Abstract
Objectives
The latent reservoir for HIV-1 in resting memory CD4+ T cells is a major barrier to eradication. In vitro models involving transformed cell lines have been used to search for small molecules that reactivate latent HIV-1. Histone deacetylase (HDAC) inhibitors can reverse HIV-1 latent infection. Most studies on HDAC inhibitors have been performed in cell line models that differ in important aspects from the resting CD4+ T cells that harbour latent HIV-1 in vivo. Therefore, we evaluated the potency and kinetics of HDAC inhibitors in a primary cell model of HIV-1 latency that involves resting CD4+ T cells.
Methods
A green fluorescent protein (GFP)-expressing reporter virus NL4-3-Δ6-drGFP was used to generate latent infection in Bcl-2-transduced primary CD4+ T cells. Seventeen HDAC inhibitors were tested in this primary cell model. The effects of these HDAC inhibitors on the reactivation of latent HIV-1 were determined and compared with anti-CD3 and anti-CD28 co-stimulation.
Results
In Bcl-2-transduced primary CD4+ T cells, short-term treatment with HDAC inhibitors resulted in very limited reactivation of latent HIV-1, while prolonged treatment greatly enhanced drug efficacy. The effects of HDAC inhibitors in reactivating latent HIV-1 correlated with their inhibitory effects on class I HDACs. Importantly, HIV-1 reactivated by HDAC inhibitors can quickly re-establish latent infection upon drug removal.
Conclusions
We identified unique features of HDAC inhibitor-induced reactivation of latent HIV-1 in primary CD4+ T cells. Our findings may be useful for the design of eradication trials.
Keywords: latent reservoir, cytotoxicity, vorinostat
Introduction
Combination antiretroviral therapy suppresses HIV-1 replication, but cannot eradicate HIV-1 infection because the virus establishes latent infection, primarily in resting memory CD4+ T cells.1–3 Due to the stability of this latent reservoir, life-time antiretroviral therapy is required. Reactivating latent HIV-1 in resting memory CD4+ T cells is the crucial first step to the elimination of this latent reservoir. Large-scale screens have been performed to search for small molecules that induce latent HIV-1 gene expression without causing T cell activation. Recruitment of histone deacetylases (HDACs) to the HIV-1 5′ long terminal repeat (LTR) represses viral transcription and facilitates the establishment and maintenance of HIV-1 latency.4,5 HDAC inhibitors are thus considered promising candidates to reactivate latent HIV-1, although there is evidence that they affect HIV-1 gene expression through a different mechanism.6
Two FDA-approved drugs that inhibit HDACs, valproic acid (VPA) and vorinostat (suberoylanilide hydroxamic acid), have been shown to reactivate latent HIV-1 in vitro in primary cell systems.6,7 Other HDAC inhibitors have been tested in cell line models of HIV-1 latency in previous studies.8–12 However, the genetic homogeneity and cell cycle and activation status of transformed cells are major limitations to their use for the study of HIV-1 latency, and cell line models have given inconsistent results. For example, some HDAC inhibitors showed great efficacy (>50% reactivation) in some cell clones, but had little effect (<5% reactivation) in others, probably because each cell clone has unique features, such as the viral integration site.8–12 Primary cell models of HIV-1 latency overcome some of these limitations and allow evaluation of HDAC inhibitors in complex cell populations. To date, few studies of HDAC inhibitors have been performed in primary cell models. Some studies showed that HDAC inhibitors, including vorinostat, trichostatin A (TsA) and VPA, had limited effects even at concentrations well above the maximum clinical concentrations.10,13
Reactivation strategies are unlikely to succeed unless the vast majority of latently infected cells are eliminated. Therefore, evaluating the fraction of latently infected primary CD4+ T cells reactivated by HDAC inhibitors is important. In studies of patient-derived CD4+ T cells treated with vorinostat, increases in viral gene expression and virus production have been detected, but the fraction of latently infected cells that are reactivated by vorinostat could not be determined.6 Vorinostat has also been investigated in a clinical trial as a latency-reversing agent. Increases in cell-associated HIV-1 RNA, but not free plasma virus, were detected in vorinostat-treated patients.14 The effect of vorinostat on the size of the latent reservoir is still unknown. In our study, we quantitatively evaluated the effects of HDAC inhibitors on the reactivation of latent HIV-1 in primary resting memory CD4+ T cells and identified some unique features of HDAC inhibitors as anti-latency agents.
