Summary
Neurogenic neuroprotection is a promising approach for treating patients with ischemic brain lesions. In rats, stimulation of the deep brain nuclei has been shown to reduce the volume of focal infarction. In this context, protection of neural tissue can be a rapid intervention that has a relatively long-lasting effect, making fastigial nucleus stimulation (FNS) a potentially valuable method for clinical application.
Although the mechanisms of neuroprotection induced by FNS remain partially unclear, important data have been presented in the last two decades. A 1-h electrical FNS reduced, by 59%, infarctions triggered by permanent occlusion of the middle cerebral artery in Fisher rats. The acute effect of electrical FNS is likely mediated by a prolonged opening of potassium channels, and the sustained effect appears to be linked to inhibition of the apoptotic cascade.
A better understanding of the neuronal circuitry underlying neurogenic neuroprotection may contribute to improving neurological outcomes in ischemic brain insults.
Keywords: cerebrovascular accident, fastigial nucleus, neurogenic neuroprotection stroke, subarachnoid hemorrhage
Introduction
The protection of brain tissue after traumatic or ischemic events currently represents a challenge for critical neurological care in medical centers worldwide (Feigin and Findlay, 2006). Neuroprotection is a strategy for re-establishing the microenvironment and conditions needed for damaged brain neurons to inhibit intrinsic undesirable programmed processes of cell death. Reduction of cellular metabolism and stimulation of trophic properties in these cells is conducive to achieving a new balance for cellular viability. To this end, many types of pharmacological and physical interventions have been employed in intensive care units (Feigin and Findlay, 2006). However, the real benefits of the current treatments are far from ideal, particularly because of difficulties in inhibiting the inexorable cascade triggered by the initial event and in controlling the complications associated with different treatment options.
Neurogenic neuroprotection opens up a new frontier for the preservation of the penumbra area in the human ischemic brain. For the purposes of this review, the penumbra area has been defined as the risk zone for cellular death that is sensitive to therapeutic procedures (Ginsberg and Pulsinelli, 1994; Baron, 1999; Kidwell et al., 2003; Baron, 2005). There are no anatomical markers that clearly map the penumbra area, and its borders may overlap with the adjacent necrotic area. This morphological feature of the penumbra area reflects the dynamic cellular and molecular events occurring within it, which can drive some of its regions toward the necrotic center or render them resistant to secondary neurodegeneration. Robust data show that electrical stimulation of the cerebellar fastigial nucleus (FN) can elicit marked global protection against brain injury in rats submitted to different types of aggression (Reis et al., 1989; Reis et al., 1991; Reis et al., 1997; Golanov et al., 1998; Glickstein et al., 1999) Long-lasting neuroprotective effects have been documented which persisted for up to 10 days after stimulation of the nuclei (Reis et al., 1998). Moreover, a 50% reduction in neuronal death following induced ischemic injury was found three days after FNS deep in the cerebellum (Glickstein et al., 1999), thus revealing a promising method for controlling secondary neuron degeneration in brain ischemia.
Neuronal circuitry
The cerebellar nuclei were first recognized by Raymond de Vieussens (1684) and called the emboliform, globose and fastigial nuclei by Stilling (1864). However, evidence of changes in the control of systemic and encephalic blood flow after stimulation or lesion of the cerebellar nuclei dates back to 1969 (Achari and Downman, 1969; Miura and Reis, 1969; Miura and Reis, 1970). These observations led to the notion of a possible effect of fastigial nucleus stimulation (FNS) on areas adjacent to a brain infarct (Reis et al., 1989). FNS for one hour in rats has been shown to reduce infarction volume by 50% compared to non-stimulated control animals (Reis et al., 1989; Reis et al., 1991; Yamamoto et al.,1993; Reis et al., 1997; Glickstein et al., 1999). The region of spared tissue is referred to as the penumbra area, whose neurons enjoy neuroprotection through inhibition of the secondary neurodegeneration process (Golanov et al., 1998). Although the effects of experimental FNS have been well established, the mechanisms underlying this neurogenic neuroprotection remain a topic of intense investigation (Golanov and Zhou, 2003).
Electrical FNS influences local neurons as well as the abundant fibers of passage within the nucleus and surrounding area (Golanov and Reis, 1996a). Stimulation of the rostromedial portion of the cerebellar FN in the rat triggers a stimulus-locked elevation of arterial pressure and heart rate (Nakai et al., 1983; Chida et al., 1986). This response, discovered by Miura and Reis (1969) and Achari and Downman (1969) in anesthetized cats, is known as “the fastigial pressor response” (FPR). In contrast, microinjection of excitatory amino acids, which only excite neuronal perikarya, decreases arterial pressure and heart rate (Chida et al., 1986; Bradley et al., 1987; Henry and Connor, 1989). These observations may explain the apparently paradoxical findings seen after indiscriminate FNS. Nevertheless, the excitation of fibers of passage seems to be as essential as the stimulation of intrinsic neurons in evoking neurogenic neuroprotection (Glickstein et al., 1999). Since the effects in the brain appear to be global, the network responsible for this action might consist of widespread brain projections from the nucleus (Golanov and Reis, 1996a). However, as this does not apply to the FN, there must be a neuronal circuitry to be found. The FN possesses relatively short projections into the vestibular nucleus, reticular dorsal and paramedian formation, many pontine nuclei including the locus coeruleus, the parabrachial nucleus, as well as the centromedial thalamic nucleus and parafascicular complex, substantia nigra and amygdala (Miura and Reis, 1969; Snider and Maiti, 1976; Batton et al., 1977; Haroian et al., 1981; Golanov and Zhou, 2003).
The same neuroprotection response observed after FNS was also evoked by stimulation of the dorsal portion of the periaqueductal gray (DPAG) and subthalamic vasodilator area (SVA) (Glickstein et al., 2001; Glickstein et al., 2003). One possibility is that DPAG stimulation-induced conditioned neuroprotection is mediated by intrinsic FN neurons. Some studies have revealed that FN efferents, labeled with radioactive amino acids, project into the lateral edge of the periaquecductal gray (PAG) in monkeys and dogs (Batton et al., 1977; Person et al., 1986), while other authors have shown that caudal FN regions involved in oculomotor systems also have fine fibers which extend into the PAG (Noda et al., 1990). Additionally, the PAG also has extensive ascending projections to the midline, intralaminar and reticular thalamic nuclei, as well as the hypothalamus and basal forebrain (Beitz, 1982). The presence of these projections supports the notion that the DPAG excites the neuroprotective SVA, which is independent of the FN (Glickstein et al., 2001).
The existence of a trans-synaptic neuronal network, currently acknowledged from a functional point of view, is consistent with the widespread neuroprotective effect of FNS (Golanov and Zhou, 2003). As the brain is highly dependent upon a continuous and abundant supply of oxygen and glucose for its metabolism, even modest reductions in regional cerebral blood flow (CBF) impair neuronal function and, if briefly sustained, result in neuronal death. Notwithstanding, there are naturalistic behaviors in which regional CBF may fall below thresholds that would normally compromise neuronal function. The fact that these reductions of regional CBF are associated with stereotyped patterns of behavior, as observed in hibernating mammals or diving vertebrates, implies the presence of neuronal networks dedicated to self-protection. Therefore, the findings regarding FNS were attributed by some authors (Reis et al., 1997) to phylogenetic maintenance of this self-protection circuitry. On the other hand, the stimulation pattern used in the cited studies is not normally observed physiologically (Reis et al., 1991; Reis et al., 1997; Golanov et al., 1998; Glickstein et al., 1999). Perhaps these experimental studies are describing the effect of neuronal circuit hyperactivation and its physiological basis, highlighting the principles underlying the coupling between neuronal activation and increased regional CBF.
