Summary
Obstacle avoidance studies have been extensively performed in normally developed subjects (N), but little work has been done on the characterization of this task in subjects with Down syndrome (DS). The aim of this study was to describe the management of walking with obstacle avoidance in adults with DS and in age-matched N subjects, considering both the lower and upper limbs. Ten subjects with DS and 16 N subjects were evaluated. The subjects walked along a walkway in two conditions: level, unobstructed walking and walking with an obstacle. The tasks were acquired using three-dimensional quantitative movement analysis. Spatiotemporal and kinematic parameters for the trunk, upper limbs and lower limbs were analyzed. The results demonstrated that the presence of a destabilizing element, the obstacle, enhanced different motor strategies in DS compared with N subjects, as shown by the parameters of the lower limbs, with a stabilization and safety strategy adopted at the upper limbs in DS.
Keywords: Down syndrome, motion analysis, motor strategy, obstacle avoidance
Introduction
Walking is a motor task characterized by highly flexible adaptation to different situations. When walking we often have to avoid obstacles, either by navigating around them or stepping over them. The ability to avoid obstacles automatically obviously involves mechanisms in the brain that represent the properties of the obstacle (size, orientation, movement, etc.) and its location relative to the body and update these representations as the body moves (Pearson and Gramlich, 2010). Knowledge of the mechanics of the locomotor system and of the control strategies adopted during this activity is helpful for a better understanding and identification of the risk factors for tripping, which is important in the prevention of falls (Chen and Lu, 2006).
Subjects with DS often exhibit both motor and perceptual difficulties that impact on their motor development and everyday life. Reduced step length and velocity are well-known features of DS walking patterns, which are related to the sense of instability perceived by DS subjects (Rigoldi et al., 2010). A tendency toward flexion of the lower limb joints during level, unobstructed walking is also well documented in DS, together with a predominance of movement in the frontal and horizontal planes (Rigoldi et al., 2010). Deficits in perceptual-motor processing and function are also documented, but remain unclear (4). From these elements it can be concluded, as indeed anecdotal evidence also suggests, that individuals with DS often have trouble avoiding obstacles, which results in increased frequency of tripping and falling (Virji-Babul and Brown, 2004).
Several studies have described the kinematics and kinetics of obstacle crossing in healthy adults (Begg et al., 1998; Chen et al., 1991; Weerdesteyn et al., 2005) and the role of vision during obstacle avoidance tasks (Patla, 1997). It is known from the literature (Patla and Prentice, 1995) that in healthy subjects limb elevation for obstacle avoidance is achieved primarily by flexing the three joints of the swing limb, although the proximal joints (hip and knee) are more flexed than the ankle joint. Thus, in healthy subjects, obstacle avoidance, as well as level, unobstructed walking, is prevalently directed in the antero-posterior direction, with a flexion-extension joint movement. Studies (Begg et al., 1998; Sparrow et al., 1996) have shown that the kinematic and kinetic characteristics of the leading limb (i.e. the limb that crosses the obstacle first) are different in a number of respects from those of the trailing limb (i.e. the support limb during leading limb crossing), highlighting different crossing strategies between them. Indeed, whereas the trailing limb moves upward as it crosses the obstacle, the leading limb begins its descent while going over the obstacle. Thus, the center of mass moves toward the supporting limb during trailing limb crossing, while it moves away from the supporting limb during leading limb crossing. This puts the leading limb in a more vulnerable situation compared to the trailing limb (Patla et al., 1996).
Chen et al. (1991) described the different crossing strategies adopted by healthy younger and older adults: neither group demonstrated any changes in approach speed as obstacle height increased, while significant decreases in crossing speed were found. This suggested a “conservative” strategy in which most of the walking parameters were kept unvaried in the approach phase. However, in the elderly group a “step-shortening” strategy was observed when the subjects were challenged by the presence of an obstacle, and this was interpreted as a safety strategy or as difficulty in interpreting the sensory input given by the obstacle.
Chou et al. (1997) found that toe clearance (i.e. the distance between the foot and the obstacle/ground in the vertical direction) increased in the presence of an obstacle compared to walking on the level ground, but remained quite constant over a wide range of obstacle heights. They noted that obstacle crossing was a more energy-consuming strategy than walking on the level; in fact, additional energy was generated to increase foot-obstacle clearance and ensure safe progression of the foot over the obstacle.