Methods
HIV-1 latent infection in Bcl-2-transduced primary CD4+ T cells
This study was approved by the Johns Hopkins Institutional Review Board (NA_00049895). Informed consent was provided by the study participants. The generation of latently infected Bcl-2-transduced primary CD4+ T cells has been described previously.13 Briefly, Bcl-2-transduced primary CD4+ T cells were infected with the green fluorescent protein (GFP)-expressing reporter virus NL4-3-Δ6-drGFP and then cultured in basal medium for 20 days. GFP-negative cells were then purified by fluorescence-activated cell sorting for future analysis. Of the GFP-negative cells, 1%–3% were latently infected and the rest were uninfected.
Reactivation of latent HIV-1 by HDAC inhibitors
HDAC inhibitors were obtained from Sigma or Selleckchem. Purified GFP-negative cells containing latently infected cells were treated with HDAC inhibitors in the absence of cytokines or activating stimuli. Cells treated with soluble anti-CD3 (2.5 mg/L) plus anti-CD28 (1 mg/L) antibodies were used as positive controls. The reactivation of latent HIV-1 was determined by measuring the fraction of GFP-positive cells by flow cytometry. Only viable cells were included for the analysis of virus reactivation/GFP expression. The strategy for the gating of viable cells is shown in Figure S1 (available as Supplementary data at JAC Online). The effects of HDAC inhibitors were normalized based on the positive control. For each sample analysed by flow cytometry in this study, at least 5 × 104 cells were examined, which usually contained 500–2000 GFP-positive cells. For samples with limited virus reactivation except negative controls, >105 cells were examined, which contained >100 GFP-positive cells.
Measurement of cell viability and cell activation
To measure the effects of HDAC inhibitors on cell viability, peripheral blood mononuclear cells (PBMCs), resting CD4+ T cells or Bcl-2-transduced CD4+ T cells were treated with HDAC inhibitors. Cell viability was measured by the MTT assay (MTS, Promega). To measure the effects of HDAC inhibitors on T cell activation, resting primary CD4+ T cells were isolated using a CD4+ T cell isolation kit and anti-CD69, anti-CD25 and anti-HLADR microbeads (Miltenyi), before being treated with HDAC inhibitors for 3 days prior to antibody staining and flow cytometry analysis.
Results and discussion
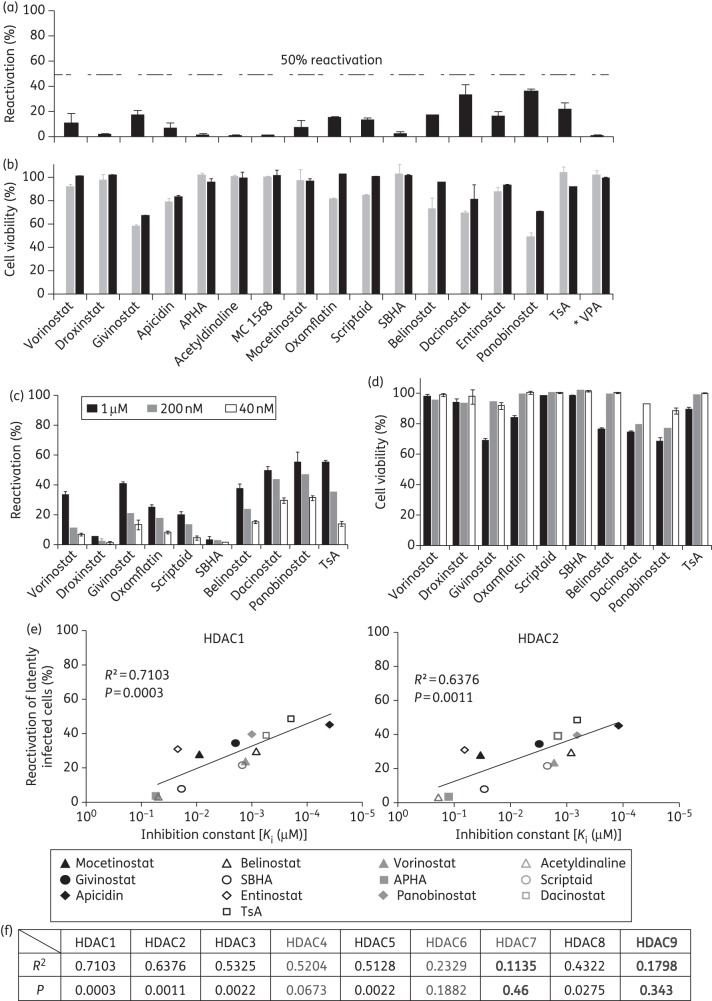
To further study the effects of HDAC inhibitors on the reactivation of latent HIV-1 in primary resting memory CD4+ T cells, we established latent HIV-1 infection in Bcl-2-transduced primary CD4+ T cells as previously reported using the GFP-expressing reporter virus NL4-3-Δ6-drGFP, which only expresses the viral genes tat and rev.13 Seventeen HDAC inhibitors that have been tested previously in cell line models of HIV-1 latency were tested in this primary cell model. Preliminary experiments showed that HDAC inhibitors at concentrations <40 nM had very limited or no effect on HIV-1 latency in our model. In addition, with the exclusion of VPA and suberohydroxamic acid (SBHA), HDAC inhibitors caused significant cell death at concentrations of ≥2 μM, which interfered with the assessment of anti-latency effects. Therefore, concentrations