In this sense, functional magnetic resonance imaging (fMRI) has advanced to become one of the leading tools for assessing brain function. Because fMRI is based on the blood oxygenation level-dependent (BOLD) effect, it does not directly record neural activity. Although generally accepted by the literature, there is no hard scientific evidence that the BOLD effect is exclusively a consequence of a regional increase in neuronal metabolism. A forgotten hypothesis, which holds that vasodilation and the consequent BOLD contrast can be trigged by the brain network itself, is totally plausible. Moreover, distinguishing BOLD signals created by cortical projection neurons from those produced by intracortical neurons has proved difficult. The existence of neuronal circuitry that controls brain microcirculation, linked to its function, is not a new concept and there is sound experimental data supporting this theory.
Two lines of evidence indicate that specific nuclei are essential for the expression of cerebrovascular vasodilation. First, electrical stimulation of the rostral ventrolateral medulla (RVLM) in intact or spinalized rats site-specifically and dose-dependently elevates regional CBF but not regional cerebral glucose utilization (CGU) (Golanov and Reis, 1996b), thereby replicating hypoxic vasodilation. This response can only be attributed to stimulation of the reticulospinal sympathoexcitatory neurons since these are the only neurons in the region excited by the agents. Second, bilateral lesions of the RVLM but not of adjacent regions, reduce hypoxemia-induced elevation of regional CBF by over 50%. The fact that such lesions do not affect vasodilation elicited by hypercarbia indicates that the response is stimulus-elective (Underwood et al., 1994; Golanov and Reis, 1996b). Therefore, cerebrovascular vasodilation elicited in the cerebral cortex by hypoxemia is largely a response to excitation of oxygen-sensitive brainstem neurons (Sun and Reis, 1993; Underwood et al., 1994), as opposed to a direct effect of hypoxia on blood vessels or stimulation of arterial chemoreceptors whose activity, while regulating blood flow to most vascular beds, has no effect on cerebral circulation.
In this context, the circuitry involved in neurogenic neuroprotection could represent the missing link. Descending neuronal projections from the DPAG innervate the parabrachial, ventromedial, and ventrolateral medulla with few direct projections into the spinal cord (Van Bockstaele et al., 1991; Golanov et al., 2000). Some observations suggest that stimulation of the RVLM might produce a neuroprotective effect (∼25%) (Yamamoto et al., 1993; Golanov and Reis, 1996b). It is possible that through these direct projections, the DPAG might excite the RVLM. On the other hand, the effect observed in other experiments is much more marked (∼50%) and independent of sympathoexcitation, which occurs in response to RVLM stimulation (Golanov et al., 2000). By comparison, the non-significant neuroprotection observed in response to stimulation of the ventral portion of the periaqueductal gray (VPAG) may be explained by the excitation of DPAG projections to the RVLM.
Additionally, both the systemic and cerebrovascular components of FNS are abolished by bilateral lesions of the RVLM (Chida et al., 1990). The neuronal elements within the FN responsible for mediating the FPR appear to be axons of brainstem neurons that project via collaterals to innervate both the cerebellum and the RVLM (Ruggiero et al., 1997) Similarly, the SVA also mediates the primary elevations of CBF elicited by hypoxic excitation of the sympathoexcitatory neurons of the RVLM without compromising the cerebral vasodilation evoked by hypercarbia (Golanov et al., 1999a). Taken together, this evidence indicates that the neuroprotective circuitry produces collaterals in order to promote changes in CBF. The functional significance of this coupling is that it might represent the neuronal circuitry involved in the control of regional blood flow and its relationship to regional neuronal activation and synchronization in a given behavior (Golanov and Reis, 1995a; Golanov and Reis, 1996a; Golanov et al., 2000). Alternatively, there is no evidence of a topographical distribution of the diffuse projections described earlier in the text. Providing such evidence would constitute a critical step toward explaining locoregional increase of blood flow in the brain.
Neurogenic neuroprotection
This entity became known as a result of extensive studies in which stimulation of the deep nucleus in the brain was followed by modification of CBF dynamics (Achari and Downman, 1969; Miura and Reis, 1970; Dormer et al., 1982; Nakai et al., 1982; Nakai et al., 1983; Chida et al., 1986). Previous reports have demonstrated the ability of the FNS to induce changes in arterial pressure at rest and in reflex control (Achari and Downman, 1969; Miura and Reis, 1969; Miura and Reis, 1970; Dormer et al., 1982). Furthermore, there is a global rise in CBF during FNS, an event that is not accompanied by an increase in CGU, thus suggesting an absence of functional activation (Nakai et al., 1983; Yamamoto et al., 1993). The first hypothesis advanced was that of an improvement in blood circulation through collateral arteries in the ischemic penumbra, without an associated increase in local functional demand, leading to beneficial action on the vascular ischemic lesion (Reis et al., 1989). Although CBF is higher in non-ischemic areas bilaterally, FNS does not raise CBF in the penumbra area of ischemic infarctions (Yamamoto et al., 1993). Consequently, a mechanism different from that linked to CBF variation may be responsible for the neuroprotection phenomenon.
In order to establish the physiological role of neurogenic neuroprotection, some authors have thought to link CBF to cerebral metabolism (Nakai et al., 1983; Yamamoto et al., 1993). The penumbra area presents a reduction of CBF compared with its cellular metabolism (Baron et al., 1981; Baron, 1999; Baron, 2005), creating what Baron et al. called a “misery-perfusion syndrome” (Baron et al., 1981). Conceptually, reduced cellular metabolism in damaged areas could restore the balance needed for cellular viability and explain the mechanism of neurogenic neuroprotection. Notwithstanding, FNS fails to reduce glucose utilization in penumbra areas, while increasing it in non-ischemic zones (Nakai et al., 1983; Chida et al., 1989; Golanov and Reis, 1996a).
Evidence exists that neuroprotection triggered by FNS is independent of CBF changes or cellular energy consumption at the ischemic penumbra (Yamamoto et al., 1993). Moreover, several experimental studies have failed to demonstrate the relationship between neuroprotection and blood pressure variations, hematocrit levels, body temperature and blood gas concentrations (Glickstein et al., 2003). However, electrical FNS does create a specific sympathetic-excitatory effect along with increased arterial blood pressure and CBF, tachycardia and activation of predatory behaviors (Achari and Downman, 1969; Dormer et al., 1982). These neurovegetative effects are not associated with a rise in metabolism (Miura and Reis, 1969; Miura and Reis 1970; Nakai et al., 1982; Nakai et al., 1983; Glickstein et al., 2003). In contrast, exclusive stimulation of intrinsic neurons of the FN evokes a sympathetic-inhibitory effect characterized by hypotension, bradycardia and global reduction of CBF (Chida et al., 1986; Chida et al., 1989; Glickstein et al., 2003). Moreover, selective injury of these neurons makes FNS non-neuroprotective, thus suggesting an association between sympathetic inhibition and the neuroprotection effect.