Although there are numerous studies dealing with obstacle avoidance in healthy subjects, only a few have addressed obstacle avoidance in pathological conditions; of these, to the best of our knowledge, only three have focused on subjects with DS (Virji-Babul and Brown, 2004; Smith and Ulrich, 2008; Wu et al., 2008). Virji-Babul and Brown (2004) examined the movement strategies and the role of vision in five children with DS (age range: 5–6 years) and in six typically developing children (age range: 4–7 years) as they crossed obstacles of two different heights: a “subtle” obstacle that was placed at a minimal distance from the floor and an “obvious” obstacle that was placed at a much greater height from the floor. They focused on the step length and toe clearance parameters. Children with DS showed a robust scaling of toe elevation to obstacle height, implying that they were able to successfully extract information about obstacle height and appropriately match this information to their movements. However, visual information about the obstacle was not used consistently to modulate movements early in the gait cycle; as a result, the children with DS maintained their “typical” gait pattern and waited until they reached the obstacle to extract the visual information needed to appropriately modulate their actions, and thus produced less smooth trajectories. Smith and Ulrich (2008) evaluated the adoption of stabilizing strategies in older adults with DS (35 to 62 years old) during obstacle avoidance, to determine whether the gait patterns of adults with DS showed early changes related to age, obesity and a sedentary lifestyle. They focused on walking speed, stride length, cadence and step width parameters and concluded that “the combined effects of ligamentous laxity, low tone, obesity, inactivity and physiological decrements associated with aging led to [...] stability-enhancing adaptations at a younger chronological age in adults with DS” compared with normally developed adults. Finally, Wu et al. (2008) analyzed the same parameters as Smith and Ulrich (2008) to evaluate different treadmill interventions to improve obstacle avoidance ability in children with DS. It thus emerges that obstacle avoidance in healthy subjects has received considerable attention in the literature, while little work has been done on the characterization of this task in DS. Furthermore, not only are DS subjects the focus of fewer studies on this topic, they have also been evaluated using only a limited number of spatiotemporal parameters, whereas lower limb evaluation in healthy subjects has involved a larger number of spatiotemporal, kinematic and kinetic parameters. Finally, there is a lack of studies on obstacle avoidance strategies in young adults with DS.
The aim of this study was to evaluate the ability to avoid obstacles during walking in subjects with DS and in age-matched normally developed (N) subjects through the evaluation of spatiotemporal and kinematic parameters, and with attention to both the lower and the upper limbs.
Materials and methods
Subjects
Ten subjects with DS and 16 age-matched N subjects were evaluated. The subjects and their legal guardians gave their informed consent to the study. The study was approved by the ethics committee of IRCSS San Raffaele Pisana, Tosinvest Sanità, Rome, Italy.
Inclusion criteria for the DS group were: teenage to adult age, no severe obesity (normal to overweight body mass index, 18.5<BMI<30), mild to moderate mental retardation, no clinical sign of dementia, no orthopedic problems.
Inclusion criteria for the N subjects were: teenage to adult age, no severe obesity (normal to overweight body mass index, 18.5<BMI<30), no clinical sign of dementia, no orthopedic problems, no reported motor and/or neurological disorders.
Table I describes the mean values for the DS and N groups.
Table I.
Characteristics of the subjects with Down syndrome (DS) and the group of normally developed subjects (N).
| Group | Age | IQ | Weight [kg] | Height [cm] | BMI (kg/m^2) |
|---|---|---|---|---|---|
| DS | 22 (6) | 56 (12) | 58 (9) | 154 (8) | 24 (3) |
| N | 25 (3) | – | 62 (14) | 170 (9) | 21 (3) |
Data are expressed as mean (standard deviation) values. IQ=intelligence quotient: BMI=Body Mass Index.