ranging from 40 nM to 1 μM were tested. The concentration range chosen overlaps the clinical concentration range for the subset of HDAC inhibitors that have been tested in clinical trials (panobinostat Cmax = 0.06 μM, givinostat Cmax = 0.2 μM, vorinostat Cmax = 1.2 μM and belinostat Cmax = 4.3 μM). At a concentration of 200 nM, some HDAC inhibitors produced only limited reactivation of latent HIV-1 in primary resting CD4+ T cells, while others had no effect (Figure 1a). None of the HDAC inhibitors reactivated >36% of latent viruses in primary resting CD4+ T cells. More effective reactivation was observed at the concentration of 1 μM (Figure 1c), which caused more cell death (see below). In previous studies, the nuclear class I HDACs 1, 2 and 3, but not the class II HDACs 4, 6 and 7, which shuttle in and out of the nucleus, were found to be recruited to the HIV-1 LTR.15–17 To further understand which HDAC isoforms are associated with latent HIV-1 infection and virus reactivation, we studied the correlation between the efficiency of virus reactivation by HDAC inhibitors and their inhibitory effect on different HDACs. The inhibition constants (Ki) of HDAC inhibitors used in our study against HDACs 1–9 were determined in a previous study.18 We found that the Ki values of the nuclear class I HDACs 1, 2 and 3 showed the best correlation with the reactivation of HIV-1 latent infection, while for the nuclear class II HDACs 4, 6 and 7 there was little correlation with the reversal of viral latency (Figure 1e and f and Figure S2, available as Supplementary data at JAC Online). The limitation of this correlation study is that we did not include some HDACs and HDAC inhibitors, specifically those that were not studied by Bradner et al.18 We also measured the expression levels of 10 different HDAC isoforms in freshly isolated resting CD4+ T cells or Bcl-2-transduced resting CD4+ T cells using serial analysis of gene expression. Expression levels of HDACs in Bcl-2-transduced cells were similar to those in freshly isolated resting CD4+ T cells (Figure S3, available as Supplementary data at JAC Online). None of the HDAC inhibitors up-regulated activation markers of CD4+ T cells, which excluded the possibility that HDAC inhibitors reactivated latent HIV-1 through T cell activation (Figure S4A, available as Supplementary data at JAC Online). We measured the viability of freshly isolated resting CD4+ T cells and Bcl-2-transduced resting CD4+ T cells after 2 days of HDAC inhibitor treatment and found that most of the HDAC inhibitors did not cause significant cytotoxicity (Figure 1b and d). A few HDAC inhibitors, such as panobinostat and dacinostat, caused substantial toxicity at concentrations that induce reactivation of latent HIV-1. More cell death was observed when prolonged treatment with HDAC inhibitors was performed (Figure 2c and Figure S4B, available as Supplementary data at JAC Online). It is worth mentioning that freshly isolated resting CD4+ T cells and Bcl-2-transduced resting CD4+ T cells are more resistant to HDAC inhibitor-induced cell death than PBMCs. It is possible that proliferating cells in freshly isolated PBMCs are rapidly and preferentially killed after HDAC inhibitor treatment.
Figure 1.