Drawing on the previous knowledge that sympathetic inhibition is triggered by stimulation of the VPAG, Glickstein et al. (2003) evaluated stimulation of this nucleus in the context of post-ischemic neuroprotection. If the association between sympathetic inhibition and the neuroprotective effect were to prove valid, then, in theory at least, brain neurovegetative effects would be causative of neuroprotection, regardless of their source. However, although Glickstein achieved inhibition of the encephalic sympathetic system, chemical stimulation of intrinsic neurons of the VPAG failed to produce neuroprotection (Glickstein et al., 2003). These observations suggest that sympathetic inhibition is not a pre-requisite for the neuroprotection induced by electrical stimulation of deep nuclei. Another important finding of the Glickstein study was that the neuroprotective effect of FNS resembled that produced by the same procedure applied to the DPAG.
The conditioned neuroprotection produced by stimulation of the DPAG shows similar features to the conditioned neuroprotection evoked by stimulation of the FN and subthalamic vasodilator area (Glickstein et al., 2001). First, the infarction volume is decreased by ∼50% even when occlusion is performed 72 h after the stimulation. Second, conditioned neuroprotection is independent of increased CBF. Third, salvage can be initiated by using similar parameters for electrical stimulation of the DPAG, FN, and subthalamic vasodilator area (Golanov and Reis, 1996a; Glickstein et al., 2001). This similarity seems to indicate the involvement of related intrinsic brain systems to evoke conditioned neuroprotection.
Other evidence also points to a positive effect of central electrical stimulation at tissue level. Stimulation of the SVA significantly reduced (by 58%) the volume of focal infarctions within the rat brain ischemic area after three days (Glickstein et al., 2001). Nevertheless, no change in neuroprotective effect was detected in FN-stimulated animals with a damaged SVA. Likewise, SVA stimulation in animals with a damaged FN induced no changes in infarction volume within the ischemic penumbra. Moreover, the most important observation was the counteraction of the FNS-induced increase of CBF in the SVA-injured rats. Therefore, these observations have experimentally confirmed that neuroprotection evoked by FNS is not dependent on changes in CBF. These data suggest the existence of an intrinsic neuroprotective network with mechanisms of action that involve neurons themselves.
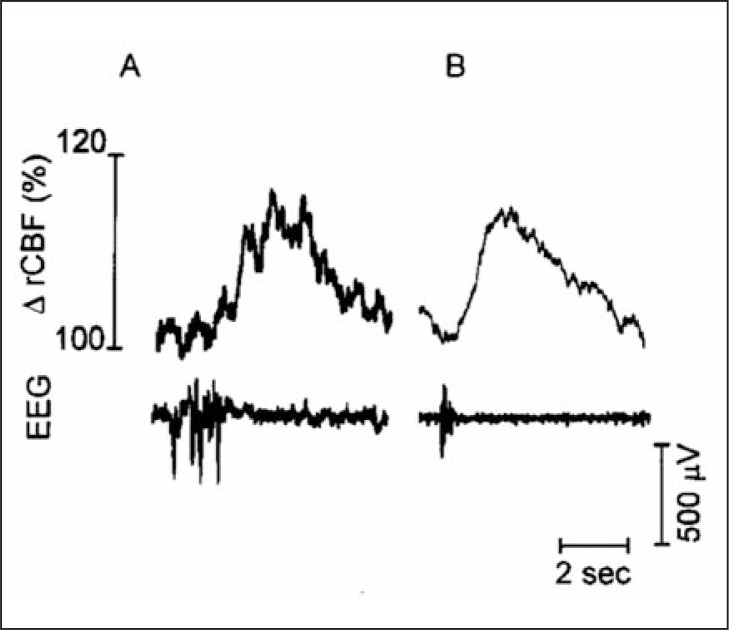
This hypothesis was corroborated by Golanov and Reis (1995a), who demonstrated spontaneous fluctuations of regional CBF in anesthetized rats. These fluctuating waves occur at a reasonably constant frequency of six events per minute with a substantial increase in CBF compared to basal values. In addition, characteristic changes in electrographic activity precede the elevation of regional CBF (Golanov and Reis, 1995a). The peak of electrographic activity precedes (by approximately 1.5 seconds) the transient elevation of CBF (Fig. 1). Electrical stimulation of the FN evokes exactly the same result. The hyperactivation of this circuitry may explain the neuroprotective effect produced by altering the electrophysiological activity of the cerebral cortex, and consequently by inducing cellular changes.
Figure 1.
Characteristic burst-wave complexes recorded in parietal cortex of anesthetized rat.
Upper row: (A) individual and (B) averaged (n = 25 sweeps) bursts recorded at slow sweep speeds followed by a single wave of vasodilation. Note that after averaging, the afterpotential of individual bursts disappears while only the initial potential remains. Reproduced with permission (Golanov and Reis, 1996a).
The electrical instability generated by focal ischemia has previously been described in the corresponding ischemic penumbra in the form of the rapid appearance of repeated depolarizing waves, also known as peri-infarction depolarizing waves (PIDs) (Leao, 1944; Ochs, 1962; Golanov and Reis, 1999). PIDs broaden throughout the cortex in the same manner as cortical spreading depression outside the infarct region. This phenomenon is initiated by acute depolarization and then maintained by secondary waves having similar electrophysiological properties, which travel slowly in normal cortex (Leao, 1944; Ochs, 1962; Golanov and Reis, 1999). PIDs are now believed to contribute to tissue damage by increasing the metabolic demand of already compromised local cells, limiting the energy available for cellular membrane repolarization.