Acquisition and instrumentation
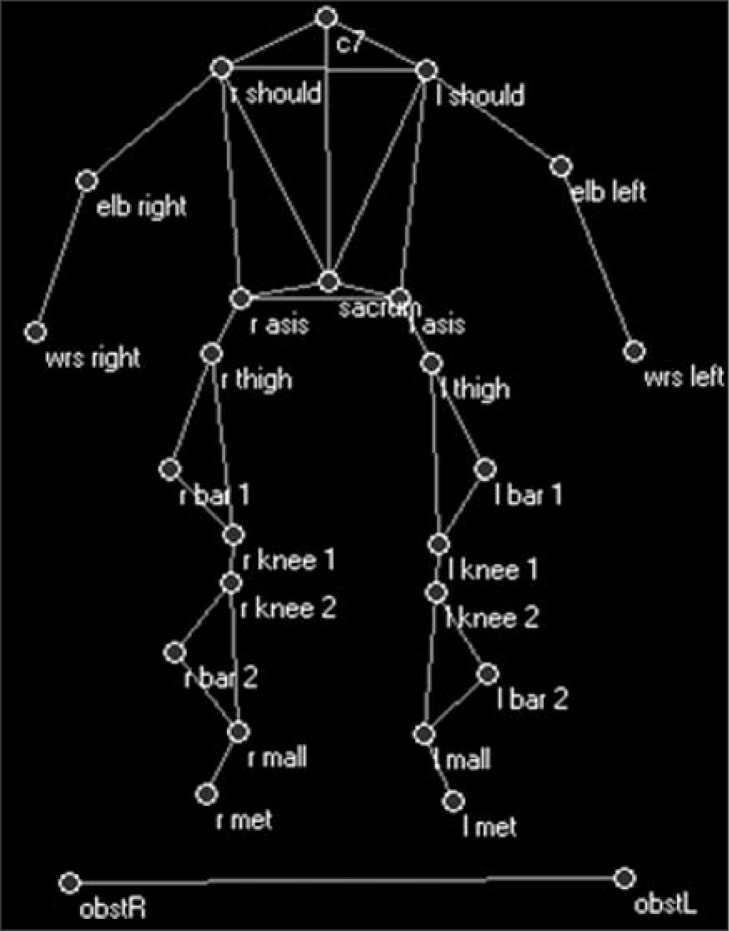
The subjects walked along a walkway in two conditions: level, unobstructed walking (WLK) and walking with an obstacle (10% of the subject’s height, OBST). The obstacle was a wooden stick approximately two meters long and with a diameter of 6 mm, which rested on two colored supports placed laterally to the walkway. The obstacle was chosen for safety reasons. In fact, the stick was simply laid on the lateral supports and fell off in the event of contact. The lateral supports had a dual function, supporting the stick and acting as a visual cue signaling the presence of the obstacle. The subjects were required to walk three times in each condition. The tasks were acquired for quantitative movement analysis using an optoelectronic system with eight infrared cameras (Elite2002, BTS, Milan). The optoelectronic system records the three-dimensional coordinates of the markers over time. Markers were placed on the subject’s body according to a modified Davis’ protocol (Davis et al., 1991) and two markers were placed one at each end of the obstacle to indicate its position relative to the subject during the movement (Fig. 1).
Figure 1.
Modified Davis’ protocol for marker placement.
Parameters
The limb that crossed the obstacle first was referred to as the leading limb, while the support limb during the crossing of the leading limb was referred to as the trailing limb. Since it has been demonstrated that the leading limb is in a more vulnerable situation compared with the trailing limb, and less likely to recover from an unexpected trip (Patla et al., 1996), we focused on the motor strategies adopted by the leading limb. The swing phase was defined as the phase during which the leading foot was crossing the obstacle (from toe-off to second ground contact) in the OBST condition, while in WLK it was defined as the phase from toe-off to the second ground contact of the foot.
From the marker coordinates several spatiotemporal and kinematic parameters were computed to describe the movement strategy adopted by the subjects.
Spatiotemporal parameters
The computed spatiotemporal parameters were:
– step length (SL) [m]: distance (in the antero-posterior, AP, direction) between consecutive contacts of the two feet, with the feet astride the obstacle. It was computed as the AP distance between the markers on the 5th metatarsi at initial contact. It measures the presence of step adaptations (i.e. step shortening/lengthening strategy) in the presence of an obstacle (Chen et al., 1991; Weerdesteyn et al., 2005).
– Max height [m]: maximum elevation of the foot (from the obstacle in OBST and from the ground in WLK), during the swing phase. It was computed as the difference between the maximum vertical height reached by the markers on the 5th metatarsus and the obstacle height/ground level. It measures the subject’s ability to estimate obstacle height and the “safety” margin adopted during crossing (Chou et al., 1997).
– Mean velocity of approach: mean velocity of the marker on the sacrum during the approach phase (before the first initial contact of the trailing limb) in the AP direction (Vmean app.X). It is a measure of “conservation” of the movement (Chen et al., 1991).
– Mean velocity during obstacle crossing: mean velocity of the marker on the sacrum during the avoidance phase (between the first and second trailing limb initial contacts) in the AP direction (Vmean obst.X).