Comparison of the ability of different HDAC inhibitors to reactivate latent HIV-1. (a–d) Limited short-term effects of HDAC inhibitors on reactivation of latent HIV-1. Latently infected Bcl-2-transduced resting memory CD4+ T cells were treated with different HDAC inhibitors at 200 nM (a) or at the indicated concentrations (c) for 2 days. The fraction of GFP-positive cells was measured by flow cytometry. The effect of HDAC inhibitors was normalized to the effect of anti-CD3 plus anti-CD28 antibodies. *The concentration of VPA was 5 mM. Error bars represent SEM, n = 3. (b) Freshly isolated resting CD4+ T cells (grey bars) or Bcl-2-transduced resting CD4+ T cells (black bars) were cultured in the presence of HDAC inhibitors at 200 nM for 2 days. (d) Bcl-2-transduced resting CD4+ T cells were cultured in the presence of HDAC inhibitors at the indicated concentrations for 2 days. Cell death was determined by MTT assay (Promega) and the value was normalized to untreated controls. Error bars represent SEM, n = 3. (e and f) Correlation between reactivation of latent HIV-1 and inhibition of the indicated HDACs by HDAC inhibitors. Reactivation of latent HIV-1 by 1 μM HDAC inhibitors was measured as described above. Values of inhibition constants were obtained from a previous study.18 APHA, 3-(1-methyl-4-phenylacetyl-1H-2-pyrrolyl)-N-hydroxypropenamide.
Figure 2.
Unique characteristics of HDAC inhibitors in reactivating latent HIV-1. (a and b) Prolonged treatment with HDAC inhibitors leads to effective virus reactivation. Latently infected Bcl-2-transduced resting memory CD4+ T cells were treated with different HDAC inhibitors for 8 days. The fraction of GFP-positive cells was measured by flow cytometry at indicated timepoints (a) or at day 8 (b). The effect of HDAC inhibitors was normalized to the effect of anti-CD3 plus anti-CD28 antibodies at day 2. *Drugs caused significant cell death. (c) Bcl-2-transduced resting CD4+ T cells were cultured in the presence of HDAC inhibitors at the indicated concentrations for 8 days. Cell death was determined by MTT assay (Promega) and the value normalized to untreated controls. Error bars represent SEM, n = 3. (d and e) Induction of latent HIV-1 gene expression by HDAC inhibitors is reversible after drug removal. (d) HIV-1 latently infected Bcl-2-transduced resting memory CD4+ T cells were treated with 1 μM vorinostat or anti-CD3 plus anti-CD28 antibodies for 4 days. Vorinostat was removed or anti-CD3 plus anti-CD28 antibodies were removed at day 4 and cells were cultured for an additional 4 days without treatment. On day 8, 1 μM vorinostat was added back or anti-CD3 plus anti-CD28 antibodies were added back to the culture. (e) Cells were treated with indicated HDAC inhibitors for 4 days and then cultured without drug for an additional 4 days. On day 8, HDAC inhibitors were added back to the culture for another 4 days. The percentage of GFP-positive cells was measured at days 4, 8 and 12. The concentrations of HDAC inhibitors used for the experiments are as follows: vorinostat, 1 μM; TsA, 200 nM; oxamflatin, 1 μM; scriptaid, 1 μM; belinostat, 200 nM; and givinostat, 200 nM. The fraction of GFP-positive cells was measured by flow cytometry at the indicated timepoints. The effect of HDAC inhibitors or anti-CD3 plus anti-CD28 antibodies over time was normalized to the effect of anti-CD3 plus anti-CD28 antibodies at day 2. Error bars represent SEM, n = 3. APHA, 3-(1-methyl-4-phenylacetyl-1H-2-pyrrolyl)-N-hydroxypropenamide.
Two days of treatment with HDAC inhibitors has a limited effect on virus reactivation, while drugs, such as prostratin and phorbol myristate acetate (PMA), that activate resting CD4+ T cells have already achieved maximum virus reactivation (Figure 2a). We noted that longer treatment with HDAC inhibitors significantly increased the efficiency of reactivation. HDAC inhibitors that caused significant toxicity after prolonged treatment (Figure 2c) were not used for the analysis of virus reactivation. In spite of limited effects at day 2, most HDAC inhibitors could reactivate >70% of latently infected cells after 6 days of treatment (Figure 2a). Some HDAC inhibitors, such as VPA and entinostat, had no effect on reversing latent infection after 2 days, but gradually became effective after a prolonged period of treatment (Figure 2a and b). Therefore, HDAC inhibitors reactivate latent HIV-1 at a slow rate in comparison with latency-reversing agents that induce T cell activation. Prolonged treatment with HDAC inhibitors is required to achieve efficient virus reactivation.
