscores the importance of host cell determinants in optimizing therapeutic approaches to this and other intracellular pathogens. Host cell transport and metabolism of antimicrobial compounds that target intracellular pathogens, and drug delivery to the specialized compartments where these microbes reside and replicate, are crucial to efficacy. Previous studies have addressed the importance of host cell drug transporters in infections caused by intracellular bacteria24,25 and viruses.26 We show that increased intracellular survival of Leishmania in human macrophages after exposure to Sb correlates with low expression of the transporter ABCB6. Sb induced abcb6 gene expression in human macrophages, similar to the up-regulation induced by the closely related metalloid As.18 shRNA gene knockdown of ABCB6 revealed increased accumulation of Sb in ABCB6KD monocytes. This, together with the previously described As-transport function of ABCB6,18 supports the function of ABCB6 as an Sb transporter. Biochemical analyses of the function of ABCB6 have shown that this transporter acts as an efflux pump in the cell membrane and as an importer when localized at intracellular membranes.17,19,21 This evidence, together with the phagolysosomal localization of ABCB6 in L. panamensis-infected macrophages, and the enhanced survival of Leishmania in ABCB6KD cells when exposed to antimonials, substantiate its role as a regulator of Sb-mediated intraphagosomal parasite killing. That the level of expression of ABCB6, a lysosomal/phagolysosomal drug transporter, alters the antileishmanial effect of antimonials in infected human macrophages highlights that the host cell-dependent regulation of drug accumulation and activation within intracellular organelles are important mechanisms of drug efficacy during the drug-mediated parasite killing.27
The antileishmanial effect of antimonials does not uniquely depend on ABCB6 expression/function; knockdown of macrophage ABCB6 did not completely abrogate the capacity of antimonials to kill intracellular Leishmania. Evaluation of the functional effect of other host transporters, including AQP-9 and SLC7A11, which were positively correlated to ABCB6 expression, showed a trend of increased parasite survival in knockdown macrophages, suggesting a contribution of these transporters in the antimicrobial effect of antimonials. It has been previously shown that the overexpression of AQP-9 in the K562 leukaemic cell line leads to intracellular accumulation of Sb and sensitizes these cells to AsIII and SbIII.28 SLC7A11, a cystine/glutamate exchanger, plays an important role in the cellular redox balance through the maintenance of reduced GSH levels;29,30 reduced GSH is essential in the GSH-mediated activation of Sb (SbV → SbIII).31 Hence, concomitant repression of aqp-9, slc7a11 and abcb6 could promote the intracellular survival of Leishmania during exposure to antimonials by reducing AQP-9-mediated drug uptake, decreasing distribution to the parasite-containing phagolysosome through ABCB6 and reducing conversion into bioactive SbIII indirectly by the function of SLC7A11.
mt2a gene expression, similarly to abcb6 expression, is strongly induced by As32,33 and MT2A-null mice have increased sensitivity to As-induced toxicity.34,35 We found that mt2a expression was strongly induced by Sb and that this increased mt2a expression negatively correlated with intracellular parasite survival. Increased levels of MT2A may promote intracellular Sb sequestration, thereby reducing cellular toxicity, while promoting efficient Sb delivery to the phagolysosome as MT2A is localized in the cytoplasm and lysosomal compartments.33 Although functional validation is necessary, and is the focus of our current research, the correlation of increased mt2a gene expression with lower rates of intracellular parasite survival within macrophages from CL patients supports this hypothesis. Consistent with the multifactorial nature of the therapeutic response, we have observed that patients with acute disease who failed treatment with meglumine antimoniate had significantly larger cutaneous lesions at the time of diagnosis than patients who responded to treatment (Table 1). Larger lesions are often accompanied by increased local immunopathology, which could contribute to a poor treatment outcome.
Among the host cell molecules identified in this study, macrophage alox5, aqp-9 and mt2a have previously been shown to be modulated by infection with Leishmania donovani.36–38 In addition, monocytes from visceral leishmaniasis patients in India from a geographical area where L. donovani infections are frequently unresponsive to Sb, and who failed treatment with antimonials, were shown to accumulate less intracellular Sb than cells from responding patients. This difference was attributed to an induction of host cell MRP-1 and P-gp expression as a result of infection with Sb-resistant L. donovani strains, but not Sb-susceptible strains, leading to active drug efflux.11 In agreement with these observations, in our study of CL and treatment failure in the context of infections with Sb-susceptible L. panamensis, we did not detect significant variation in intracellular Sb or gene expression of mrp-1 and p-gp in macrophages from patients who failed or responded to meglumine antimoniate. Together, these results portray the complexity of the host cell mechanisms underlying the antileishmanial effect of antimonials and the potential impact of the infecting Leishmania species and strain. Based on these findings, we suggest two non-exclusive host cell-dependent mechanisms operating to promote the intracellular survival of Leishmania during antimonial drug exposure: (i) the survival of Sb-resistant parasites could be attributed to both intrinsic or acquired mechanisms of drug resistance coupled with reduced intramacrophage accumulation of the drug through up-regulation of host cell MRP-1 and P-gp; and (ii) the survival of Sb-susceptible parasites could result from reduced exposure of the intraphagosomal parasite to the drug through decreased expression of host cell ABCB6.