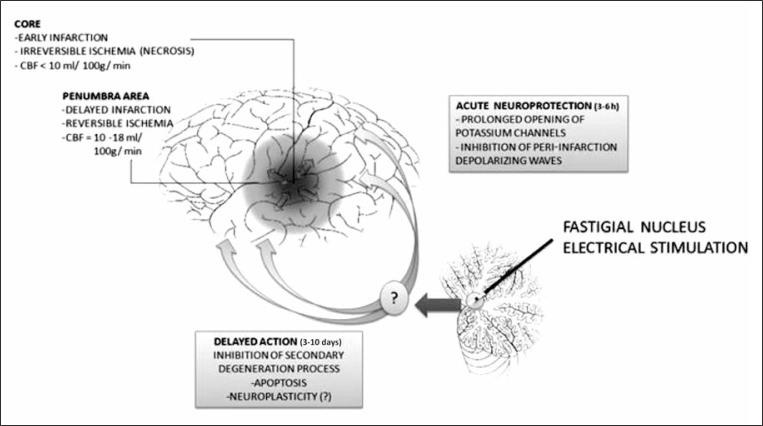
At both electrophysiological and pharmacological levels, the neuroprotective effect of FNS is reversible by a prior intraventricular injection of a potassium channel blocker, i.e. glibenclamide, when the stimulus is delivered immediately before middle cerebral artery (MCA) occlusion in rats (Golanov et al., 1999b). This finding is consistent with the possibility that FNS can evoke a prolonged opening of potassium channels acutely, leading to hyperpolarization and reduced neuronal excitability. In addition, glibenclamide may enhance cortical spreading depression-associated hyperemia (Shimizu et al., 2000) and Golanov and Reis (1999) corroborated this theory, demonstrating that FNS can reduce PID by prolonging the latency of its appearance and reducing the frequency of events. However, glibenclamide has failed to revert neuroprotection when administered immediately after MCA occlusion in rats, submitted to stimulation three days earlier. This finding suggests the existence of at least two possible cellular mechanisms involved in neurogenic neuroprotection (Fig. 2). While a possible acute mechanism may involve modulation of potassium channels, thus accounting for rapid but short-lived neuroprotection, a subsequent and long-term response may depend on intracellular signaling and changes in gene expression. In addition, FNS-mediated responses may modify the apoptotic cascade flow. Furthermore, acute excitability suppression may not constitute a critical event underlying neuroprotection, as demonstrated in stimulated rats that showed inhibition of spreading depolarization waves (Golanov and Reis, 1999). The changes in excitability could be an epiphenomenon or, alternatively, part of the neuroprotection initiation process.
Figure 2.
Neurogenic neuroprotection
A 1-h electrical FNS (1 s on, 1 s off; 0.5 ms pulse durations, 75–150 mA) reduced infarctions triggered by permanent occlusion of the middle cerebral artery by 48–55% in Sprague-Dawley rats and by 59% in Fisher rats. The salvaging effect of FNS was long-lasting and reduced the volume of infarctions 72 h or 10 days later by 58 and 26%, respectively (Glickstein et al., 2001). As the effect appears to be global in the brain and given that the FN possesses relatively short projections, other relays must be involved in this network.
Brain inflammatory responses
It has long been recognized that the threshold for the occurrence of inflammation in the brain is higher than in other tissues. Some authors, however, have suggested that perturbation or dysfunction of certain brain areas could be a contributory factor to the initiation, progression, or lack of resolution of inflammatory responses (Galea et al., 1998a,b). Ischemia triggers an inflammatory process in which capillaries (the blood-brain barrier) play a specific role. Also, ischemia induces the production of several pro-inflammatory cytokines, such as IL-β (Ginsberg and Pulsinelli, 1994; Galea et al., 1998b), which may be involved in the expression of isoform-2 of nitric oxide synthase (iNOS or NOS2) (Iadecola et al., 1995) and of the adhesion molecule ICAM-1 in local capillaries. Blockade of the expression of these components seems to be able to reduce the volume of ischemic infarction. Therefore, these mechanisms have been explored in order to understand the effect of FNS at molecular level.
Electrical FNS prior to the ischemic episode reduced NOS2 mRNA and protein expression by over 90%, where this decrease was restricted to the penumbra area (Galea et al., 1998a). These results demonstrated that FNS is able to modify ischemia-dependent inflammatory gene expression, suggesting that the neuroprotective effects of FNS could be due, in part, to attenuation of NOS2 expression and of other proinflammatory molecules (Galea et al., 1998a). However, whether decreased NOS2 expression was a direct consequence of FN activation (i.e. FNS reduced the capacity of the microvasculature to express NOS2 or the presence of NOS2-expressing leukocytes) or was secondary to the ability of FNS to reduce ischemic damage was not established.
In order to distinguish between these two mechanisms, the effects of FNS on cerebrovascular inflammatory responses were measured in the absence of ischemia. Galea et al. (1998b) demonstrated that FNS renders the brain microvasculature of rats refractory to inflammation generated by the presence of IL-β, by reducing the ability of cerebral microvessels to express both cell adhesion molecules as well as proinflammatory molecules, including NOS2. Furthermore, increased IkBα mRNA induction suggests that FNS modifies the transcriptional machinery involved in the expression of IkBα (an inhibitory protein which prevents NFkB activation).
Apoptosis
The penumbra area has a partially preserved blood supply and is hypermetabolic. This condition evokes sublethal damage ultimately promoting delayed, apoptotic cell death. Apoptosis is a cell-intrinsic process that is essential for animal development and tissue homeostasis. As the tissue salvaged by FNS after focal ischemic insult follows the contours of the presumed ischemic penumbra, the hypothesis that FN not only suppresses inflammatory reactions (Galea et al., 1998a,b) but also attenuates apoptotic processes gains merit.
One evolutionarily conserved component of the apoptotic pathway is the involvement of the members of the caspase family. Measurement of caspase activation serves as a valid early apoptotic marker, while morphological change resulting from caspase activation occurs later (Reed, 2000). Caspase-3 activity is low in freshly isolated brain slice cultures, and slowly increases in a linear fashion over a 24-h period, most likely through spontaneously occurring apoptosis (Zhou et al., 2001). In contrast, robust caspase-3 activation is seen when the slice cultures are challenged with an apoptosis inducer such as staurosporine. This increases intracellular calcium, reactive oxygen species and the release of mitochondrial cytochrome c with subsequent activation of caspase-9 and -3 (Krohn et al., 1998).
The activation of caspase-3 was suppressed in brain tissues removed 72 h post-FNS compared with controls submitted to stimulation of the dentate nucleus (DN) (Zhou et al., 2001), indicating that FNS suppresses apoptosis. Nevertheless, the author found no reproducible or consistent pattern of changes in levels of Bcl-2 family proteins between samples from both FN- and control DN-stimulated animals (Zhou et al., 2001). Therefore, changes in the overall levels of Bcl-2 protein family members are unlikely to contribute to the enhanced mitochondrial resistance conferred by FNS (Zhou et al., 2005). On the other hand, FNS decreases Bax insertion into mitochondria (Zhou et al., 2005), a critical step in the initiation of apoptosis. Although observations in isolated mitochondria may not be directly applicable to the experiments in which Bax insertion was investigated in brain slices, these findings may provide a molecular link between FNS and reduction in caspase-3 activation (Zhou et al., 2001; Zhou et al., 2005).
FNS also reduces staurosporine-induced cytochrome c release from mitochondria compared with DN-stimulated rats (Zhou et al., 2001). This may result from a direct effect of FNS, rendering the mitochondria more resilient to the insult. Alternatively, FNS may have an indirect effect, increasing the threshold of cellular stress essential to evoke mitochondrial dysfunction. Seeking insights into the molecular mechanisms of FNS, Zhou et al. used isolated rat brain mitochondria to demonstrate that FNS increases the ability of mitochondria to maintain their functional state during Ca2+ loading, and inhibits mitochondrial pathways from triggering apoptosis (Zhou et al., 2005).
Mitochondria have emerged as critical organelles in determining cell fate. During ischemia and excitotoxic injury, Ca2+ enters neurons through NMDA receptors, and the overloading of Ca2+ into mitochondria leads to opening of the permeability transition pore and loss of mitochondrial membrane potential. As a result, the outer mitochondrial membrane (OMM) becomes permeable to intermembrane space proteins, such as cytochrome c, a change which marks the beginning of mitochondrial dysfunction. Interventions enhancing OMM integrity would clearly offer neuroprotection against injury.