Since HDAC inhibitors reactivate latent HIV-1 independent of T cell activation, it is important to understand whether HIV-1 gene expression induced by HDAC inhibitors is sustained or temporary in resting CD4+ T cells. Therefore, latently infected resting CD4+ T cells were treated with vorinostat for 4 days and then vorinostat was removed. We have previously shown that reversing latency with vorinostat is not sufficient to cause the death of infected resting CD4+ T cells.19 Although HIV-1 gene expression was induced after vorinostat treatment, viruses rapidly re-established latent infection after removal of vorinostat, as indicated by the decrease in the fraction of GFP-positive cells. The virus stably remained latent because no GFP-positive cells re-emerged unless vorinostat was added back to the culture (Figure 2d). This decrease in GFP-positive cells upon withdrawal of vorinostat was due to the re-establishment of latency, but not because of the death of infected cells (Figure S5, available as Supplementary data at JAC Online). We tested six HDAC inhibitors and found that the viral gene expression induced by all six in resting CD4+ T cells was not sustained after drug removal (Figure 2e).
Our study demonstrates that HDAC inhibitors can potently reactivate latent HIV-1 in primary resting memory CD4+ T cells. The potency of HDAC inhibitors in reactivating latent HIV-1 is correlated with their inhibitory effects on class I HDACs. It is also possible that forms of cell stress induced by the multiple effects of these HDAC inhibitors contribute in an indirect manner to the reactivation of latent HIV-1. These results are consistent with previous studies performed in cell line models of latency and in resting CD4+ T cells isolated from HIV-1 infected patients.17,20 More importantly, we found that efficient reactivation of latent HIV-1 by HDAC inhibitors requires prolonged treatment. We also show here that the reactivation of latent HIV-1 by HDAC inhibitors is a reversible process, because the virus can rapidly re-enter a latent state after drug removal. It remains unclear how well in vitro cell models mimic HIV-1 latency in vivo. It is possible that multiple mechanisms enforce HIV-1 latency in vivo and that not all of them are captured by a given in vitro model. Therefore, the evaluation of latency-reversing agents in cells from patients on antiretroviral therapy is important and the ability of the HDAC inhibitors studied here as single agents to reactivate latent HIV-1 in vivo remains unclear. Nevertheless, our results suggest that HDAC inhibitors may be useful components of pharmacological strategies to purge the latent reservoir.
Funding
This work was supported by NIH grant AI043222, the Howard Hughes Medical Institute, the Foundation for AIDS Research (amFAR) and the Martin Delaney CARE Collaboratory.
Transparency declarations
None to declare.
Supplementary data
Figures S1 to S5 are available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
References
- 1.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 2.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS. 2011;6:25–9. doi: 10.1097/COH.0b013e328341242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mbonye U, Karn J. Control of HIV latency by epigenetic and non-epigenetic mechanisms. Curr HIV Res. 2011;9:554–67. doi: 10.2174/157016211798998736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contreras X, Schweneker M, Chen CS, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284:6782–9. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ylisastigui L, Archin NM, Lehrman G, et al. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–8. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 8.Reuse S, Calao M, Kabeya K, et al. Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PLoS One. 2009;4:e6093. doi: 10.1371/journal.pone.0006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savarino A, Mai A, Norelli S, et al. ‘Shock and kill’ effects of class I-selective histone deacetylase inhibitors in combination with the glutathione synthesis inhibitor buthionine sulfoximine in cell line models for HIV-1 quiescence. Retrovirology. 2009;6:52. doi: 10.1186/1742-4690-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnett JC, Lim KI, Calafi A, et al. Combinatorial latency reactivation for HIV-1 subtypes and variants. J Virol. 2010;84:5958–74. doi: 10.1128/JVI.00161-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber K, Doyon G, Plaks J, et al. Inhibitors of histone deacetylases: correlation between isoform specificity and reactivation of HIV type 1 (HIV-1) from latently infected cells. J Biol Chem. 2011;286:22211–8. doi: 10.1074/jbc.M110.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matalon S, Rasmussen TA, Dinarello CA. Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir. Mol Med. 2011;17:466–72. doi: 10.2119/molmed.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang HC, Xing S, Shan L, et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest. 2009;119:3473–86. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–5. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coull JJ, Romerio F, Sun JM, et al. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74:6790–9. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams SA, Chen LF, Kwon H, et al. NF-κB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–49. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keedy KS, Archin NM, Gates AT, et al. A limited group of class I histone deacetylases acts to repress human immunodeficiency virus type 1 expression. J Virol. 2009;83:4749–56. doi: 10.1128/JVI.02585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradner JE, West N, Grachan ML, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–43. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Archin NM, Keedy KS, Espeseth A, et al. Expression of latent human immunodeficiency type 1 is induced by novel and selective histone deacetylase inhibitors. AIDS. 2009;23:1799–806. doi: 10.1097/QAD.0b013e32832ec1dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.