Kinematic parameters
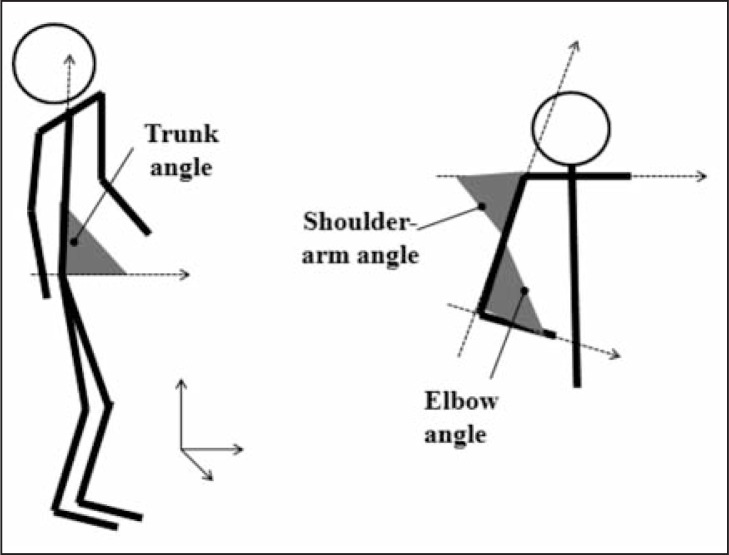
Starting from the coordinates of the markers placed according to Davis’ protocol, the maximum (Max), minimum (Min) and range of motion (ROM) angular values in the three planes of movement for the pelvis (P), hip (H), knee (K) and ankle (A) joints during the swing phase were calculated for the leading limb during the swing phase. For a detailed description of the definition and calculation of these angles, see Davis et al. (1991). Max, Min and ROM values during the swing phase were computed for the trunk flexion angle, defined as the angle between the vector connecting the sacrum and c7 markers and the vector in the AP direction of the laboratory’s reference system (Fig. 2).
Figure 2.
Definition of the trunk angle, shoulder-arm angle and elbow angle.
To evaluate upper limb kinematics, the Max, Min and ROM angular values of the shoulder-arm (S-A) angle and of the elbow (E) angle (Fig. 2) during the swing phase were calculated for the limb ipsilateral to the leading limb (leading arm) and for the contralateral limb (trailing arm).
For each side, the S-A angle was defined as the angle between the vector connecting the markers on the shoulders and the vector connecting the elbow and shoulder markers. The E angle was defined as the angle between the vector connecting the elbow and shoulder markers and the vector connecting the elbow and wrist markers. We hypothesized that if the presence of the obstacle challenged the subjects’ stability, the subjects would react by lifting their arms (decreasing S-A angles at both sides) to stabilize their center of mass and flexing their elbows (decreasing E angles at both sides) to prepare for possible falls, as described by Marigold et al. (16) in a study describing gait perturbations in healthy adults.
Statistical analysis
The distribution of the data was verified using the Kolmogorov-Smirnov test of normality. The result showed that the data were not normally distributed. However, given the limited number of samples, it might be hypothesized that a larger number of subjects would result in a normal distribution of the data. For this reason, we employed a 2 conditions x 2 groups ANOVA to analyze the presence of statistically significant differences (p-value <0.05) between the two groups (N and DS) in the two conditions (WLK and OBST). Post-hoc analysis, with Bonferroni’s correction for multiple comparisons, was then used for further investigation. Post-hoc power analysis was conducted to determine the power of the study.
Results
All the subjects completed the tasks successfully. The power for the parameters was found to be above 79%. For the sake of brevity, only the statistically significant results most meaningful for for the present analysis are presented. Table II shows the spatiotemporal parameter values (medians, 25th and 75th percentiles) recorded in the DS and N groups in the WLK and OBST conditions. A reduced and more variable step length was found in both conditions in the DS compared with the N subjects. Analysis of the WLK condition showed that the DS subjects had lower excursions (Max height parameter) of the feet compared with the N group. While the N subjects maintained a similar foot excursion between the WLK and OBST conditions, the DS subjects increased their maximum foot height in OBST, reaching the clearance values recorded by the N group in the OBST condition.
Table II.
