Summary
Secondary non-response (SNR) to botulinum toxin (BoNT) in cervical dystonia (CD) lacks a universal definition. We conducted a retrospective survey to develop a definition based on clinicians’ practice. Fifty-seven neurologists completed a 17-item questionnaire. In defining SNR, insufficiently improved posture was considered to be more relevant (98% of physicians) than insufficiently improved pain (86%). The most frequently used diagnostic test for SNR was the frontalis test (68%); antibody testing was performed by only 13% of physicians. Three consecutive unsuccessful injection cycles were considered the most appropriate indicator of SNR (55% of physicians). Physicians reported that 5.9% (median) of patients treated in 2008 became secondary non-responders to BoNT-A. The most common strategy for SNR was optimization of physiotherapy, considered by 98% of the physicians. On the basis of our findings, SNR can be defined as insufficiently improved posture after ≥3 unsuccessful injection cycles in CD patients previously achieving satisfactory results.
Keywords: botulinum toxin, cervical dystonia, non-response
Introduction
There is a considerable amount of published data documenting the fact that botulinum toxin type A (BoNT-A) preparations are the treatment of choice for cervical dystonia (CD) (Albanese et al., 2011), having a well-established long-term efficacy and safety profile (Mohammadi et al., 2009). Since CD is a chronic disease, long-term efficacy of BoNT-A treatment is crucial both for patients and for treating physicians. BoNT-A treatment is considered to be safe and efficacious even when repeated treatments are performed over many years (Albanese et al., 2011). However, with repeated treatments, some previously responsive patients can fail to respond, i.e. develop a phenomenon known as secondary non-response. Secondary non-response has been broadly defined as a lack of clinical response to further treatment in a patient who previously showed adequate clinical improvement (Brin, 2007; Cordivari et al., 2006).
Reported rates of secondary non-response vary in the literature. In a long-term study of CD patients treated with BoNT-A (abobotulinumtoxinA; Dysport®, Ipsen Pharma, Slough, Berkshire, UK) for up to 10 years, only 3% of initially responsive patients subsequently became refractory to injections and were classified as secondary non-responders (Hausermann et al., 2004). Conversely, 7.5% of patients treated with BoNT-A (onabotulinumtoxinA; Botox®, Allergan Inc., Irvine, California, USA) for a range of movement disorders, primarily CD, reported secondary non-response to treatment during a follow-up period of 10 years (Hsiung et al., 2002). This latter study was unusual in that a specific definition was used to determine secondary non-response: achievement of at least 50% improvement for at least two treatment cycles followed by less than 25% improvement after two or more subsequent treatment cycles (as defined by clinical evaluation and questionnaires).
What causes secondary non-response is still not well established. Historically, it was assumed that treatment failure was caused by the formation of neutralizing anti-bodies (NAbs) against the heavy or light chains of BoNT-A (Lange et al., 2009). However, results from a recent study, which tested for the presence of NAbs in a large cohort of patients, revealed that in more than half of them, lack of efficacy was not due to NAb formation (Lange et al., 2009). In this study, patients were classified as secondary non-responders to BoNT-A by their treating physicians and were required to have at least two unsuccessful treatments subsequent to treatments that had given satisfactory results. Similarly, in a prospective, longitudinal study, the majority of subjects deemed clinically non-responsive to onabotulinumtoxinA by investigators were found to be antibody negative (Brin et al., 2008), further suggesting that factors other than NAbs account for secondary non-response.
Proposed alternative explanations for the development of non-response include disease progression (Dressler, 2004), changes in the pattern of muscle hyperactivity (Gelb et al., 1991), and even inappropriate dosing and target muscle selection. Anyhow, there is clearly a need for a better understanding of secondary non-response to BoNT-A, from its prevalence right through to the mechanisms responsible, in order to optimize the diagnosis, management, and prevention of this problem.
What emerges from the literature is that, not only does the operational definition of secondary non-response vary considerably between studies, but also the choice of possible clinical tests [such as the frontalis test (Brin, 2007) or the extensor digitorum brevis test (Gordon et al., 2002)] to confirm secondary non-response has not been firmly established. Thus, we felt that there was a clinical need to develop a new operational definition of secondary non-response in CD based on current practice in several centers in Europe. We were also interested in evaluating what therapeutic practices are used to overcome this phenomenon.