Zhou et al. (2005) used mastoparan and Ca2+ as inducers of cytochrome c release. Earlier studies by others had shown that mastoparan induces the mitochondrial permeability transition (Pfeiffer et al., 1995) and releases mitochondrial proteins. FN-conditioned mitochondria release less cytochrome c in response to mastoparan and Ca2+, suggesting that FNS is able to stabilize protein tyrosine phosphatases and enhance the integrity of the OMM. This ability attenuates the inhibition of respiration produced by Ca2+ loading, maintains adenosine triphosphate production, and may reduce free radical production during ischemia or excitotoxicity. Taken together, these data provide evidence that the neuroprotection exerted by FNS is mediated by an effect on mitochondria, resulting in an enhanced Ca2+ buffering capability, improved respiration in the presence of excess Ca2+, reduced Bax insertion, and attenuation of cytochrome c release (Zhou et al., 2005).
This suppression of apoptosis by FNS was moderate, leading to reductions of approximately 30% compared with DN-stimulated controls, a considerably lower level than anticipated from in vivo studies, where maximum reductions in cell death are greater than 50% (Reis et al., 1991; Reis et al., 1998; Glickstein et al., 1999).This may reflect the nature of the in vitro system, in that adult neurons are fragile in culture, so the full protective potential of FNS is not completely displayed in this system. As FN neuroprotection is at least in part attributed to the prevention of caspase activation primarily in glial cells (Zhou et al., 2001), changes in the microenvironment may also play an important role. On the other hand, in vivo neuroprotection by FNS may involve multiple protective mechanisms besides anti-apoptosis, such as the systemic suppression of inflammatory responses (Galea et al., 1998a,b).
Clinical perspectives
As regards their experimental design, the studies cited above adopted the stimulation-followed-by-lesion paradigm, which seems to be of little value from a clinical point of view. To identify endogenous mechanisms of protection and repair, and to make use of these mechanisms therapeutically, biomedical investigators have developed preconditioning strategies. Classically, preconditioning denotes a procedure through which a noxious stimulus, close to, but below, the threshold of damage, is applied to the tissue. However, there are also other ways of “preconditioning” the brain. Shortly after preconditioning or after a delay, the organ (and therefore the organism) develops resistance or tolerance to the same, similar, or even different noxious stimuli administered beyond the threshold of damage. Preconditioning thereby protects against subsequent injury. In this context, FNS can be considered a preconditioning measure which does not run the risk of inducing sub-lethal damage. However, FNS can also be used to change the chain of events when it is still reversible.
Alterations in CBF and metabolism after subarachnoid hemorrhage (SAH) are well known and have been extensively described (Feigin and Findlay, 2006). Cerebral vasospasm remains one of the most serious complications after SAH. It is the classic cause of delayed neurological deterioration after aneurysmal SAH and it leads to cerebral ischemia and infarction with poor outcomes, and occasionally to death. Over the past decades, several pharmacological approaches have been investigated for the prevention of cerebral vasospasm (Feigin and Findlay, 2006; Lee et al., 2006). Despite advances in understanding of the physiopathological nuances of cerebral vasospasm, it has been correlated with a 1.5- to threefold increase in death within the first two weeks of the ictal event. Unfortunately, no efficacious treatment is available for this critical patient group (Lee et al., 2006). Classically, vasospasm follows a typical time course, in that its onset usually occurs within one week of the hemorrhage, reaches maximum severity between days 7 and 14 post-SAH, and usually dissipates after 14 to 28 days (Fisher et al., 1980; Feigin and Findlay, 2006; Lee et al., 2006). Accordingly, brain preconditioning can be an important tool in the treatment of these patients. In this sense, observations from spinal cord stimulation (SCS) suggest that this may be an interesting strategy. SCS with epidural electrodes has been used to treat chronic pain syndrome and peripheral vascular disease. In addition to its effects on the peripheral vasculature, cervical SCS seems to have similar effects on the cerebral vasculature. Since the first report by Hosobuchi (1985), the ability of SCS to augment CBF has been demonstrated in several clinical and experimental studies (Matsui and Hosobuchi, 1989; Visocchi et al., 1994; Takanashi and Shinonaga, 2000; Visocchi et al., 2001). A number of clinical reports describe the use of SCS to treat patients with cerebral ischemia. However, despite the promise of clinical benefit from SCS in the treatment of cerebral ischemia, its effective use is hampered by a lack of understanding of its mechanism(s) of action.
Evidence from studies of the peripheral vasculature suggests that SCS produces a reversible functional sympathectomy (Linderoth et al., 1995). Modulation of the superior cervical ganglion at a cervical-thoracic transitional level, suppressing efferent signals from their origin to the sympathetic chain, may be one explanation. Further support for such a pathway stems from the finding that concurrent stimulation of the cervical sympathetic chain overcomes the CBF response to SCS (Visocchi et al., 1994). However, although the sympathetic system may play an important role in the pathogenesis of vasospasms (Treggiari et al., 2003), a reversible sympathectomy cannot be considered the sole mechanism involved in mediating both SCS-induced CBF increase and vasospasm prevention effects. The genesis of vasospasm involves vasomotor changes and an inflammatory cascade promoted by the presence of blood in the brain basal cisterns (Feigin and Findlay, 2006).
The results of a study by Patel et al. (2004) demonstrated that surgical interruption of cervical sympathetic outflow has no effect on SCS-induced CBF augmentation. At baseline, surgical sympathectomy did not significantly alter CBF. When SCS was applied after surgical ablation of the superior cervical ganglion, the resultant CBF response remained robust, thus indicating that the CBF response to SCS is not significantly dependent on cervical sympathetic outflow. Furthermore, the effects of SCS on CBF are completely abolished by interruption of the spinal cord pathways above the level of stimulation. SCS performed after spinalization failed to elicit any increase in CBF (Patel et al., 2004). These lines of evidence suggest that cervical SCS produces a significant cerebrovascular effect and that this effect may involve alterations in sympathetic tone as well as in indirect activation of brainstem or cerebellar vasomotor centers.
The primary vasodilation which occurs as an oxygenconserving response, such as in the diving reflex, has been well described in diving vertebrates. A rapid set of autonomic reflex adjustments involving differentiated excitations of sympathetic neurons is critical. This sympathetically mediated vasomotor response can be replicated by electrical or chemical stimulation of a small population of neurons, mostly adrenergic, lying within a small subnucleus representing the C1 area of the RVLM (Reis et al., 1994). These neurons also mediate much of the sympathetic and cerebrovascular response to hypoxemia. These neuronal pathways, through which excitation of oxygen-sensitive neurons in the RVLM reflexively protects the cerebral cortex from hypoxia, are indirect (Golanov and Reis, 1996b). Projection is polysynaptic, reaching the cortex through as-yet-undefined projections to the upper brainstem and/or thalamus. Additionally, the subcortical-cortical efferent vasodilator pathway does not directly regulate cerebral vessels. Rather, it appears to excite a small population of cortical neurons that seem to be dedicated to transducing a neuronal signal into vasodilation (Chida et al., 1986; Golanov and Reis, 1996a,b).