Spatiotemporal parameter values recorded in the subjects with Down syndrome (DS) and the group of normally developed subjects (N).
| DS | N | DS vs N | |||
|---|---|---|---|---|---|
|
| |||||
| WLK | OBST | WLK | OBST | ||
| SL [m] | 0.49 | 0.60 | 0.69 | 0.70 | 1,2 |
| (0.44,0.54) | (0.50,0.80) | (0.64,0.71) | (0.69,0.70) | ||
| Max height [m] | 0.19 | 0.25 | 0.24 | 0.25 | 1 |
| (0.18,0.19) | (0.21,0.29) # | (0.22,0.24) | (0.21,0.27) | 1 | |
| Vmean app.X [m/s] | 0.80 | 0.60 | 1.30 | 1.20 | 1,2 |
| (0.60,0.80) | (0.50,0.98) # | (1.15,1.35) | (1.08,1.30) | ||
| Vmean obst.X [m/s] | 0.80 | 0.50 | 1.30 | 1.00 | 1,2 |
| (0.60,0.80) | (0.33,0.78) # | (1.15,1.35) | (0.90,1.10) # | ||
Data are expressed as median (25th percentile, 75th percentile) values. SL=step length; Max height [m]=maximum elevation of the foot during the swing phase; Vmean app.X [m/s]=mean velocity of approach; Vmean obst.X [m/s]=mean velocity during obstacle clearance; WLK=level walking condition; OBST=walking with an obstacle.
=p<0.05 WLK vs OBST; 1=p<0.05 DS_WLK vs N_WLK; 2=p<0.05 DS_OBST vs N_OBST.
The mean forward velocity of approach was lower in the DS than in the N subjects in both conditions. In the DS group, this velocity further decreased in OBST (reaching values corresponding to approximately 50% of those of the N subjects). Instead, the N subjects maintained an unvaried approach velocity across the conditions.
The mean forward velocity during obstacle crossing decreased for both groups in OBST but, as seen with the approach phase, the values were consistently lower in the DS than the N subjects. Variability was higher across all conditions in the DS compared with the N subjects. Table III shows the kinematic parameter values (medians, 25th and 75th percentiles) recorded in the WLK and OBST conditions.
Table III.
Leading limb kinematic parameter values in the subjects with Down syndrome (DS) and the group of normally developed subjects (N).
| DS | N | DS vs N | |||
|---|---|---|---|---|---|
|
| |||||
| WLK | OBST | WLK | OBST | ||
| Trunk flexion [ ° ] | |||||
| Max | 83.25 (81.38,84.95) | 84.8 (83.90,87.6) | 87.4 (85.4,89.2) | 87.70 (84.75,89.30) | 1,2 |
| Min | 80.5 (77.13,81.65) | 76.05 (73.13,77.85) # | 83.7 (82.63,85.80) | 80.5 (77.9,83.3) # | 1,2 |
| Pelvic tilt [ ° ] | |||||
| Max | 18.28 (16.36,20.15) | 16.93 (16.01,21.3) | 8.74 (7.21,10.72) | 9.77 (7.88,14.29) | 1,2 |
| Min | 13.48 (11.22,14.98) | 3.33 (−3.82,5.84) # | 6.35 (3.93,7.35) | 2.60 (−0.63,4.19) | 1 |
| Pelvic obliquity [ ° ] | |||||
| Max | 4.64 (2.77,4.85) | 13.05 (9.79,14.60) # | 4.81 (3.49,5.77) | 8.48 (5.64,9.74) # | 2 |
| Min | −3.36 (−5.21, −2.69) | −1.28 (−2.07, 0.25) | −4.64 (−5.61, −3.90) | −2.76 (−4.00, −1.45) | |
| Pelvic rotation [ ° ] | |||||
| Max | 8.51 (6.02,9.27) | 15.84 (12.32,30.50) # | 7.22 (5.08,8.68) | 13.92 (10.59,20.16) # | 2 |
| Min | −7.61 (−8.59, −5.98) | −3.97 (−10.03, −1.82) | −5.80 (−7.05, −4.92) | −8.77 (−10.58, −3.53) | |
| Hip flex-extension [ ° ] | |||||
| Max | 38.79 (27.93,41.26) | 81.82 (76.41,83.35) # | 27.58 (23.95,30.01) | 68.35 (64.79,73.15) # | 1,2 |
| Min | 1.85 (−14.49,7.66) | 7.38 (−0.54,13.93) # | −15.05 (−17.28, −13.38) | −13.87 (−16.88, −9.47) | 1,2 |
| Hip ab-adduction [ ° ] | |||||
| Max | −1.78 (−3.10,1.17) | −3.64 (−7.20, −0.99) | −0.74 (−2.46,1.61) | −2.86 (−4.67,2.86) | |