Materials and methods
In order to establish what is common practice, in terms of the definition of secondary non-response in CD, we conducted an international survey among 57 neurologists from nine countries (Czech Republic [2], France [6], Italy [18], Poland [3], Portugal [3], Romania [1], Russia [3], Spain [20] and Thailand [1]). All the physicians were based in departments with BoNT clinics and are experienced in the treatment of this condition with BoNT-A; 96% of the neurologists had more than 5 years’ treatment experience. The median number of patients treated by physicians was 42 per year in 2008. The survey was undertaken in 2009.
The neurologists were asked to complete a 17-item questionnaire, which was designed to document each physician’s pragmatic definition of secondary non-response. The questions required physicians to provide responses regarding their use of patient feedback and clinical examination to define BoNT-A failure, what factors are considered to contribute to the perception of treatment failure (based on both feedback from patients and physicians’ perspectives), whether they considered a decrease in interval between injections as relevant, and whether they use clinical tests – e.g. Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) (Consky and Lang, 1994) or the Tsui scale (Tsui et al., 1986) – to confirm treatment failure, and whether they conduct antibody testing. Each outcome was rated on a 4-item scale: never = ≤10%; sometimes = 10% to 50%; often = ≥50% to 90%, or always = ≥90%. Physicians were asked to indicate the average number of injection cycles (with no or less benefit) after which they would consider BoNT-A therapy to have failed. The questionnaire also included questions regarding the incidence of secondary non-response in their centers and their therapeutic practice in the event of secondary non-response. Physicians were not asked specifically whether the treatment options in the event of secondary non-response outlined in the questionnaire were available in their country. The full questionnaire is reproduced in the appendix at the end of this article. Descriptive statistics were calculated; no formal additional analyses were conducted.
Results
Definition of secondary non-response
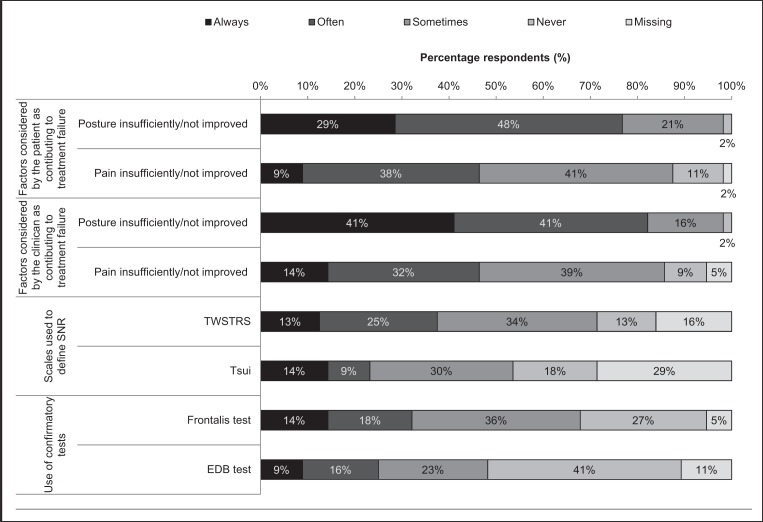
Clinical opinion suggests that patients frequently consider both insufficiently improved head/neck posture and lack of improvement in pain to be relevant to treatment failure, although insufficient improvement in posture was rated as more relevant than insufficient improvement in pain; 98% vs 88%, respectively (Fig. 1). There was no clear difference between patients’ and clinicians’ perceptions.
Figure 1.
Clinicians’ practice in establishing secondary non-response (n=56).
Abbreviations: SNR=secondary non-response; TWSTRS=Toronto Western Spasmodic Torticollis Rating Scale; EDB=extensor digitorum brevis.
More than half of the surveyed neurologists (55%) recommend that three consecutive injection cycles with reduced or no benefit are necessary to define secondary non-response. Two, four or five injection cycles were each considered necessary by <15% of respondents. A decrease in the intervals between injections did not seem to be considered a relevant factor for establishing treatment failure (ratings for sometimes, often, and always were 48%, 14%, and 2% of physicians, respectively).
Clinical examination of head and neck posture is often performed to define secondary non-response, more frequently using the TWSTRS (71%) than the Tsui scale (54%) (Fig. 1). Clinicians who use a confirmatory test to establish secondary non-response used the frontalis test more frequently than the extensor digitorum brevis test (68% vs 48%, Fig. 1). Antibody testing is rarely performed; 86% of clinicians never conduct antibody testing and 13% only sometimes do.