This system also appears to relay the central neurogenic vasodilation elicited from other brain regions, including the FN and nucleus tractus solitarius areas (Golanov and Reis, 1995b). Neurons of the RVLM are the principal relay for the potent vasopressor and cerebrovascular vasodilator responses evoked by electrical stimulation of the cerebellar FN (Golanov and Reis, 1996b). Both the systemic and cerebrovascular components of the fastigial effects are abolished by bilateral lesions of the RVLM (Chida et al., 1990). The neuronal elements within the FN responsible for mediating the FPR appear to be axons of brainstem neurons that project via collaterals to innervate the cerebellum and also the RVLM, since microinjection of excitatory amino acids into the FN fails to replicate the FPR (Chida et al., 1990). However, unlike conditioned central neurogenic protection, activation of neurons of the RVLM appears to be less cytoprotective (Yamamoto et al., 1993). Cervical SCS likely has a limited effect only on the RVLM, and does not reflect the true potential of this neuronal circuitry in patients experiencing ischemic damage due to vasospasm. Deep FNS seems to be the best target in order to promote a dual effect on the brain parenchyma: vasodilation and neuroprotection. Experimental investigations on neurogenic neuroprotection demonstrated a maximum effect, in terms of the counteraction of neuronal death, provided the electrical stimulation had occurred three days before the MCA occlusion in rats (Reis et al., 1991; Reis et al., 1997). Knowing that ischemic events due to vasospasm occur at least three days after aneurysm rupture, there may be a therapeutic window, and a new horizon for neurogenic neuroprotection. If experimental results were extrapolated to clinical situations, then a substantial reduction of neuronal death, in the order of about 50%, could be achievable in humans, a level not attained by any other pharmacological interventions. Such a reduction in mortality would represent a remarkable feat of translational neuroscience, leading to a substantial reduction of mortality in brain ischemia in clinical situations.
Nevertheless, as outlined earlier, there are times when we cannot predict events even in the near future. Malignant MCA infarction is a large hemispheric infarction with poor outcome due to the ischemic inflammation/edema that causes an early rise in intracranial pressure and subsequent brain herniation and death (Ginsberg and Pulsinelli, 1994; Baron, 1999; Vahedi et al., 2007). No clinical therapy has proven able to effectively prevent neuronal death in the penumbra area and thereby improve patient outcome. Surgical decompression techniques have been proposed to relieve high intracranial pressure, but surgical decompression is only a critical intervention in an inexorable process (Vahedi et al., 2007). The only method currently used to prevent this outcome is unblocking of the compromised vessel. However, although recombinant tissue-type plasminogen activator (rt-PA) has been approved for acute ischemic stroke, less than 5% of eligible patients actually receive rt-PA. Several factors have been identified to explain the underuse of this therapeutic approach for ischemic stroke: the short therapeutic window, insufficient public awareness and knowledge of stroke symptoms, the limited number of centers able to perform thrombolysis on a 24-hour basis, and excessive fear of hemorrhagic complications. Although in clinical practice this complication may be less frequent than failure of treatment to recanalize occluded cerebral artery or early (within 48 hours) reocclusion, intracerebral hemorrhage seems to represent a significant obstacle to the generalization of thrombolytic therapy. Those patients ineligible for thrombolysis are condemned to an ineffective group of systemic measures in the intensive care unit.
Acute intervention by means of FNS, leading to control of the apoptotic cycle of compromised neurons and of the inflammatory cascade taking place in the penumbra area, is feasible in neurosurgical clinical practice and may change the course of this malignant evolution.
Nowadays, the implantation of electrodes for stimulation of deep brain nuclei (deep brain stimulation, DBS) is mainly performed in patients with movement disorders and psychiatric disturbances. The fact that DBS is used in elective situations should not be an impediment to its application in critical situations. MRI, utilized for anatomical programming, is available in emergency situations, and 3D target localization can be calculated in seconds with computational assistance. Another important point is that although DBS surgery for patients with movement disorders usually implies the need for patients to be awake, DBS for neurogenic neuroprotection could be performed safely with patients under general anesthesia. The only variable that must be measured, besides the patient’s own anatomy, is the increase of CBF that occurs when we stimulate the FN. This variable can be easily measured by intraoperative transcranial Doppler ultrasound.
Translation of these experimental data to a clinical setting, albeit implying an enormous increase in cost and complexity of patient care in the emergency department, could indeed lead to change. An association between thrombolysis and FNS may have the potential to alter the pathological chain of events leading to decompressive craniectomy.
Concluding remarks
In conclusion, it is difficult to conceive of a brain-protective intrinsic circuit which is activated to render the brain less susceptible to traumatic or ischemic events. It is more likely that the findings of these experimental studies are the result of neuronal circuit hyperactivation. This effect seems to be related to inhibition of apoptosis and regulation of the inflammatory cascade. Perhaps, the neurogenic neuroprotection circuitry has a physiological function related to the regulation of microvasculature and modulation of brain metabolism in order to promote a more receptive regional microenvironment and prevent neuronal excitotoxicity caused by repeated neuronal activity. The stimulation of deep brain nuclei to obtain changes in ischemic brain lesion outcomes and to promote changes in regional metabolism heralds a new era in functional neurosurgery. However, further studies are needed to translate these experimental data to changes into clinical practice.
Acknowledgments
We acknowledge support from FAPESP (State of São Paulo Research Foundation) and CNPQ (Brazilian National Council for Technological and Scientific Development).