| Min | −9.93 (−12.05, −8.05) | −14.73 (−18.70, −11.11) # | −9.94 (−10.57, −8.69) | −9.65 (−13.50, −8.18) | 2 |
| Hip rotation [ ° ] | |||||
| Max | 19.11 (6.90,23.63) | 16.20 (11.22,25.02) | 6.22 (0.04,11.75) | 10.40 (−1.33,15.42) | 1,2 |
| Min | 6.51 (−6.12,10.61) | 1.50 (−8.98,11.75) | −3.67 (−10.54, −1.25) | −3.40 (−12.73,1.34) | 1,2 |
| Knee flex-extension [ ° ] | |||||
| Max | 48.55 (39.50,54.57) | 88.73 (87.93,89.52) # | 55.31 (51.36,58.43) | 89.18 (88.84,89.51) # | 1 |
| Min | 5.96 (0.30,10.71) | 10.40 (−3.31,11.13) | −4.50 (−6.23, −0.64) | −2.33 (−5.93, −0.10) | 1,2 |
| Ankle dorsi-plantar-flexion [ ° ] | |||||
| Max | 1.47 (−0.61,4.66) | 8.30 (6.86,13.41) # | −0.03 (−2.40,1.94) | 9.73 (4.83,12.42) # | |
| Min | −12.27 (−15.16, −8.24) | −23.45 (−28.54, −17.00) # | −17.33 (−21.05, −12.49) | −22.55 (−24.04, −10.86) # | |
Data are expressed as median (25th percentile, 75th percentile) values. WLK=level walking condition; OBST=walking with an obstacle.
=p<0.05 WLK vs OBST; 1=p<0.05 DS_WLK vs N_WLK; 2=p<0.05 DS_OBST vs N_OBST.
The DS subjects were more flexed at the trunk than the N subjects in both conditions, and also showed a more anterior pelvic tilt. In DS, the decrease in the pelvic tilt minimum value in OBST determined an increased ROM of the pelvis in the sagittal plane. Pelvic obliquity and pelvic rotation increased in both groups in the OBST condition, but with higher values for the DS than for the N subjects.
The hip joint was more flexed in OBST in both groups, due to the need to avoid the obstacle by raising the limb; however, the DS subjects were more flexed than the N subjects in both WLK and OBST, with patterns shifted towards flexion throughout the crossing. Hip adduction was greater in OBST with respect to WLK in both groups, with the DS subjects found to be more adducted than the N group in all conditions. Hip intra-rotation was greater in DS in both conditions.
At the knee joint, the DS subjects were more flexed than the N subjects in WLK, but both groups showed increased flexion values in OBST, recording similar values. At the ankle joint, both groups showed increased dorsiflexion in the OBST condition.
Table IV (over) shows the kinematic parameter values (medians, 25th and 75th percentiles) of the leading and trailing arms in the WLK and OBST conditions.
Table IV.
Kinematic parameter values of the leading and trailing arms in the subjects with Down syndrome (DS) and the group of normally developed subjects (N).
| DS | N | DS vs N | |||
|---|---|---|---|---|---|
|
| |||||
| WLK | OBST | WLK | OBST | ||
| Elbow angle [ ° ] | |||||
| Leading arm_Min | 138.90 (137.03,141.75) | 119.80 (105.30,125.30) # | 136.80 (128.85,140.30) | 133.30 (120.60,139.90) | 2 |
| Trailing arm_Min | 145.25 (141.65,148.55) | 123.70 (114.80,130.30) # | 132.40 (128.65,137.40) | 129.20 (114.73,134.73) | 2 |
| Shoulder-arm angle [ ° ] | |||||
| Leading arm_Min | 66.05 (63.03,69.75) | 58.30 (56.40,65.70) # | 68.00 (66.30,71.45) | 68.80 (67.20,72.83) | 1,2 |
| Trailing arm_Min | 65.60 (57.60,69.53) | 53.20 (50.80,58.20) # | 75.20 (65.65,76.20) | 69.90 (63.25,74.03) | 1,2 |
Data are expressed as median (25th percentile, 75th percentile) values. WLK=level walking condition; OBST=walking with an obstacle.
=p<0.05 WLK vs OBST; 1=p<0.05 DS_WLK vs N_WLK; 2=p<0.05 DS_OBST vs N_OBST.