Occurrence of secondary non-response and treatment practice
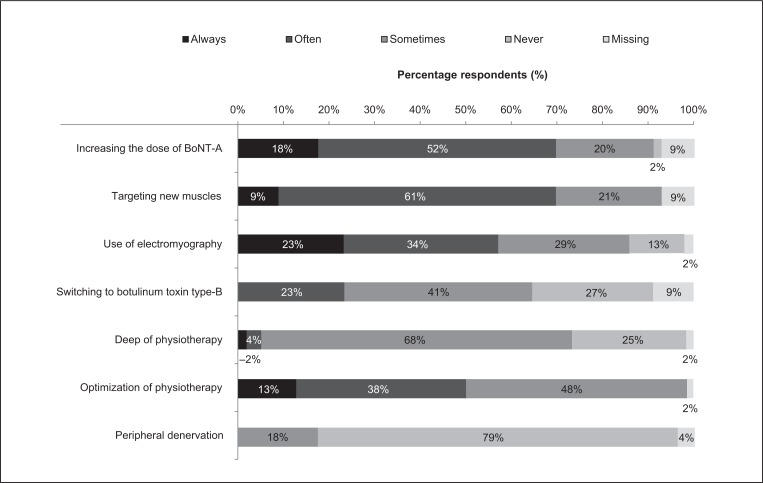
The database records of those surveyed showed that secondary non-response was reported in 5.9% of treated patients (median). Treatment practices adopted in the event of secondary non-response are shown in figure 2. The most common treatment practice, considered at least sometimes by 98% of physicians, was optimization of physiotherapy. In addition to physiotherapy, other common treatment practices included increasing the dose of BoNT-A (89% of physicians), targeting new muscles with the treatment (91%), and using EMG (86%). Switching to BoNT-B and deep brain stimulation were considered by 64% and 73% of physicians, respectively. Peripheral denervation was considered by only 18% of physicians. However, it was not documented whether all the alternative treatment approaches were available to all the physicians.
Figure 2.
Clinicians’ practice for management of secondary non-response (n=56).
Abbreviations: BoNT-A=botulinum toxin type A.
Discussion
According to the international panel of neurologists we surveyed, secondary non-response in CD is considered to have occurred following BoNT-A treatment in the presence of insufficiently improved head/neck posture after three injection cycles. In their practice, the frontalis test is the additional examination most frequently applied to confirm the diagnosis, although it is not used to confirm all cases. Antibody testing in cases of secondary non-response is not generally conducted in clinical practice.
The frequency of secondary non-response reported in this survey reflects clinical practice (5.9%) and contradicts some findings reporting significantly higher rates of secondary non-response. Changing the administration technique (targeting new muscles or using EMG) or increasing the BoNT dose following secondary non-response appear to be common practice, suggesting that clinicians assume this phenomenon is related to dose or administration rather than to true pharmacological resistance. Interestingly, among those surveyed, shorter intervals between injections were not considered to be a relevant factor for establishing treatment failure.
Our proposed definition of secondary non-response could be used in routine practice as well as in clinical research. Indeed, we are currently conducting an international, retrospective, non-interventional case-control study using our definition to identify factors influencing secondary non-response to BoNT-A injections in subjects suffering from idiopathic CD. This study will provide more detailed insight into patients with secondary non-response and will collect demographic information on patients experiencing secondary non-response as well as detailed information on BoNT-A treatment dosing history.
With respect to our findings, it should be borne in mind that the questionnaire utilized in this survey has not been validated in independent studies. Moreover, as with all non-controlled studies with a retrospective design, the potential for bias (for example, bias arising from reliance on written history or the recall of individuals) and the presence of confounding factors cannot be excluded. Accordingly, given the implications of secondary non-response for both patients and treating physicians, we feel that there is a need for further prospective studies to identify the real factors contributing to a decrease in the benefit after subsequent treatments and provide therapeutic guidance on treatment strategies in secondary non-response.
In conclusion, the results of this survey indicate that in clinical practice, secondary non-response can be defined as an insufficiently improved posture after ≥3 unsuccessful injection cycles in CD patients previously achieving satisfactory results. This definition is being used in an ongoing study to further investigate factors influencing secondary non-response to BoNT-A injections in subjects suffering from idiopathic cervical dystonia.
Acknowledgments
Editorial assistance for the preparation of this manuscript was provided by Ogilvy Healthworld Medical Education; funding was provided by Ipsen.
APPENDIX.