Notwithstanding the individual function of the molecules validated in this study, the translation of these observations to therapeutic outcomes and the generalization of these data to infections with other Leishmania species and clinical manifestations will require consideration within the context of a multimarker profile defining immune as well as pharmacological functions. These issues are not confined to leishmaniasis; the influence of host–pathogen–drug interactions on the pharmacological response challenges the exclusive focus of antimicrobial strategies on the targeting of the pathogen without consideration or exploitation of host cell determinants of efficacy.
Funding
This work received financial support from COLCIENCIAS grant # 222949326161 and the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) Re-entry grant # B00032. L. J. R. and D. A. V. are recipients of COLCIENCIAS Young Investigator Awards, Contract #797-2009 and #0040-2012. V. M. B. and M. A. G. were supported by Swiss National Science Foundation grant #IZ70ZD_131421 and Fogarty/National Institutes of Health (NIH) training grant D43 TW006589.
Transparency declarations
None to declare.
Supplementary data
Acknowledgements
We gratefully acknowledge the patients who participated in this study, the support of Dr David Gregory (Department of Environmental Health, Harvard University) for training in gene silencing through shRNA, the CIDEIM BioBank team for the culture, typing and evaluation of drug susceptibility of clinical strains and Dr Neal Alexander (Epidemiology and Biostatistics Unit, CIDEIM) for insightful discussions.
References
- 1.Murray HW, Delph-Etienne S. Roles of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2000;68:288–93. doi: 10.1128/iai.68.1.288-293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray HW, Jungbluth A, Ritter E, et al. Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment. Infect Immun. 2000;68:6289–93. doi: 10.1128/iai.68.11.6289-6293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray HW, Montelibano C, Peterson R, et al. Interleukin-12 regulates the response to chemotherapy in experimental visceral leishmaniasis. J Infect Dis. 2000;182:1497–502. doi: 10.1086/315890. [DOI] [PubMed] [Google Scholar]
- 4.Bacellar O, Lessa H, Schriefer A, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–40. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz YR, Rojas R, Valderrama L, et al. T-bet, GATA-3, and Foxp3 expression and Th1/Th2 cytokine production in the clinical outcome of human infection with Leishmania (Viannia) species. J Infect Dis. 2010;202:406–15. doi: 10.1086/653829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvar J, Aparicio P, Aseffa A, et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–59. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rojas R, Valderrama L, Valderrama M, et al. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J Infect Dis. 2006;193:1375–83. doi: 10.1086/503371. [DOI] [PubMed] [Google Scholar]
- 8.Robledo SM, Valencia AZ, Saravia NG. Sensitivity to Glucantime of Leishmania viannia isolated from patients prior to treatment. J Parasitol. 1999;85:360–6. [PubMed] [Google Scholar]
- 9.Jackson JE, Tally JD, Ellis WY, et al. Quantitative in vitro drug potency and drug susceptibility evaluation of Leishmania ssp. from patients unresponsive to pentavalent antimony therapy. Am J Trop Med Hyg. 1990;43:464–80. doi: 10.4269/ajtmh.1990.43.464. [DOI] [PubMed] [Google Scholar]
- 10.Cruz A, Rainey PM, Herwaldt BL, et al. Pharmacokinetics of antimony in children treated for leishmaniasis with meglumine antimoniate. J Infect Dis. 2007;195:602–8. doi: 10.1086/510860. [DOI] [PubMed] [Google Scholar]
- 11.Mookerjee Basu J, Mookerjee A, Banerjee R, et al. Inhibition of ABC transporters abolishes antimony resistance in Leishmania infection. Antimicrob Agents Chemother. 2008;52:1080–93. doi: 10.1128/AAC.01196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez O, Diaz-Toro Y, Valderrama L, et al. Novel approach to in vitro drug susceptibility assessment of clinical strains of Leishmania spp. J Clin Microbiol. 2012;50:2207–11. doi: 10.1128/JCM.00216-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy G, Dumas C, Sereno D, et al. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol Biochem Parasitol. 2000;110:195–206. doi: 10.1016/s0166-6851(00)00270-x. [DOI] [PubMed] [Google Scholar]
- 14.Yu Z, Genest PA, ter Riet B, et al. The protein that binds to DNA base J in trypanosomatids has features of a thymidine hydroxylase. Nucleic Acids Res. 2007;35:2107–15. doi: 10.1093/nar/gkm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H, DeLoid G, Browning E, et al. Genome-wide RNAi screen in IFN-γ-treated human macrophages identifies genes mediating resistance to the intracellular pathogen Francisella tularensis. PLoS One. 2012;7:e31752. doi: 10.1371/journal.pone.0031752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moffat J, Grueneberg DA, Yang X, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–98. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamurthy PC, Du G, Fukuda Y, et al. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–9. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 18.Chavan H, Oruganti M, Krishnamurthy P. The ATP-binding cassette transporter ABCB6 is induced by arsenic and protects against arsenic cytotoxicity. Toxicol Sci. 2011;120:519–28. doi: 10.1093/toxsci/kfr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paterson JK, Shukla S, Black CM, et al. Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry. 2007;46:9443–52. doi: 10.1021/bi700015m. [DOI] [PubMed] [Google Scholar]