In the DS group, the elbow angle minimum values for both arms were higher in WLK than in OBST. In the OBST condition, the DS subjects flexed their elbows more than the N subjects did. No difference across conditions was found in the N group.
In the DS group, the shoulder-arm angle minimum values for both arms were lower in the OBST than in the WLK condition. The DS subjects showed lower values than the N subjects in both conditions.
Considerations
Spatiotemporal parameters
Reduced step length and velocity are well-known features of DS walking patterns, which are related to these subjects’ perceived instability (3). In both conditions, but especially in OBST, the DS subjects, compared with the N group, displayed higher variability and lower values of step length during obstacle crossing, and lower velocity in the approach and crossing phases. Conversely, the N subjects maintained similar step length and approach velocity values in both conditions, while only mean velocity in the crossing phase decreased (but with values that remained higher than those recorded in DS). These results demonstrated the presence of a more conservative strategy in the N subjects.
During WLK, maximum foot elevation was lower in the DS than in the N subjects. This may be a cause of increased tripping in DS. The DS subjects increased their foot elevation in OBST, reaching N values. Thus, the presence of the obstacle elicited a correct motor response in DS. In the N group, the safety margin remained unvaried across conditions.
Kinematic parameters
A tendency towards flexion of the lower limb joints during level, unobstructed walking is a well-documented feature of DS (Rigoldi et al., 2010), and was confirmed by our study. In the DS group, the trunk was more forward-flexed and the hip showed an increased intra-rotation in WLK.
In the OBST condition, the N subjects adjusted their movement to the presence of the obstacle by increasing hip and knee flexion, and by increasing ankle dorsiflexion to avoid hitting the obstacle with their foot. As the limb was being lifted and moved forward, pelvic obliquity and intra-rotation increased. Trunk flexion decreased as they straightened their trunk to allow hip flexion. An increased plantar-flexion of the ankle at final stance allowed the N subjects to land with the L foot in “tiptoe” position.
In the OBST condition, trunk, pelvis and particularly hip joint flexion continued to be a feature characterizing the DS compared with the N subjects. The DS subjects increased their hip and knee flexion, and increased dorsiflexion of the ankle to avoid the obstacle. Thus, in the sagittal plane, a strategy similar to that of the N subjects was found, even though the flexion values were higher in the DS group. In the other planes, pelvic obliquity and rotation increased together with hip abduction, leading to values higher than those of the N subjects. Hip intrarotation remained higher than in the N group.
The upper limb parameters revealed that the DS subjects perceived the presence of the obstacle as a threat to their stability. Indeed, they lifted their arms (decreasing the shoulder-arm angles at both sides) to stabilize their center of mass and flexed their elbows (decreasing elbow angles at both sides) in anticipation of a possible fall.
Discussion
The above results showed that the presence of a destabilizing element, the obstacle, enhanced different motor strategies in DS compared to N subjects. At least four main differences were found between the strategies adopted by the two groups.
First, as regards the kinematic parameters, major differences were found in the pelvis and hip joint patterns in the DS compared with the N subjects. Whereas the N subjects modified their movement only in the main plane of movement, i.e. the sagittal plane, consistently with the study by Patla and Prentice (1995), the DS subjects showed increased values for the sagittal, frontal and horizontal planes.
Second, analysis of the spatiotemporal parameters revealed a conservative strategy in the N but not in the DS subjects, who, in the presence of the obstacle, reduced their velocity of approach and decreased their step length. This “step-shortening” strategy has been interpreted in the literature, in the case of elderly subjects, as a safety strategy or as difficulty in interpreting the sensory input given by the obstacle (Chen et al., 1991). Thus, the DS subjects may have been varying their gait parameters due to programming and decision-making problems, or to uncertainty in the integration of the sensory information. The finding, in agreement with Virji-Babul and Brown (2004), that DS and N showed similar foot elevations in the presence of the obstacle may indicate that the visual information is correctly extracted, which would suggest that the deficit is more linked to subsequent motor programming.
Third, although the foot elevation was similar in the two groups, the N subjects exploited it to progress forward (longer step lengths); the DS subjects on the other hand, showing shorter step lengths, did not exploit the elevation in order to land with their foot further forward. Obstacle avoidance is already more costly, in terms of energy consumption, than level, unobstructed walking (Chou et al., 1997) and the DS subjects’ non-exploitation of the limb elevation presumably means that they expend even more energy than the N subjects.