BEST RESPONDERS - CERVICAL DYSTONIA (CD): DEFINITION OF SECONDARY NON-RESPONS E TO BOTULINUM TOXIN TYPE A (BONT-A)
| COUNTRY | PRE-IDENTIFIED BY IPSEN | ||
| INVESTIGATOR’S NAME | PRE-IDENTIFIED BY IPSEN (1 QUESTIONNAIRE PER PHYSICIAN) | ||
| INVETSIGATOR’S SPECIALITY | □NEUROLOGY | □REHABILITATION | □NEUROSURGERY |
| WHEN DID YOU TREAT YOUR 1ST CD PATIENT WITH BONT-A? | □ |
 YEAR YEAR |
|
| DATE OF QUESTIONNAIRE COMPLETION |
 DAY DAY
 MONTH MONTH
 YEAR YEAR |
||
| SIGNATURE | |||
Guidance for completion of the questionnaire:
Never=<10% - Sometimes=10% to 50% - Often=>50% to 90% - Always=>90%
HOW WOULD YOU DEFINE A TREATMENT FAILURE WITH BoNT-A?
| TICK ONLY 1 BOX PER LINE | Never | Some-times | Often | Always |
|---|---|---|---|---|
| 1) ON THE BASIS OF PATIENT FEEDBACK (DISSATISFIED, REDUCED/LACK OF EFFICACY)? | ||||
| 1a) WHAT, FOR THE PATIENT, ARE THE FACTORS THAT CONTRIBUTE MOST TO PERCEIVED TREATMENT FAILURE? | ||||
| PAIN INSUFFICIENTLY IMPROVED OR NOT IMPROVED? | ||||
| POSTURE INSUFICIENTLY IMPROVED OR NOT IMPROVED? | ||||
| OTHER? | ||||
| 2) ON THE BASIS OF YOUR CLINICAL EXAMINATION? | ||||
| IF SO, PERFORMED USING TWSTRS? | ||||
| TSUI? | ||||
| OTHER? | ||||
| 2a) WHAT, FOR YOU, ARE THE FACTORS THAT CONTRIBUTE MOST TO PERCEIVED TREATMENT FAILURE? | ||||
| PAIN INSUFFICIENTLY IMPROVED OR NOT IMPROVED? | ||||
| POSTURE INSUFICIENTLY IMPROVED OR NOT IMPROVED? | ||||
| OTHER? | ||||
| 3) DO YOU CONSIDER REDUCING THE INTERVAL BETWEEN INJECTIONS TO BE A RELEVANT FACTOR FOR DEFINITION OF TREATMENT FAILURE? | ||||
| 4) DO YOU CONFIRM THE DIAGNOSIS OF TREATMENT FAILURE WITH: | ||||
| 4a) FRONTALIS TEST? | ||||
| 4b) EXTENSOR DIGITORUM BREVIS TEST? | ||||
| 4c) OTHER TEST? | ||||
| 5) DO YOU PERFORM NEUTRALIZING ANTIBODY TESTING? |
| ON AVERAGE, AFTER HOW MANY INJECTION CYCLES (WITH NO OR REDUCED BENEFIT) WOULD YOU CONSIDER BoNT-A THERAPY TO HAVE FAILED? | ||||||
| (TICK ONLY ONE BOX) | □1 | □2 | □3 | □4 | □5 | □6 |
INCIDENCE OF TREATMENT FAILURE IN YOUR CENTER IN 2008
| HOW MANY PATIENTS SUFFERING FROM CERVICAL DYSTONIA DO YOU HAVE IN YOUR CLINICAL DATABASE? |

|
| HOW MANY PATIENTS SUFFERING FROM CERVICAL DYSTONIA DID YOU TREAT WITH BoNT-A IN 2008? |

|
| HOW MANY DE NOVO PATIENTS SUFFERING FROM CERVICAL DYSTONIA DID YOU TREAT WITH BoNT-A IN 2008? |

|
| HOW MANY OF THESE PATIENTS (DE NOVO & PREVIOUSLY TREATED) WOULD YOU CONSIDER TO BE TREATMENT FAILURES (ACCORDING TO THE ABOVE DEFINITON)? |

|
WHAT IS YOUR USUAL THERAPEUTIC PRACTICE IN THE EVENT OF SECONDARY TREATMENT FAILURE?