References
- Achari NK, Downman CB. Autonomic responses evoked by stimulation of fastigial nuclei in the anaesthetized cat. J Physiol. 1969;204:130. [PubMed] [Google Scholar]
- Baron JC, Bousser MG, Rey A, et al. Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke. 1981;12:454–459. doi: 10.1161/01.str.12.4.454. [DOI] [PubMed] [Google Scholar]
- Baron JC. Mapping the ischaemic penumbra with PET: implications for acute stroke treatment. Cerebrovasc Dis. 1999;9:193–201. doi: 10.1159/000015955. [DOI] [PubMed] [Google Scholar]
- Baron JC. How healthy is the acutely reperfused ischemic penumbra? Cerebrovasc Dis. 2005;20(Suppl 2):25–31. doi: 10.1159/000089354. [DOI] [PubMed] [Google Scholar]
- Batton RR, 3rd, Jayaraman A, Ruggiero B, et al. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol. 1977;174:281–305. doi: 10.1002/cne.901740206. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Pascoe JP, Paton JF, et al. Cardiovascular and respiratory responses evoked from the posterior cerebellar cortex and fastigial nucleus in the cat. J Physiol. 1987;393:107–121. doi: 10.1113/jphysiol.1987.sp016813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida K, Iadecola C, Underwood MD, et al. A novel vasodepressor response elicited from the rat cerebellar fastigial nucleus: the fastigial depressor response. Brain Res. 1986;370:378–382. doi: 10.1016/0006-8993(86)90498-1. [DOI] [PubMed] [Google Scholar]
- Chida K, Iadecola C, Reis DJ. Global reduction in cerebral blood flow and metabolism elicited from intrinsic neurons of fastigial nucleus. Brain Res. 1989;500:177–192. doi: 10.1016/0006-8993(89)90312-0. [DOI] [PubMed] [Google Scholar]
- Chida K, Iadecola C, Reis DJ. Lesions of rostral ventrolateral medulla abolish some cardio- and cerebrovascular components of the cerebellar fastigial pressor and depressor responses. Brain Res. 1990;508:93–104. doi: 10.1016/0006-8993(90)91122-w. [DOI] [PubMed] [Google Scholar]
- de Vieussens R. Neurographia universalis. Certe, Lyons: 1684. [Google Scholar]
- Ginsberg MD, Pulsinelli WA. The ischemic penumbra, injury thresholds, and the therapeutic window for acute stroke. Ann Neurol. 1994;36:553–554. doi: 10.1002/ana.410360402. [DOI] [PubMed] [Google Scholar]
- Dormer KJ, Foreman RD, Ohata CA. Fastigial nucleus stimulation and excitatory spinal sympathetic activity in dog. Am J Physiol. 1982;243:R25–33. doi: 10.1152/ajpregu.1982.243.1.R25. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Findlay M. Advances in subarachnoid hemorrhage. Stroke. 2006;37:305–308. doi: 10.1161/01.STR.0000200558.38774.d5. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by CT scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Galea E, Golanov EV, Feinstein DL, et al. Cerebellar stimulation reduces inducible nitric oxide synthase expression and protects brain from ischemia. Am J Physiol. 1998a;274:H2035–2045. doi: 10.1152/ajpheart.1998.274.6.H2035. [DOI] [PubMed] [Google Scholar]
- Galea E, Glickstein SB, Feinstein DL, et al. Stimulation of cerebellar fastigial nucleus inhibits interleukin-1beta-induced cerebrovascular inflammation. Am J Physiol. 1998b;275:H2053–2063. doi: 10.1152/ajpheart.1998.275.6.H2053. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Golanov EV, Reis DJ. Intrinsic neurons of fastigial nucleus mediate neurogenic neuroprotection against excitotoxic and ischemic neuronal injury in rat. J Neurosci. 1999;19:4142–4154. doi: 10.1523/JNEUROSCI.19-10-04142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein SB, Ilch CP, Reis DJ, et al. Stimulation of the subthalamic vasodilator area and fastigial nucleus independently protects the brain against focal ischemia. Brain Res. 2001;912:47–59. doi: 10.1016/s0006-8993(01)02602-6. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Ilch CP, Golanov EV. Electrical stimulation of the dorsal periaqueductal gray decreases volume of the brain infarction independently of accompanying hypertension and cerebrovasodilation. Brain Res. 2003;994:135–145. doi: 10.1016/j.brainres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Reis DJ. Vasodilation evoked from medulla and cerebellum is coupled to bursts of cortical EEG activity in rats. Am J Physiol. 1995a;268:R454–467. doi: 10.1152/ajpregu.1995.268.2.R454. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Reis DJ. Synchronization of EEG activity in cerebral cortex by stimulation of nucleus tractus solitarii (NTS) in rat: coupling to cerebral blood flow and mediation by rostral ventrolateral medulla (RVL) Society for Neuroscience Abstracts. 1995b;21:1669. [Google Scholar]
- Golanov EV, Reis DJ. Cerebral cortical neurons with activity linked to central neurogenic spontaneous and evoked elevations in cerebral blood flow. Neurosci Lett. 1996a;209:101–104. doi: 10.1016/0304-3940(96)12611-2. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Reis DJ. Contribution of oxygen-sensitive neurons of the rostral ventrolateral medulla to hypoxic cerebral vasodilatation in the rat. J Physiol. 1996b;495:201–216. doi: 10.1113/jphysiol.1996.sp021585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golanov EV, Liu F, Reis DJ. Stimulation of cerebellum protects hippocampal neurons from global ischemia. Neuroreport. 1998;9:819–824. doi: 10.1097/00001756-199803300-00010. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Christensen J, Reis DJ. Neurons of a subthalamic cerebrovasodilator area (SCA) mediate elevations in cerebral flood flow (rCBF) evoked by excitation of neurons of rostral ventrolateral medulla. Soc Neurosci. 1999a;25:12. doi: 10.1523/JNEUROSCI.21-11-04032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golanov EV, Christensen JD, Reis DJ. Role of potassium channels in the central neurogenic neuroprotection elicited by cerebellar stimulation in rat. Brain Res. 1999b;842:496–500. doi: 10.1016/s0006-8993(99)01871-5. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Reis DJ. Neuroprotective electrical stimulation of cerebellar fastigial nucleus attenuates expression of periinfarction depolarizing waves (PIDs) and inhibits cortical spreading depression. Brain Res. 1999;818:304–315. doi: 10.1016/s0006-8993(98)01169-x. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Ruggiero DA, Reis DJ. A brainstem area mediating cerebrovascular and EEG responses to hypoxic excitation of rostral ventrolateral medulla in rat. J Physiol. 2000;529:413–429. doi: 10.1111/j.1469-7793.2000.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golanov EV, Zhou P. Neurogenic neuroprotection. Cell Mol Neurobiol. 2003;23:651–663. doi: 10.1023/A:1025088516742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroian AJ, Massopust LC, Young PA. Cerebellothalamic projections in the rat: an autoradiographic and degeneration study. J Comp Neurol. 1981;197:217–236. doi: 10.1002/cne.901970205. [DOI] [PubMed] [Google Scholar]
- Henry RT, Connor JD. Axons of passage may be responsible for fastigial nucleus pressor response. Am J Physiol. 1989;257:R1436–1440. doi: 10.1152/ajpregu.1989.257.6.R1436. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y. Electrical stimulation of cervical spinal cord increases cerebral blood flow in humans. Appl Neurophysiol. 1985;48:372–376. doi: 10.1159/000101161. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Xu S, et al. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab. 1995;15:378–384. doi: 10.1038/jcbfm.1995.47. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- Krohn AJ, Preis E, Prehn JH. Staurosporine-induced apoptosis of cultured rat hippocampal neurons involves caspase-1- like proteases as upstream initiators and increased production of superoxide as a main downstream effector. J Neurosci. 1998;18:8186–8197. doi: 10.1523/JNEUROSCI.18-20-08186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Lee KH, Lukovits T, Friedman JA. “Triple-H” therapy for cerebral vasospasm following subarachnoid hemorrhage. Neurocrit Care. 2006;4:68–76. doi: 10.1385/NCC:4:1:068. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Gherardini G, Ren B, et al. Preemptive spinal cord stimulation reduces ischemia in an animal model of vasospasm. Neurosurgery. 1995;37:266–272. doi: 10.1227/00006123-199508000-00011. [DOI] [PubMed] [Google Scholar]