Fourth, in DS the presence of an obstacle enhanced stabilization and safety strategies at the level of the upper limbs, which were elevated forward and outward in an attempt to stabilize the center of mass and prepare for possible falls, in line with the upper limb movement strategies described by Marigold et al. (2003) in healthy subjects challenged with perturbations during gait.
Thus, walking with obstacle avoidance seems to be more destabilizing for subjects with DS, for whom adaptation to the presence of an obstacle seems to be more energy consuming than it is for N subjects. These results suggest that a rehabilitation therapy based on obstacle avoidance tasks could be helpful in subjects with DS to improve their stability and motor strategies, and to decrease their risk of falling.
References
- Begg RK, Sparrow WA, Lythgo ND. Time-domain analysis of foot-ground reaction forces in negotiating obstacles. Gait Posture. 1998;7:99–109. doi: 10.1016/s0966-6362(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Chen HC, Ashton-Miller JA, Alexander NB, et al. Stepping over obstacles: gait patterns of healthy young and old adults. J Gerontol. 1991;46:M196–203. doi: 10.1093/geronj/46.6.m196. [DOI] [PubMed] [Google Scholar]
- Chen HL, Lu TW. Comparisons of the joint moments between leading and trailing limb in young adults when stepping over obstacles. Gait Posture. 2006;23:69–77. doi: 10.1016/j.gaitpost.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Chou LS, Draganich LF, Song SM. Minimum energy trajectories of the swing ankle when stepping over obstacles of different heights. J Biomech. 1997;30:115–120. doi: 10.1016/s0021-9290(96)00111-x. [DOI] [PubMed] [Google Scholar]
- Davis B, Õunpuu S, Tyburski D, et al. A gait 367 analysis data collection and reduction technique. Hum Mov Sci. 1991;10:575–587. [Google Scholar]
- Marigold DS, Bethune AJ, Patla AE. Role of the unperturbed limb and arms in the reactive recovery response to an unexpected slip during locomotion. J Neurophysiol. 2003;89:1727–1737. doi: 10.1152/jn.00683.2002. [DOI] [PubMed] [Google Scholar]
- Patla AE. Understanding the roles of vision in the control of human locomotion. Gait Posture. 1997;5:54–69. [Google Scholar]
- Patla AE, Prentice SD. The role of active forces and intersegmental dynamics in the control of limb trajectory over obstacles during locomotion in humans. Exp Brain Res. 1995;106:499–504. doi: 10.1007/BF00231074. [DOI] [PubMed] [Google Scholar]
- Patla AE, Rietdyk S, Martin C, et al. Locomotor patterns of the leading and the trailing limbs as solid and fragile obstacles are stepped over: some insights into the role of vision during locomotion. J Mot Behav. 1996;28:35–47. doi: 10.1080/00222895.1996.9941731. [DOI] [PubMed] [Google Scholar]
- Pearson K, Gramlich R. Updating neural representations of objects during walking. Ann N Y Acad Sci. 2010;1198:1–9. doi: 10.1111/j.1749-6632.2009.05422.x. [DOI] [PubMed] [Google Scholar]
- Rigoldi C, Galli M, Albertini G. Gait development during lifespan in subjects with Down syndrome. Res Dev Disabil. 2011;32:158–163. doi: 10.1016/j.ridd.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Smith BA, Ulrich BD. Early onset of stabilizing strategies for gait and obstacles: older adults with Down syndrome. Gait Posture. 2008;28:448–455. doi: 10.1016/j.gaitpost.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow WA, Shinkfield AJ, Chow S, et al. Characteristics of gait in stepping over obstacles. Hum Mov Sci. 1996;15:605–622. [Google Scholar]
- Virji-Babul N, Brown M. Stepping over obstacles: anticipatory modifications in children with and without Down syndrome. Exp Brain Res. 2004;159:487–490. doi: 10.1007/s00221-004-1971-5. [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, Nienhuis B, Duysens J. Advancing age progressively affects obstacle avoidance skills in the elderly. Hum Mov Sci. 2005;24:865–880. doi: 10.1016/j.humov.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Wu J, Ulrich DA, Looper J, et al. Strategy adoption and locomotor adjustment in obstacle clearance of newly walking toddlers with Down syndrome after different treadmill interventions. Exp Brain Res. 2008;186:261–272. doi: 10.1007/s00221-007-1230-7. [DOI] [PubMed] [Google Scholar]