| TICK ONLY 1 BOX PER LINE | Never | Sometimes | Often | Always |
|---|---|---|---|---|
| WITH REGARD TO THE DOSE OF BoNT-A | ||||
| NO CHANGE | □ | □ | □ | □ |
| I INCREASE THE DOSE | □ | □ | □ | □ |
| I DECREASE THE DOSE | □ | □ | □ | □ |
| WITH REGARD TO THE INJECTED MUSCLES | ||||
| NO CHANGE | □ | □ | □ | □ |
| I INJECT NEW MUSCLES | □ | □ | □ | □ |
| WITH REGARD TO EMG | ||||
| I CONSIDER STARTING TO USE EMG TO OPTIMIZE MUSCLE TARGETING | □ | □ | □ | □ |
| WITH REGARD TO THE BoNT-A PREPARATION | ||||
| I CONTINUE WITH THIS PREPARATION | □ | □ | □ | □ |
| I TEMPORARILY INTERRUPT THIS PREPARATION | □ | □ | □ | □ |
| I SWITCH TO ANOTHER BoNT-A PREPARATION | □ | □ | □ | □ |
| I SWITCH TO A BoNT-B PREPARATION | □ | □ | □ | □ |
| I STOP BoNT THERAPY (WHATEVER THE SEROTYPE) | □ | □ | □ | □ |
IF YOU ASSUME BoNT-A FAILURE
| Never | Sometimes | Often | Always | |
|---|---|---|---|---|
| DO YOU REFER YOUR PATIENT TO AN OTHER BoNT-A CENTER? | □ | □ | □ | □ |
| DO YOU REFER YOUR PATIENT FOR PERIPHERAL D ENERVATION? | □ | □ | □ | □ |
| DO YOU R EFER Y OUR P ATIENT F OR D EEP BRAIN STIMULATION? | □ | □ | □ | □ |
| DO YOU OPTIMIZE PHYSICAL THERAPY? | □ | □ | □ | □ |
| OTHER? | □ | □ | □ | □ |
References
- Albanese A, Asmus F, Bhatia KP, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- Brin MF. Treatment of Dystonia. In: Jankovic J, Tolosa E, editors. Parkinson’s Disease and Movement Disorders. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 348–355. [Google Scholar]
- Brin MF, Comella CL, Jankovic J, et al. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord. 2008;23:1353–1360. doi: 10.1002/mds.22157. [DOI] [PubMed] [Google Scholar]
- Consky ES, Lang AE. Clinical assessments of patients with cervical dystonia. In: Jankovic J, Hallett M, editors. Therapy with Botulinum Toxin. M. Dekker; New York: 1994. pp. 211–237. [Google Scholar]
- Cordivari C, Misra VP, Vincent A, et al. Secondary non-responsiveness to botulinum toxin A in cervical dystonia: the role of electromyogram-guided injections, botulinum toxin A antibody assay, and the extensor digitorum brevis test. Mov Disord. 2006;21:1737–1741. doi: 10.1002/mds.21051. [DOI] [PubMed] [Google Scholar]
- Dressler D. Clinical presentation and management of antibody-induced failure of botulinum toxin therapy. Mov Disord. 2004;19(Suppl 8):S92–S100. doi: 10.1002/mds.20022. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Yoshimura DM, Olney RK, et al. Change in pattern of muscle activity following botulinum toxin injections for torticollis. Ann Neurol. 1991;29:370–376. doi: 10.1002/ana.410290407. [DOI] [PubMed] [Google Scholar]
- Gordon PH, Gooch CL, Greene PE. Extensor digitorum brevis test and resistance to botulinum toxin type A. Muscle Nerve. 2002;26:828–831. doi: 10.1002/mus.10231. [DOI] [PubMed] [Google Scholar]
- Haussermann P, Marczoch S, Klinger C, et al. Long-term follow-up of cervical dystonia patients treated with botulinum toxin A. Mov Disord. 2004;19:303–308. doi: 10.1002/mds.10659. [DOI] [PubMed] [Google Scholar]
- Hsiung GY, Das SK, Ranawaya R, et al. Long-term efficacy of botulinum toxin A in treatment of various movement disorders over a 10-year period. Mov Disord. 2002;17:1288–1293. doi: 10.1002/mds.10252. [DOI] [PubMed] [Google Scholar]
- Lange O, Bigalke H, Dengler R, et al. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol. 2009;32:213–218. doi: 10.1097/WNF.0b013e3181914d0a. [DOI] [PubMed] [Google Scholar]
- Mohammadi B, Buhr N, Bigalke H, et al. A long-term follow-up of botulinum toxin A in cervical dystonia. Neurol Res. 2009;31:463–466. doi: 10.1179/174313209X405137. [DOI] [PubMed] [Google Scholar]
- Tsui JK, Eisen A, Stoessl AJ, et al. Double-blind study of botulinum toxin in spasmodic torticollis. Lancet. 1986;2:245–247. doi: 10.1016/s0140-6736(86)92070-2. [DOI] [PubMed] [Google Scholar]