- Matsui T, Hosobuchi Y. The effects of cervical spinal cord stimulation (cSCS) on experimental stroke. Pacing Clin Electrophysiol. 1989;12:726–732. doi: 10.1111/j.1540-8159.1989.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Miura M, Reis DJ. Cerebellum: a pressor response elicited from the fastigial nucleus and its efferent pathway in brainstem. Brain Res. 1969;13:595–599. doi: 10.1016/0006-8993(69)90269-8. [DOI] [PubMed] [Google Scholar]
- Miura M, Reis DJ. A blood pressure response from fastigial nucleus and its relay pathway in brainstem. Am J Physiol. 1970;219:1330–1336. doi: 10.1152/ajplegacy.1970.219.5.1330. [DOI] [PubMed] [Google Scholar]
- Nakai M, Iadecola C, Reis DJ. Global cerebral vasodilation by stimulation of rat fastigial cerebellar nucleus. Am J Physiol. 1982;243:H226–235. doi: 10.1152/ajpheart.1982.243.2.H226. [DOI] [PubMed] [Google Scholar]
- Nakai M, Iadecola C, Ruggiero DA, et al. Electrical stimulation of cerebellar fastigial nucleus increases cerebral cortical blood flow without change in local metabolism: evidence for an intrinsic system in brain for primary vasodilation. Brain Res. 1983;260:35–49. doi: 10.1016/0006-8993(83)90762-x. [DOI] [PubMed] [Google Scholar]
- Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990;302:330–348. doi: 10.1002/cne.903020211. [DOI] [PubMed] [Google Scholar]
- Ochs S. The nature of spreading depression in neural networks. Int Rev Neurobiol. 1962;4:1–69. [Google Scholar]
- Patel S, Huang DL, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery. 2004;55:201–206. doi: 10.1227/01.neu.0000126949.28912.71. [DOI] [PubMed] [Google Scholar]
- Person RJ, Andrezik JA, Dormer KJ, et al. Fastigial nucleus projections in the midbrain and thalamus in dogs. Neuroscience. 1986;18:105–120. doi: 10.1016/0306-4522(86)90182-x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer DR, Gudz TI, Novgorodov SA, et al. The peptide mastoparan is a potent facilitator of the mitochondrial permeability transition. J Biol Chem. 1995;270:4923–4932. doi: 10.1074/jbc.270.9.4923. [DOI] [PubMed] [Google Scholar]
- Reed J. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis DJ, Underwood MD, Berger SB, et al. Fastigial nucleus stimulation reduces the volume of cerebral infarction produced by occlusion of the middle cerebral artery in rat. In: Seylaz J, MacKenzie ET, editors. Neurotransmission and Cerebrovascular Function I. New York: Elsevier Science; 1989. pp. 401–404. [Google Scholar]
- Reis DJ, Berger SB, Underwood MD, et al. Electrical stimulation of cerebellar fastigial nucleus reduces ischemic infarction elicited by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 1991;11:810–818. doi: 10.1038/jcbfm.1991.139. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Golanov EV, Ruggiero DA, et al. Sympatho-excitatory neurons of the rostral ventrolateral medulla are oxygen sensors and essential elements in the tonic and reflex control of the systemic and cerebral circulations. J Hypertens Suppl. 1994;12:S159–180. [PubMed] [Google Scholar]
- Reis DJ, Golanov EV, Galea E, et al. Central neurogenic neuroprotection: central neural systems that protect the brain from hypoxia and ischemia. Ann NY Acad Sci. 1997;835:168–186. doi: 10.1111/j.1749-6632.1997.tb48628.x. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Kobylarz K, Yamamoto S, et al. Brief electrical stimulation of cerebellar fastigial nucleus conditions long-lasting salvage from focal cerebral ischemia: conditioned central neurogenic neuroprotection. Brain Res. 1998;780:159–163. [PubMed] [Google Scholar]
- Ruggiero DA, Anwar M, Golanov EV, et al. The pedunculopontine tegmental nucleus issues collaterals to the fastigial nucleus and rostral ventrolateral reticular nucleus in the rat. Brain Res. 1997;760:272–276. doi: 10.1016/s0006-8993(97)00397-1. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Bari F, Busija DW. Glibenclamide enhances cortical spreading depression-associated hyperemia in the rat. Neuroreport. 2000;11:2103–2106. doi: 10.1097/00001756-200007140-00009. [DOI] [PubMed] [Google Scholar]
- Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. J Neurosci Res. 1976;2:133–146. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- Stilling B. Untersuchungen über den Bau des kleinen Gehirns des Menschen. T. Fischer; Cassel: 1864. [Google Scholar]
- Sun MK, Reis DJ. Hypoxic excitation of medullary vasomotor neurons in rats are not mediated by glutamate or nitric oxide. Neurosci Lett. 1993;157:219–222. doi: 10.1016/0304-3940(93)90741-3. [DOI] [PubMed] [Google Scholar]
- Takanashi Y, Shinonaga M. Spinal cord stimulation for cerebral vasospasm as prophylaxis. Neurol Med Chir (Tokyo) 2000;40:352–357. doi: 10.2176/nmc.40.352. [DOI] [PubMed] [Google Scholar]
- Treggiari MM, Romand JA, Martin JB, et al. Cervical sympathetic block to reverse delayed ischemic neurological deficits after aneurysmal subarachnoid hemorrhage. Stroke. 2003;34:961–967. doi: 10.1161/01.STR.0000060893.72098.80. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Iadecola D, Reis DJ. Lesions of the rostral ventrolateral medulla reduce the cerebrovascular response to hypoxia. Brain Res. 1994;635:217–223. doi: 10.1016/0006-8993(94)91442-7. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Vicaut E, Mateo J, et al. Sequential-design multicenter randomized controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial) Stroke. 2007;38:2506–2517. doi: 10.1161/STROKEAHA.107.485235. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Aston-Jones G, Pieribone VA, et al. Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J Comp Neurol. 1991;309:305–327. doi: 10.1002/cne.903090303. [DOI] [PubMed] [Google Scholar]
- Visocchi M, Cioni B, Pentimalli L, et al. Increase of cerebral blood flow and improvement of brain motor control following spinal cord stimulation in ischemic spastic hemiparesis. Stereotact Funct Neurosurg. 1994;62:103–107. doi: 10.1159/000098604. [DOI] [PubMed] [Google Scholar]
- Visocchi M, Argiolas L, Meglio M, et al. Spinal cord stimulation and early experimental cerebral spasm: the “functional monitoring” and the “preventing effect”. Acta Neurochir (Wien) 2001;143:177–185. doi: 10.1007/s007010170126. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Golanov EV, Reis DJ. Reductions in focal ischemic infarctions elicited from cerebellar fastigial nucleus do not result from elevations in cerebral blood flow. J Cereb Blood Flow Metab. 1993;13:1020–1024. doi: 10.1038/jcbfm.1993.128. [DOI] [PubMed] [Google Scholar]
- Zhou P, Qian L, Glickstein SB, et al. Electrical stimulation of cerebellar fastigial nucleus protects rat brain, in vitro, from staurosporine-induced apoptosis. J Neurochem. 2001;79:328–338. doi: 10.1046/j.1471-4159.2001.00585.x. [DOI] [PubMed] [Google Scholar]
- Zhou P, Qian L, Zhou T, et al. Mitochondria are involved in the neurogenic neuroprotection conferred by stimulation of cerebellar fastigial nucleus. J Neurochem. 2005;95:221–229. doi: 10.1111/j.1471-4159.2005.03358.x. [DOI] [PubMed] [Google Scholar]