- 20.Jalil YA, Ritz V, Jakimenko A, et al. Vesicular localization of the rat ATP-binding cassette half-transporter rAbcb6. Am J Physiol Cell Physiol. 2008;294:C579–90. doi: 10.1152/ajpcell.00612.2006. [DOI] [PubMed] [Google Scholar]
- 21.Kiss K, Brozik A, Kucsma N, et al. Shifting the paradigm: the putative mitochondrial protein ABCB6 resides in the lysosomes of cells and in the plasma membrane of erythrocytes. PLoS One. 2012;7:e37378. doi: 10.1371/journal.pone.0037378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della Valle MC, Sleat DE, Zheng H, et al. Classification of subcellular location by comparative proteomic analysis of native and density-shifted lysosomes. Mol Cell Proteomics. 2011;10:M110.006403. doi: 10.1074/mcp.M110.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye P, Scott P. Leishmaniasis: complexity at the host–pathogen interface. Nat Rev Microbiol. 2011;9:604–15. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 24.Van Bambeke F, Glupczynski Y, Plesiat P, et al. Antibiotic efflux pumps in prokaryotic cells: occurrence, impact on resistance and strategies for the future of antimicrobial therapy. J Antimicrob Chemother. 2003;51:1055–65. doi: 10.1093/jac/dkg224. [DOI] [PubMed] [Google Scholar]
- 25.Van Bambeke F, Michot JM, Tulkens PM. Antibiotic efflux pumps in eukaryotic cells: occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics and toxicodynamics. J Antimicrob Chemother. 2003;51:1067–77. doi: 10.1093/jac/dkg225. [DOI] [PubMed] [Google Scholar]
- 26.Ford J, Khoo SH, Back DJ. The intracellular pharmacology of antiretroviral protease inhibitors. J Antimicrob Chemother. 2004;54:982–90. doi: 10.1093/jac/dkh487. [DOI] [PubMed] [Google Scholar]
- 27.Lemaire S, Kosowska-Shick K, Appelbaum PC, et al. Cellular pharmacodynamics of the novel biaryloxazolidinone radezolid: studies with infected phagocytic and nonphagocytic cells, using Staphylococcus aureus, Staphylococcus epidermidis, Listeria monocytogenes, and Legionella pneumophila. Antimicrob Agents Chemother. 2010;54:2549–59. doi: 10.1128/AAC.01724-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharjee H, Carbrey J, Rosen BP, et al. Drug uptake and pharmacological modulation of drug sensitivity in leukemia by AQP9. Biochem Biophys Res Commun. 2004;322:836–41. doi: 10.1016/j.bbrc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Sato H, Shiiya A, Kimata M, et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280:37423–9. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- 30.Mandal PK, Seiler A, Perisic T, et al. System xc− and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J Biol Chem. 2010;285:22244–53. doi: 10.1074/jbc.M110.121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira Cdos S, Martins PS, Demicheli C, et al. Thiol-induced reduction of antimony(V) into antimony(III): a comparative study with trypanothione, cysteinyl-glycine, cysteine and glutathione. Biometals. 2003;16:441–6. doi: 10.1023/a:1022823605068. [DOI] [PubMed] [Google Scholar]
- 32.Albores A, Koropatnick J, Cherian MG, et al. Arsenic induces and enhances rat hepatic metallothionein production in vivo. Chem Biol Interact. 1992;85:127–40. doi: 10.1016/0009-2797(92)90057-r. [DOI] [PubMed] [Google Scholar]
- 33.Sabolic I, Breljak D, Skarica M, et al. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals. 23:897–926. doi: 10.1007/s10534-010-9351-z. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Liu Y, Habeebu SM, et al. Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice. Toxicology. 2000;147:157–66. doi: 10.1016/s0300-483x(00)00194-3. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Liu Y, Goyer RA, et al. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci. 2000;55:460–7. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 36.Gregory DJ, Sladek R, Olivier M, et al. Comparison of the effects of Leishmania major or Leishmania donovani infection on macrophage gene expression. Infect Immun. 2008;76:1186–92. doi: 10.1128/IAI.01320-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Fadili K, Imbeault M, Messier N, et al. Modulation of gene expression in human macrophages treated with the anti-Leishmania pentavalent antimonial drug sodium stibogluconate. Antimicrob Agents Chemother. 2008;52:526–33. doi: 10.1128/AAC.01183-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaussabel D, Semnani RT, McDowell MA, et al. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–81. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.